Introduction: Life threatening hyperinflammation occurs in hemophagocytic lymphohistiocytosis (HLH) and during acute rejection of hematopoietic stem cell (HSC) grafts. Both conditions reflect acute activation of the immune system, in part due to overproduction of proinflammatory cytokines, particularly interferon gamma (IFNγ). Emapalumab, a fully human monoclonal antibody that binds to IFNγ, is approved by the FDA for refractory or recurrent primary HLH (pHLH) or in patients intolerant to conventional therapy. Off-label use of emapalumab has been reported for treatment of secondary HLH (sHLH) and for HSC graft rejection. The European Medicines Agency did not approve emapalumab for the treatment of HLH and many other regulatory authorities, including the Israeli Ministry of Health, have yet to approve emapalumab for clinical use. Pending such regulatory approval, emapalumab is available in Israel exclusively though the Managed Access Request Program (MAP) on a compassionate basis sponsored by Sobi (Swedish Orphan Biovitrum AB,Reg. no. 556038-9321). Experience that accumulates through on- and off-label use of emapalumab obtained via MAP enriches the knowledge base regarding the uses of this drug in the real-world clinical setting.
Methods:
We summarized the data of patients receiving emapalumab at our tertiary pediatric center. The medication was received after application to the MAP sponsored by Sobi International with import approval of the Israel Ministry of Health on a named-patient basis.
Results:
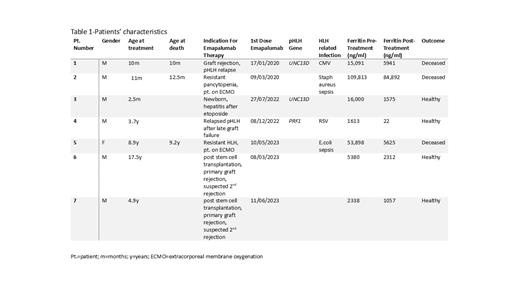
Seven patients (Pt) aged 2.5 months-17.5 years were treated with emapalumab between 3/2020-6/2023. Patients 1-5 fulfilled HLH-2004 diagnostic criteria. Indications for the use of emapalumab included treatment of HLH in a newborn with severe hepatotoxicity after treatment with the standard HLH-94 protocol (Pt. 3), treatment of acute graft rejection with recurrence of HLH in a patient with pHLH (Pt.1), secondary HSC graft failure and disease relapse in a child with pHLH (Pt. 4), sHLH with multi-organ failure (MOF) requiring extracorporeal membrane oxygenation (ECMO) (Pts 2&5) and HSC primary graft rejection with suspected 2 nd rejection (Pts.6&7). Table 1 summarizes the clinical characteristics of all cases. The patient outcomes were closely related to their clinical status at treatment initiation. Pt.3 was able to reach remission and successfully bridge to SCT without complications after only 4 doses of VP-16 with continuation therapy using only dexamethasone and emapalumab. Pt.4, who experienced relapsed pHLH with concomitant HSC graft loss, attained complete remission with emapalumab, dexamethasone and IVIG, facilitating a successful second HSC graft. Pt. 1 expired after initial graft failure, severe sepsis and relapse of pHLH. Pts. 2&5, despite showing improvement of their HLH criteria after treatment with emapalumab, succumbed to MOF that was already present when they were referred to our center for ECMO support. Following unsuccessful emapalumab treatment for primary graft rejection, Pts. 6&7 underwent successful 2 nd HSC transplants with early emapalumab treatment for suspected re-rejection.
Conclusions:
Emapalumab is a safe, effective, and well tolerated treatment for various HLH scenarios, and for similar hyper-inflammatory medical conditions. Administration of emapalumab early in the disease course is likely to be more effective as compared to administration of the drug when MOF has occurred or when the HSC graft has already been rejected. Since these diseases are life-threatening, eliminating delays in procurement of emapalumab is essential to expedite treatment before irreversible organ failure ensues.
OffLabel Disclosure:
Jordan:Sobi: Consultancy, Research Funding. Krauss:Sanofi: Consultancy.
Emapalumab is a fully human, anti-IFNγ monoclonal antibody that binds to both free and receptor-bound IFNy, neutralizing its biologic activity. It was approved by the Food and Drug Administration (FDA) in 2018 for the treatment of adult and pediatric patients with primary HLH with refractory, recurrent or progressive disease or intolerance with conventional HLH therapy. It's administration in secondary HLH or similar hyper-imflammatory states is considered off-label.