Introduction
Young black patients with acute myeloid leukemia (AML) have remarkably worse outcomes than white patients of the same age. Compared to white patients, the death rate of young black patients is fivefold more within 30 days of starting treatment and twofold more within five years. Here we studied the demographic representation of different races in the AML and myelodysplastic syndrome (MDS) clinical trials that led to drug approval by the US Food and Drug Administration (FDA).
Methods
Data were collected from all the trials submitted to the FDA to support the approval of AML and MDS drugs from 2006 to 2022. A total of 17 trials were screened, and 10 trials that did not mention the race or ethnicity of the participants were excluded. Our analysis included six trials of AML drugs and one trial of MDS drugs. The methodological quality of the included studies was evaluated using the NIH quality assessment tool. The inter-study variance was calculated using the Der Simonian-Laird Estimator. Analysis for this study was performed using R version 4.1.2 and R Studio version 1.1.423 (R Project for Statistical Computing).
Results:
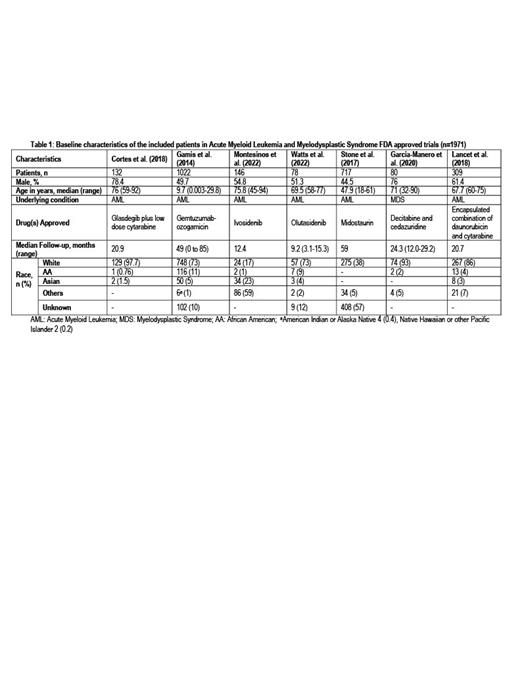
Seven FDA trials reported the race or ethnicity of the participants, and a total of 2484 patients from these trials were analyzed. (Table 1) Olutasidenib, glasdegib plus low dose cytarabine, gemtuzumab-ozogamicin, liposome-encapsulated combination of daunorubicin and cytarabine, ivosidenib, and midostaurin were approved for AML. An oral combination of decitabine and cedazuridine was approved for MDS. The median age of the participants was 69.5 years (0.003-94). The median follow-up time was 20.9 months (0-85.2), and 51% (n=1267) of the participants were male and female participants were 49 % (n=1217). The racial analysis of included patients showed 63.4% (n=1574) white, 5.7% (n=141) black, 3.9% (n=97) Asian, 0.2% (n=4) American Indian/Alaska Native, and 0.1% (n=2) Native Hawaiian or other Pacific Islander while 5.9% (n=147) of the patients belonged to other races. Data about race was not reported for 20.8% (n=519) of the patients. Cortes et al. (2018) reported the highest racial disparity with 97.7% (n=129) white patients and only 0.76% black patients (n=1), while Gamis et al. (2014) reported the highest number of black patients (n=116).
Conclusion:
Despite being affected adversely to a significant degree by AML, black patients are underrepresented in drug trials. More efforts should be made to include them in trials so that better treatment plans can be formulated for this underrepresented group of patients.
Disclosures
Jaglal:Janssen: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; SOBI: Membership on an entity's Board of Directors or advisory committees.