Background: The treatment of acute myeloid leukemia (AML) in older and/or unfit patients consists of a combination of a hypomethylating agent (HMA), such as decitabine and azacitidine, with venetoclax, which can be safely administered in the ambulatory setting. In this study, we aimed to compare the outcomes of the treatment of patients with AML who received chemotherapy with venetoclax and an HMA in an ambulatory setting at an academic center or at a community practice with academic center support.
Methods: This was a retrospective review of patients with de novo or relapsed/refractory AML treated with a combination of venetoclax and either decitabine or azacitidine between February 2018 and June 2023. Patients were included if they received treatment at an academic center or if they received their treatment at a community practice with ongoing consultation at an academic center. Drugs were administered per the standard of care and local practice. The primary objective was to evaluate the overall response rate (ORR). Secondary objectives were to evaluate differences in chemotherapy management between locations, potential socioeconomic disparities, and to identify adverse events that affect the dosing or administration of therapy.
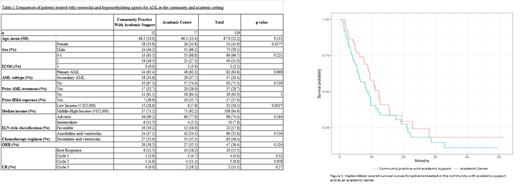
Results: A total of 129 patients received venetoclax plus HMA for AML for a minimum of one cycle as seen in table 1. Of these patients, 77 (60%) received treatment at an academic center, and 52 (40%) received treatment in the community setting. The average age at treatment initiation was 67 years. Disease risk was stratified according to the 2022 European LeukemiaNet (ELN) guidelines. While not statistically significant, there was a tendency for more patients at the academic center to have adverse risk disease (77.9% vs. 69.2%, p=0.364). They were also more likely to have had exposure to a previous HMA (n=10 vs. n=7, p=1) and prior AML treatment (n=20 vs. n=17, p=0.529). Additionally, patients were more likely to have been diagnosed with secondary AML in an academic setting (n=29 vs. n=18, p=0.868). In a multivariate Cox regression model, patients in the middle- and high-income groups, defined as a median household income greater than $52,000, had a 17% lower relative risk regarding survival probability than those in the lower-income group (HR=0.83, 95% CI 0.39-1.73, p=0.622). Overall, patients in the academic setting had a 33% higher relative risk regarding survival probabilities than the community-treated with covariate consideration of performance status, ELN risk, poverty status and exposure to prior-treatments (HR=1.33, 95% CI 0.78-2.26, p=0.285). The OS of all patients was 9.67 months (95% CI 8.33-12.7), with no significant difference between the groups as seen in figure 1. Additionally, the overall response rates (ORR) of the two groups were similar at 38.5% (n=20) and 35.1% (n=27) in community and academic settings, respectively. The complete remission (CR) rate in the community was 11%, while there was an increase of 18% in the academic setting. There were also increased CR rates in the academic compared to the community setting after cycle 1 (4.7 vs. 2.9%, p=0.33), cycle 2 (11.1 vs. 4.8%, p=0.858), and cycle 3 (18.2 vs. 0%, p=0.27). Adverse events in the study were similar except for increased rates of fatigue in the academic setting (76.6% vs. 55.8%, p=0.021). Only 67% of patients underwent a bone marrow biopsy after cycle 1 in the community versus 83% in the academic setting (p=0.101). Lastly, 67% of patients underwent dose reduction with the decitabine with venetoclax regimen in the community setting versus 31% in the academic setting (p=0.012).
Conclusion: This study shows that the use of venetoclax plus HMA allows patients to safely receive AML treatment in the community with support from an academic center. Although there are some significant differences in management, such as increased rates of dose reduction in the community setting, the OS and ORR are similar. However, CR rates remain lower in the community setting. It is important to note that the patients in our study, who were treated at an academic center, had disease characteristics that were more resistant to treatment. Ultimately, this study identifies our efforts in creating community partnerships. Our goal is to further evaluate this partnership with quality improvement projects to bridge the gap in AML care between the community and academic practices.
Disclosures
El Chaer:PharmaEssentia: Research Funding; DAVA Oncology: Other: Travel grant; BioSight: Research Funding; Fibrogen: Research Funding; Celgene: Research Funding; Bristol Myers Squib: Research Funding; Amgen: Consultancy, Research Funding; Novartis: Research Funding; MEI Pharma: Research Funding; Arog Pharmaceuticals: Research Funding; Association of Community Cancer Centers: Consultancy; Sanofi: Research Funding; Sumitomo Pharma Oncology: Consultancy, Research Funding. Reed:Amgen: Research Funding. Keng:Amgen: Research Funding.