In this issue of Blood, Donkó et al report the clinical and biological features of a unique cohort of 54 patients from 37 families with Ras-related C3 botulinum toxin substrate 2 (RAC2) pathogenic variants.1
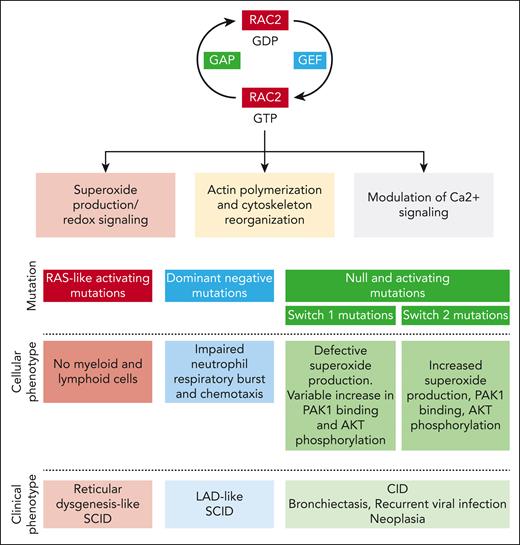
RAC2 is a highly conserved rat sarcoma virus (RAS)-related GTPase whose expression is limited primarily to immune cells and lymphoid tissue. This protein acts as a molecular switch regulating multiple downstream processes, such as superoxide production (RAC2 being a critical component of the phagocyte reduced NAD phosphate [NADPH] oxidase), F-actin polymerization, and modulation of Ca2+ signaling (through stimulation of phospholipase Cγ2) (see figure).2,3 RAC2 activity is finely tuned by multiple regulatory mechanisms, including posttranslational modifications (mainly isoprenylation), protein degradation, and cross talk mechanisms with other Rho GTPases.4
Rac2 function in immune cells and consequences of RAC2 mutation on the cellular and clinical phenotype. GAP, GTPase-activating protein; GEF, guanine nucleotide exchange factor; LAD, leukocyte adhesion deficiency.
Rac2 function in immune cells and consequences of RAC2 mutation on the cellular and clinical phenotype. GAP, GTPase-activating protein; GEF, guanine nucleotide exchange factor; LAD, leukocyte adhesion deficiency.
Since the first clinical description of the D57N RAC2 mutation, at the beginning of the 21st century, in an infant with severe bacterial infections,5 impaired wound healing, and defective neutrophil chemotaxis, the phenotype of RAC2-associated pathogenic variants has considerably expanded. The data collection of Donkó et al brings invaluable precision for further delineating the phenotype of this complex disease, which depends on the functional consequence of the mutation. Hence, variants inducing constitutively active RAC2 proteins are responsible for severe combined immunodeficiency (SCID) mimicking reticular dysgenesis; the dominant negative D57N mutation is classically associated with a leukocyte adhesion deficiency–like SCID, whereas both homozygous null or heterozygous activating mutations with variable stability are linked to a combined immunodeficiency (CID) phenotype.
One merit of the study by Donkó et al is to provide a clinical picture of the evolution of patients with RAC2 pathogenic variants. With no surprises, all but 1 patient with the SCID phenotype underwent allogeneic hematopoietic stem cell transplantation (HSCT). Recognition of RAC2 dysfunction as a cause of SCID is long overdue, and its inclusion among the classic cause of SCID by the International Union of Immunological Societies dates only from 2019,6 almost 20 years after the first description! Of note, patients with the CID phenotype can have various disease courses, with the main clinical manifestations being recurrent airway infections leading to frequent bronchiectasis (34.9% of the cohort), recurrent viral infection (55.8%) with herpes simplex virus and human papillomavirus, and neoplasia (anogenital neoplasia, cutaneous squamous cell carcinoma, and B-cell lymphoma). The severity of the phenotype is attested by the notable mortality (6 patients among 43) and the need for allogeneic HSCT (9 patients). Regardless of presentation, T lymphopenia is the most consistent biological finding, and interestingly, some patients have been identified by newborn screening for T-cell receptor excision circles, offering a chance for early diagnosis and therapeutic intervention.
The study by Donkó et al shows that most identified mutations affect 3 domains: the guanine nucleotide-binding P loop and switch I and switch II. These switch regions play a critical role in RAC2 function through their binding to guanine nucleotide exchange factors, which drive GDP-to-GTP exchange, GTPase-activating proteins, which accelerate GTP hydrolysis, and downstream effectors.
Using heterologous expression and functional assays, Donkó et al highlight the diversity of the functional consequences of RAC2-reported pathogenic variants. First, different gene variants affect the stability of the RAC2 protein diversly: although the variants harboring constitutively active mutations (Q61 and G12R) display increased stability, most mutant RAC2 proteins exhibit decreased stability. Second, depending on the location of the mutations, different downstream effects are observed. Pathogenic variants in the switch I region (which binds p67phox to activate phagocyte NADPH oxidase) inhibit phorbol-12-myristate-13-acetate (PMA)-induced superoxide production. On the clinical level, this is consistent with the report that patients with a mutation near the switch I region (C18W, I21S, and G30R) display bacterial infections usually occurring in patients with chronic granulomatous disease. Switch I region mutations also variably increase RAC2 binding to its downstream effector P21 (RAC1) activated kinase 1 (PAK1), which regulates cytoskeleton remodeling and AKT phosphorylation. Conversely, the switch II region variant increases superoxide production under basal and PMA-induced conditions, enhances RAC2 binding to PAK1, and enhances phosphorylation of AKT. On the cellular level, switch II region mutations are associated with an increased macropinosome formation and membrane ruffling.
The greater accessibility of genetic investigation for patients with immunologic disease has considerably accelerated the discovery of new inborn errors of immunity (IEIs) since 2009, with 30 to 40 new immunologic genetic diseases reported each year. Although novelty is exciting, studies such as the one from Donkó et al, which offer a state-of-the-art clinical and biological assessment of a disease described >20 years ago, remain crucial. They give a much-needed view of natural history and biological intricacies necessary for adequate decision-making regarding patient management. Paul Valéry once said, “Everything that’s simple is wrong, but everything that isn’t is unusable.” We always tend to oversimplify things to ease our comprehension. The study by Donkó et al managed the touchy in between, delivering comprehensive and efficient insights into the pathophysiology of a complex IEI in an elegant manner.
Conflict-of-interest disclosure: F.T. declares no competing financial interests.