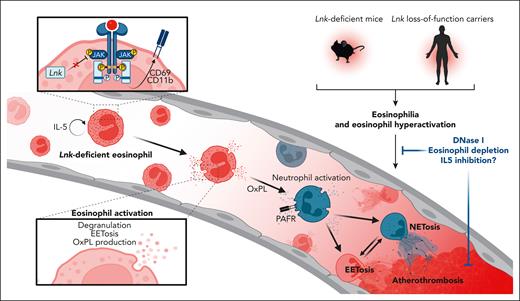
In this issue of Blood, Dou et al1 reveal a decisive role for hematopoietic lymphocyte adapter protein (LNK) deficiency in promoting eosinophilia, systemic eosinophil activation, and arterial thrombus formation. The authors use pharmacological and genetic eosinophil depletion to reveal that eosinophil-specific LNK deficiency exacerbates arterial thrombus formation through reciprocal eosinophil-neutrophil activation and extracellular trap (ET) formation.
Eosinophils protect the host against parasitic infections, but eosinophilia and hyperactivation of circulating eosinophils, as observed in autoimmune diseases like eosinophilic granulomatosis with polyangiitis, are associated with collateral self-damage. This is, at least in part, driven by a prothrombotic state, including clinically relevant increases in myocardial infarction and stroke.2 Moreover, eosinophils are enriched in arterial thrombi from patients with stroke and myocardial infarction. Recent studies have highlighted mechanistic details into how eosinophils modulate cardiovascular disease including thrombosis and associated inflammation: although eosinophils have been shown to aggravate the formation of experimental atherosclerotic lesions and arterial thrombus formation,3-5 they also have been shown to have beneficial effects on myocardial remodeling in the setting of chronic coronary artery ligation in a mouse model of myocardial infarction.6 The effect of eosinophils may be different in the setting of myocardial ischemia-reperfusion injury. This dichotomous role of eosinophils in cardiovascular disease warrants further investigation in a context-dependent manner.
SH2B3/LNK is a negative regulator of Janus kinase (JAK)/signal transducer and activator of transcription proteins (STAT) signaling. Carriers of a single nucleotide polymorphism with functional loss of SH2B3/LNK have an increased risk for developing myeloproliferative disorders and are often identified due to elevated leukocyte counts with prominent eosinophilia. Importantly, carriers also suffer from an increased risk of cardiovascular disease, including myocardial infarction.2 However, the functional link between eosinophilia in LNK-deficient individuals and the observed increases in thrombotic events are not wholly understood.
Mimicking the genetic background of T-allele carriers with LNK loss of function, Dou et al show that hematopoietic LNK deficiency increases peripheral eosinophil counts and eosinophil activation under steady-state conditions. The eosinophilia and eosinophil activation phenotypes become even more pronounced during metabolic disarray or during a chronic inflammatory state as induced by high-fat diet. Further, unleashed JAK/STAT signaling in response to interleukin-5 (IL-5) renders LNK-deficient mice more prone to forming eosinophil extracellular traps (EETs). Next, the authors used an elegant combination of genetic and pharmacological eosinophil ablation strategies, including both well-defined models such as anti-Siglec F antibody injection and ΔdbIGata1 mice. To dissect the specific contribution of LNK deficiency in eosinophils to arterial thrombosis in this setting, an eoCre-Lnkfl/fl mouse was generated. In all of the models, eosinophil depletion consistently alleviated arterial thrombus formation in ferric chloride-induced arterial thrombosis.
An interesting finding is that in addition to increased EET formation, infiltrating neutrophils and neutrophil extracellular traps (NETs) were also frequently observed in Lnk-deficient mice. In line with this, the authors found that in response to eosinophil depletion, thrombus infiltration of both eosinophils and neutrophils was markedly reduced. Further, both EET and NET formation was alleviated following eosinophil depletion, pointing toward reciprocal activation loops of neutrophils and eosinophils in Lnk-deficient thrombi. Mechanistic in vitro experiments confirmed that Lnk-deficient eosinophils potently induced NET formation. On a cellular level, oxidized phospholipids (OxPL) generated by activated Lnk-deficient eosinophils promoted neutrophil activation in a platelet-activating factor receptor (PAFR)-dependent manner, leading to enhanced neutrophil (trans)migration and exacerbating NET formation (see figure). This observation is in line with previous work from the same authors that highlights neutrophil activation and enhanced NET formation through platelet-derived OxPL-mediated PAFR signaling.7 However, the reciprocal communication between eosinophils and neutrophils is still not completely understood, but it is a relevant issue beyond cardiovascular diseases.
Loss-of-function mutations of SH2B3/LNK in human carriers and LNK-deficient murine models promote eosinophilia and activate peripheral eosinophils via uncontrolled JAK/STAT signaling and downstream upregulation of surface receptors like CD11b and CD69. This activated eosinophil phenotype leads to degranulation and formation of oxidized phospholipids and promotes EET-osis. Eosinophil-released OxPLs promote neutrophil activation via PAFR-mediated signaling, leading to increased neutrophil recruitment and NET formation. Reciprocal eosinophil and neutrophil activations then culminate in a prothrombotic state, leading to atherothrombosis. Eosinophil depletion, pharmacological degradation of ETs by DNase I, or targeted approaches like inhibition of IL-5 may therefore serve as novel antithrombotic therapeutic concepts in eosinophil-driven diseases.
Loss-of-function mutations of SH2B3/LNK in human carriers and LNK-deficient murine models promote eosinophilia and activate peripheral eosinophils via uncontrolled JAK/STAT signaling and downstream upregulation of surface receptors like CD11b and CD69. This activated eosinophil phenotype leads to degranulation and formation of oxidized phospholipids and promotes EET-osis. Eosinophil-released OxPLs promote neutrophil activation via PAFR-mediated signaling, leading to increased neutrophil recruitment and NET formation. Reciprocal eosinophil and neutrophil activations then culminate in a prothrombotic state, leading to atherothrombosis. Eosinophil depletion, pharmacological degradation of ETs by DNase I, or targeted approaches like inhibition of IL-5 may therefore serve as novel antithrombotic therapeutic concepts in eosinophil-driven diseases.
Finally, the authors used human-induced pluripotent stem cells carrying wild-type or mutated LNK alleles to generate eosinophils with or without functional LNK, emphasizing the translational relevance of their findings. Indeed, LNK-deficient human eosinophils were characterized by an increased activation state, as assessed by CD11b and CD69 surface expression as well as JAK/STAT signaling, and prone to form EETs in response to PAF stimulation.
The role of ETs in promoting arterial and venous thrombosis through a variety of mechanisms, including direct activation of the coagulation system, degradation of antithrombotic proteins, as well as platelet recruitment, is well known.8,9 Conversely, soluble and platelet-derived mediators like PAF promote ET formation. The mechanism proposed by the authors—OxPL formation by hyperactivated eosinophils, subsequent neutrophil binding and PAFR-mediated activation—appears consistent with their previous work, and the in vitro experiments promote this. However, in vivo, PAFR-mediated activation may also affect platelets, which in turn may serve as a further culprit activator of neutrophils. Thus, untangling the direct or indirect effects of platelets in the interplay between eosinophils and neutrophils remains an open question.
This study emphasizes the context dependency of the intricate interplay between immune cells and coagulation and the need for specifically investigating this interplay in the right context—for example, specific genetic backgrounds and the metabolic disarray induced by a Western-type diet. It is worth noting that a previous study found no effect of eosinophil depletion on venous thrombosis in a model of inferior vena cava stenosis in wild-type mice.5 However, given the increased risk for venous thrombus formation and thromboembolism in eosinophilia and the association with LNK single-nucleotide polymorphisms including the T allele,2,10 taming eosinophils through clinically established therapeutics such as IL-5 antagonists may serve as a novel, targeted antithrombotic approach in select patients. This generates an interesting link to other settings of eosinophilic inflammation, such as asthmatic disorders or chronic obstructive pulmonary disease, which also share risk factors with cardiovascular diseases, and cases in which IL-5 inhibition is an established treatment approach and might potentially affect cardiovascular outcomes. Moreover, boosting LNK-mediated suppression of JAK/STAT signaling in genetically defined individuals at risk of thrombosis and thromboembolism could serve as an elegant precision medicine approach. However, future studies that investigate antithrombotic but also antihemostatic properties of these novel therapeutic approaches will be necessary before clinical leverage can be attempted. Further, it remains unclear whether the mechanistic insights provided by Dou et al are also applicable to patients with other disease entities driven by eosinophil hyperactivation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.