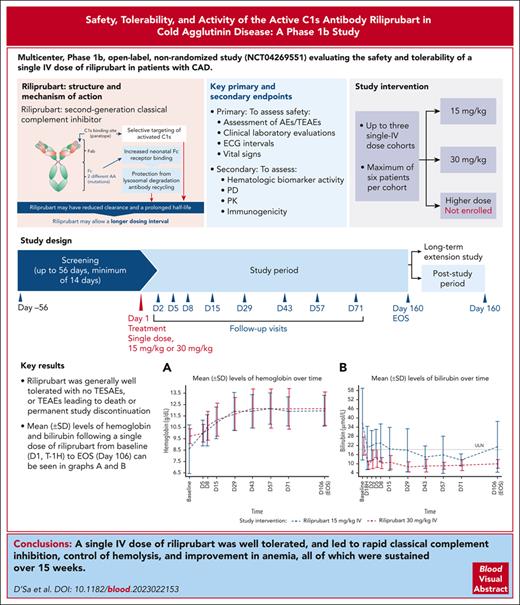
Riliprubart (SAR445088, BIVV020) was generally well tolerated in patients with cold agglutinin disease.
A single dose led to complement pathway inhibition, control of hemolysis, and improvement in anemia, sustained over 15 weeks.
Visual Abstract
Cold agglutinin disease is a rare autoimmune hemolytic anemia characterized by complement pathway-mediated hemolysis. Riliprubart (SAR445088, BIVV020), a second-generation classical complement inhibitor, is a humanized monoclonal antibody that selectively inhibits only the activated form of C1s. This Phase 1b study evaluated the safety, tolerability, and effect on hemolysis of riliprubart in adult patients with cold agglutinin disease. On day 1, 12 patients received a single IV dose of either 30 mg/kg (n = 6) or 15 mg/kg (n = 6) of riliprubart and were subsequently followed for 15 weeks. Riliprubart was generally well tolerated; there were no treatment-emergent serious adverse events, or treatment-emergent adverse events leading to death or permanent study discontinuation. There were no reports of serious infections, encapsulated bacterial infections including meningococcal infections, hypersensitivity, or thromboembolic events. Rapid improvements in hemoglobin (day 5) and bilirubin (day 1) were observed in both treatment cohorts. Mean hemoglobin levels were maintained at >11.0 g/dL from day 29 and mean levels of bilirubin were normalized by day 29; both responses were maintained throughout the study. Improvements in clinical markers closely correlated with a sustained reduction in the 50% hemolytic complement (CH50) throughout the study. Mean C4 levels, an in vivo marker of treatment activity, increased 1 week after treatment with either dose of riliprubart and were sustained throughout the study. In conclusion, a single IV dose of riliprubart was well tolerated, and led to rapid classical complement inhibition, control of hemolysis, and improvement in anemia, all of which were sustained over 15 weeks. This trial was registered at www.ClinicalTrials.gov as #NCT04269551.
Introduction
Cold agglutinin disease (CAD) is a rare, autoimmune hemolytic anemia mediated by the classical complement pathway (CP).1,2 Immunoglobulin M (IgM) autoantibodies, known as cold agglutinins, preferentially bind to the I antigen on erythrocytes at temperatures of 37°C or lower, resulting in erythrocyte agglutination.1-3 IgM is a potent trigger of the classical CP, whereby antigen-IgM complexes on erythrocytes bind to complement protein complex C1, composed of C1q, C1r, and C1s, and result in CP activation, thereafter, C1s activates C4 and C2, generating C3 convertase C4b2a, which cleaves C3 into C3a and C3b.2,3 In CAD, this cascade predominantly results in extravascular hemolysis mediated by macrophages of the mononuclear phagocytic system, which removes C3b–coated red blood cells.2,4 Intravascular hemolysis occurs to a lesser extent via the terminal complement cascade, whereby increased levels of C3b contribute to C5 convertase formation and membrane attack complex (C5b-9) production.1,2
All patients with CAD have complement-mediated hemolysis. Only a few patients are not anemic because the hemolysis is fully compensated, depending on the balance between hemolytic activity and bone marrow compensation.5 Clinical features of CAD mediated by the CP include anemia, profound fatigue, dyspnea, and jaundice.6,7 These patients may also experience cold-induced circulatory symptoms (non-complement-mediated) including acrocyanosis and Raynaud phenomenon.5,6 Medically-attended anxiety or depression is more likely to occur in patients with CAD, compared with the general population, which may impact their quality of life.8 In addition, these patients have a higher risk of thromboembolic events and early mortality by any cause compared with the general population.3 Symptoms of CAD contribute to a high burden on health care resource utilization including inpatient, outpatient, and emergency services.6
Until recently, there were no approved therapies to treat chronic hemolysis in patients with CAD. Sutimlimab (BIVV009, TNT009) is a humanized, monoclonal antibody that selectively inhibits the CP at C1s and is the first approved treatment for CAD.9,10 The efficacy and safety of sutimlimab were investigated in 2 phase 3 studies; CARDINAL was an open-label, single-arm study for patients with CAD with a history of recent blood transfusion (≥1 blood transfusion within 6 months of enrollment) and CADENZA was a randomized, double blind, placebo-controlled study for patients with CAD without a history of recent blood transfusion (0 within 6 months prior to enrollment).9,10 In both studies, sutimlimab was investigated at a dose of 6.5 g (patients with body weight <75 kg) or 7.5 g (patients with body weight ≥75 kg) via 500 mL IV infusion every 2 weeks for the duration of the studies. At this approved recommended dose, the half-life of sutimlimab, which is dependent on the plasma concentration, is 21 days.11 Sutimlimab response was rapid and sustained regardless of transfusion history, and included increased hemoglobin levels, improved markers of hemolysis, and clinically meaningful improvements in fatigue.9,10 Treatment was generally well tolerated, with the type and frequency of treatment-emergent adverse events (TEAEs) consistent with an older and medically complex patient population.9,10 These data support the targeted inhibition of C1s as an effective and well tolerated therapeutic approach for CAD management.9,10 Sutimlimab is currently approved for the treatment of CAD in the United States, European Union, Japan, Switzerland, and South Korea.12-15
Riliprubart (SAR445088, BIVV020) is a humanized monoclonal antibody that selectively inhibits the activated form of C1s.16,17 As a second-generation classical complement inhibitor, it has a modified mechanism of action compared to sutimlimab (which binds to both the nonactivated and activated forms of C1s). Riliprubart contains mutations that increase neonatal Fc receptor binding, promoting antibody recycling through protection from lysosomal degradation and enabling riliprubart to be recycled back into the system.18 As a result of this modified mechanism of action and selective targeting of only the activated form of C1s, riliprubart may have reduced clearance and a prolonged half-life, which may allow a longer dosing interval compared with sutimlimab, thus potentially alleviating some of the treatment burden that patients experience with regular infusions, such as infusion-related reactions and difficulty accessing infusion centers.
The aim of this study was to evaluate the safety, tolerability, and effect of a single IV dose of riliprubart on anemia and hemolysis in patients with CAD, to facilitate dose and dosing regimen selection for future clinical studies, such as the current phase 1 study on the long-term effects of riliprubart (NCT04802057) and a planned phase 3 study.
Methods
Study design and patients
This was a multicenter, phase 1b, open-label, nonrandomized study (NCT04269551) evaluating the safety and tolerability of a single IV dose of riliprubart in patients with CAD. The study objective was to assess the safety, impact of riliprubart on complement-mediated hemolysis, and generate data to determine doses that may be used in the next phase of studies. The study design initially included up to 3 single-dose cohorts, with a maximum of 6 patients dosed in any cohort. The first cohort, starting with 3 patients, received a dose of 30 mg/kg IV; on-study decisions about selection of the next cohort and/or expansion within a cohort were based on a review of all available safety, biomarker activity, and pharmacodynamics (PD) data after the day 15 visit. Following the first cohort, additional IV dose levels could be initiated; a second cohort of patients received a lower dose of 15 mg/kg IV but a third higher-dose cohort was not enrolled. Final determination of doses beyond the starting dose was made at a dose-selection meeting based upon a review of available data.
To be enrolled in the study, patients were required to be ≥18 years of age with a confirmed diagnosis of CAD and, at the time of screening, have a hemoglobin level ≤11.0 g/dL and a total bilirubin level above the normal reference range secondary to hemolysis. Patients were excluded from the study if they presented with secondary CAD (cold agglutinin syndrome), were pregnant or lactating, had a clinically relevant infection of any kind within 1 month preceding screening, or were treated with anti-CD20 monotherapy, systemic immunosuppressive agents (targeting B- or T-cell function and/or cytotoxic agents), a complement system inhibitor, or systemic corticosteroids (other than the equivalent of ≤10 mg/d prednisone) within 3 months of screening.
Prior to initiating dosing in the study, patients underwent a screening period of up to 56 days to establish eligibility and ensure adequate prophylactic vaccination against encapsulated bacterial pathogens (Streptococcus pneumoniae and Neisseria meningitidis). Provided all eligibility criteria were met, patients received treatment with riliprubart on day 1 and had follow-up visits on days 2, 5, 8, 15, and then every 2 weeks up to day 71, with an end-of-study (EOS) visit on day 106 (week 15). After the EOS visit, any patients who did not enroll in the long-term extension study were required to enter the post-study period in which they were followed from EOS to day 160.
All patients provided written informed consent prior to enrollment, and the study was conducted in accordance with the protocol and consensus ethical principles derived from international guidelines including the Declaration of Helsinki, and the International Council for Harmonization Guideline for Good Clinical Practice, all applicable laws, rules, and regulations. The trial protocol and its amendments were approved by the institutional review board and independent ethics committee prior to the study being initiated.
Study objectives and endpoints
Primary safety endpoints included assessment of adverse events (AEs)/TEAEs, physical examination, clinical laboratory evaluations including hematology, biochemistry, systemic lupus erythematosus (SLE) panel testing (as with the inhibition of C1s, there is a theoretical risk of the development of SLE based on data from patients with congenital CP deficiencies19) and urinalysis, electrocardiographic intervals, and vital signs (blood pressure and heart rate).
Secondary endpoints included hemolytic biomarker activity (total bilirubin and hemoglobin), PD (CP, CH50, total C4 and complement alternative pathway [AP]), and pharmacokinetic (PK) parameters including but not limited to Cminss, Cmaxss, and AUCss. CP and AP activity were determined by Wieslab Classical or Alternative Pathway kits, respectively.
Exploratory endpoints included PD related to C1s and the ratio of C1s-C1INH to C1 level in collected blood samples, which were quantified with specific C1s and C1INH liquid chromatography coupled to tandem mass spectrometry assays and mathematically calculated based on assayed C1s and C1INH levels.
Statistical analysis
Endpoints were summarized using descriptive statistics (arithmetic mean, standard deviation, standard error of the mean, median, minimum and maximum or confidence intervals for quantitative parameters, frequency and percentage for qualitative parameters) by dose cohort. AEs were coded according to the Medical Dictionary for Regulatory Activities (version 24.1). Severity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v5.0. Sample size for this study was based on empirical considerations and no sample size calculation was performed.
Results
Patient disposition and baseline characteristics
Twelve patients received a single IV dose of riliprubart (n = 6, 30 mg/kg; n = 6, 15 mg/kg). All patients completed the study, and were analyzed as part of the PD, PK, PK/PD, biomarker activity, and safety populations. Demographic and patient characteristics at baseline were similar between the 2 dose cohorts, as shown in Table 1. Most patients were female (91.7%, n = 11), the median age was 69 years (range, 54-80), and median (range) hemoglobin was 9.60 g/dL (4.8-10.9). Most patients (91.7%, n = 11) had a body mass index value of <30 kg/m2.
Safety
There were no treatment-emergent serious AEs, TEAEs leading to death, or TEAEs leading to permanent study discontinuation (Table 2). Overall, 58 TEAEs were reported by 11 patients (83.3%; n = 5, 30 mg/kg; 100%; n = 6, 15 mg/kg) (supplemental Table 1, available on the Blood website). TEAEs reported by ≥2 patients included headache, acrocyanosis, rash, arthralgia, and fatigue. Hematuria was observed in 1 patient (1 event) in the 15 mg/kg IV cohort and 1 patient (10 events) in the 30 mg/kg IV cohort (supplemental Table 1). One (16.7%) patient in the 15 mg/kg cohort experienced a pretreatment severe AE (grade 3) of hemolytic anemia that began prior to riliprubart administration and was ongoing during the treatment period (Table 2). The remainder of reported TEAEs were mild or moderate in severity (grade 1 or 2). AEs of special interest (AESI) were reported in 2 (33.3%) patients in the 15 mg/kg IV cohort (Table 2): one of these patients reported 2 episodes of polyarthritis; the first episode occurred during the screening period prior to riliprubart administration and the second episode on day 34 of the study (grade 2), which represented a worsening from baseline symptoms. Both episodes were assessed by the investigator as not related to riliprubart. The other patient reported a pretreatment AESI of increased double stranded (ds) DNA; this patient had a positive anti–dsDNA laboratory test result at baseline prior to riliprubart administration. The test result of the dsDNA antibody remained positive throughout the study. The patient had no clinical signs of SLE. No AESI was reported in the 30 mg/kg IV cohort (Table 2).
No meaningful changes or trends in the electrocardiogram, urinalysis, or vital signs parameters were observed during the study. No serious infections, encapsulated bacterial infections including meningococcal infections, hypersensitivity, or thromboembolic events, were reported.
Hematologic biomarker activity
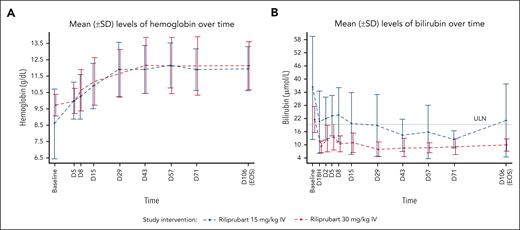
Mean levels of hemoglobin and bilirubin following a single dose of riliprubart from baseline (day (D) 1, time (T)–1 hour [H]) to EOS (day 106) are shown in Figure 1. After a single dose of 30 mg/kg IV riliprubart, mean hemoglobin increased from 9.73 g/dL at baseline to 10.0 g/dL by day 5, reaching a plateau at ∼12.0 g/dL by day 29, which was sustained to the EOS visit. A single dose of 15 mg/kg IV riliprubart increased mean hemoglobin from 8.59 g/dL at baseline to 10.0 g/dL by day 5, reaching a plateau of ∼12.0 g/dL by day 29, which was sustained to the EOS visit (Figure 1A). Eight patients (66.7%; n = 4/6 for each dose cohorts) had an increase in mean hemoglobin >1.0 g/dL from baseline to day 15; 9 patients (66.7%; n = 4/6, 30 mg/kg IV cohort; 83.3%; n = 5/6, 15 mg/kg IV cohort) had an increase in mean hemoglobin ≥1.5 g/L from baseline to day 106 (EOS visit).
Mean (SD) levels of hemoglobin and bilirubin following a single dose of riliprubart from baseline (D1, T–1H) to EOS (day 106). (A) Hemoglobin levels. (B) Bilirubin levels. ULN of total bilirubin is 18.81 μmol/L. Baseline was defined as the D1, T–1H assessment value. For 1 patient, the baseline of hemoglobin was the screening instead of D1, T–1H. D, day; H, hour; SD, standard deviation; T, time; ULN, upper limit of normal.
Mean (SD) levels of hemoglobin and bilirubin following a single dose of riliprubart from baseline (D1, T–1H) to EOS (day 106). (A) Hemoglobin levels. (B) Bilirubin levels. ULN of total bilirubin is 18.81 μmol/L. Baseline was defined as the D1, T–1H assessment value. For 1 patient, the baseline of hemoglobin was the screening instead of D1, T–1H. D, day; H, hour; SD, standard deviation; T, time; ULN, upper limit of normal.
Total mean bilirubin decreased from 21.18 to 8.46 μmol/L by day 1 in the 30 mg/kg IV cohort, and this was sustained to the EOS visit (9.41 μmol/L). A decrease in total mean bilirubin from 35.91 to 19.81 μmol/L from baseline by day 1 in the 15 mg/kg IV cohort was also observed, and this was sustained to the EOS visit (20.52 μmol/L) (Figure 1B). The upper limit of normal range for total bilirubin is 18.81 μmol/L.
PD
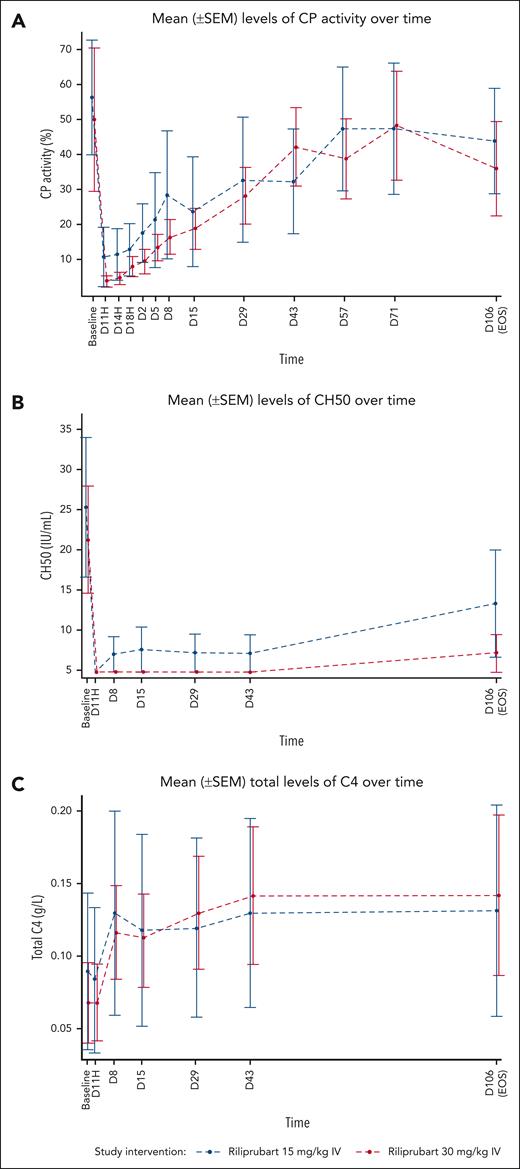
Mean levels of PD markers of complement activity following a single dose of riliprubart from baseline (D1, T–1H) to the EOS visit (day 106) are shown in Figure 2. Following a single dose of riliprubart 30 mg/kg, mean percent CP activity decreased from 49.70% at baseline to <10% by 1 hour and this was sustained through 24 hours, followed by gradual rebound of CP activity through to the EOS visit (Figure 2A). In the 15 mg/kg IV cohort, mean percent CP activity decreased from 56.09% at baseline to 10.23% by 1 hour, followed by gradual rebound of CP activity through to the EOS visit (Figure 2A). The mean CH50 level decreased from baseline (21.7 IU/mL) to <10 IU/mL by 1 hour through to the EOS visit in the 30 mg/kg IV cohort (Figure 2B). In the 15 mg/kg IV cohort, mean CH50 level decreased from baseline (25.7 IU/mL) to <10 IU/mL by 1 hour through 42 days and was 13.6 IU/mL at the EOS visit. Mean C4 levels increased from baseline within the first week after administration for both cohorts and this was sustained through to the EOS visit (Figure 2C). The C4 values increased from 0.07 g/L and 0.09 g/L at baseline to 0.14 g/L and 0.13 g/L at the EOS visit in the 30 and 15 mg/kg IV cohorts, respectively.
Mean (±SEM) levels of PD markers of complement activity following a single dose of riliprubart from baseline (D1, T–1H) to EOS (day 106). (A) Wieslab CP levels. (B) CH50 levels. (C) C4 levels. C, complement; CH50, 50% hemolytic complement; CP, classical complement pathway; SEM, standard error of the mean.
Mean (±SEM) levels of PD markers of complement activity following a single dose of riliprubart from baseline (D1, T–1H) to EOS (day 106). (A) Wieslab CP levels. (B) CH50 levels. (C) C4 levels. C, complement; CH50, 50% hemolytic complement; CP, classical complement pathway; SEM, standard error of the mean.
AP activity, as measured by the Wieslab AP kit, did not significantly change from baseline for the duration of the study in the 30 and 15 mg/kg cohorts.
Both the endogenous C1 inhibitor and riliprubart bound to active C1s. In all patients, a decrease in C1s-C1INH to C1s ratio from the pre-dose baseline level was observed at the first timepoint (1 hour) after riliprubart dose. However, the baseline C1s-C1INH to C1s ratio level, and the magnitude and duration of this observed decrease in C1s-C1INH to C1s ratio were variable among the 12 patients.
PK
Riliprubart (estimated half-life of 8-15 weeks16) mean plasma concentrations proportionally increased between both dose cohorts (15 and 30 mg/kg) (Figure 3). Patients with CAD had similar concentration-time profiles to those of healthy participants, except for 1 patient in the 30 mg/kg cohort, who had low riliprubart exposure from the first timepoint.
Patient-level concentration-time profiles of riliprubart mean plasma concentrations over the study period (15 weeks). 90% inhibitory concentration for CH50 is 57.0 μg/mL; this value correlates to inhibition of the CP that may provide therapeutic effect to patients with CAD. With the exception of 1 patient in the 30 mg/kg cohort who had low riliprubart exposure from the first timepoint, patients with CAD had similar concentration-time profiles to those of healthy participants. CH50, 50% hemolytic complement.
Patient-level concentration-time profiles of riliprubart mean plasma concentrations over the study period (15 weeks). 90% inhibitory concentration for CH50 is 57.0 μg/mL; this value correlates to inhibition of the CP that may provide therapeutic effect to patients with CAD. With the exception of 1 patient in the 30 mg/kg cohort who had low riliprubart exposure from the first timepoint, patients with CAD had similar concentration-time profiles to those of healthy participants. CH50, 50% hemolytic complement.
Discussion
In this phase 1b study, selective inhibition of the activated form of C1s by a single dose of the complement inhibitor riliprubart was generally well tolerated and led to rapid and long-lasting classical complement inhibition, control of hemolysis, and improvement in anemia in adult patients with CAD.
No safety concerns were identified following a single dose of riliprubart. Almost all TEAEs reported were mild or moderate in severity (grade 1 or 2), and there were no treatment-emergent serious AEs, TEAEs leading to death, or TEAEs leading to permanent study intervention discontinuation.
Classical complement inhibition, control of hemolysis, and improvement in anemia were observed after a single IV dose of either 30 or 15 mg/kg of riliprubart and sustained through 15 weeks (EOS visit). The response to riliprubart was rapid, as demonstrated by improvements in hemoglobin (by day 5) and bilirubin (by day 1) in both treatment cohorts. Mean hemoglobin levels were maintained at ∼12.0 g/dL from day 29 through to the EOS visit at day 106, and mean levels of bilirubin, a marker of hemolysis, normalized by day 29 and were sustained through to the EOS visit.
Following a single dose of riliprubart, mean levels of all assessed PD markers of complement activity in patients with CAD returned to levels consistent with those observed in a healthy population, indicating improvements in the patients’ disease. Improvements in clinical markers following riliprubart exposure were associated with a sustained reduction in CH50. The relationship was observed more closely with CH50 than with CP, demonstrating that CH50 is the more relevant PD marker of the clinical effect of riliprubart. The level of C1s-C1INH to C1s ratio after treatment exposure was calculated to provide information on riliprubart engagement with the target, as both endogenous C1 inhibitor and riliprubart bind active C1s. A consistent decrease in the ratio of C1s-C1sINH to C1s was observed in all patients immediately after treatment, indicating target engagement. The specificity of riliprubart for the CP was demonstrated by the lack of change in Wieslab AP activity from baseline through to EOS in both treatment cohorts. This indicates that the AP is not affected by riliprubart and remains intact for immune surveillance, potentially reducing the risk of infection.
As C4 is the first substrate to be cleaved following activation of C1s, low levels of C4 are expected in patients with CAD.9 The inhibition of activated C1s by riliprubart in this study led to an increase in mean C4 to levels consistent with those observed in healthy participants across both cohorts (30 and 15 mg/kg), providing an in vivo marker of treatment activity. This observation is similar to what has been observed with sutimlimab, another inhibitor of C1s that is currently approved for the treatment of CAD.9,10
Riliprubart is a second-generation classical complement inhibitor. It has a modified mechanism of action compared with the recently approved, first-in-class CAD therapy, sutimlimab, which targets nonactivated and activated forms of C1s.9-11,20 By selectively targeting only the activated form of C1s,16 riliprubart may potentially offer patients with CAD a favorable dosing schedule due to low clearance and a long half-life. Exploratory PD analysis of C1s-C1INH to C1s ratio suggested fast (within 1 hour of dosing) engagement of riliprubart with active C1s, demonstrated by a decrease in C1s-C1INH to C1s ratio after riliprubart exposure compared with the pre-dose baseline level. However, it should be noted that the magnitude and duration of this engagement cannot be determined from these data as results were variable among the 12 patient samples assessed. Further analysis is, therefore, required to validate these findings.
This study provides information on the safety, tolerability, and effect on hemolysis of a single IV dose of riliprubart in adults with CAD, while facilitating dose and dosing regimen selection for future clinical trials. A limitation of this study is the small patient population; however, this is consistent with a phase 1 study in a rare disease. Further research in a larger study would be needed to confirm the data described here.
In conclusion, selective inhibition of the activated form of C1s by riliprubart, via a single IV dose, led to rapid classical complement inhibition, control of hemolysis, and improvement in anemia that was sustained over 15 weeks. Moreover, riliprubart was generally well tolerated with no safety concerns.
Acknowledgments
The authors thank the investigators, health care providers, research staff, and patients and families who participated in the study. Medical writing support was provided by Blake Thornley of Lucid Group Communications Ltd.
This study, and medical writing support, was funded by Sanofi.
Authorship
Contribution: M.W., L.P., G.B., M.Z., M.S., and T.C. contributed to the design and implementation of the research; and all authors contributed to the analysis of the results and to the writing of the manuscript.
Conflict-of-interest disclosure: S.D. received consulting fees from Sanofi. J.M.I.V. received research support from BeiGene, AbbVie/Genmab and consulting fees/honoraria from Bristol Myers Squibb, Janssen, Amgen, and Sanofi; all of these grants and honoraria were provided to the institution. W.B. received consulting fees from Alexion, Apellis, Novartis, Roche, Bioverativ, Sanofi, and Sobi. M.W. and M.S. are employees of and own stocks and/or shares in Sanofi. L.P., G.B., M.Z., and T.C. are employees of Sanofi. A.R. received honoraria from Alexion, Amgen, Apellis, Novartis, Roche, Sanofi, and Sobi; and advisory board fees from Alexion, Amgen, Apellis, Novartis, Roche, Bioverativ, Sanofi, and Sobi.
Correspondence: Shirley D’Sa, UCLH Centre for Waldenström’s Macroglobulinaemia and Related Conditions, University College London Hospitals NHS Foundation Trust, 250 Euston Rd, London NW1 2PG, United Kingdom; email: s.d'sa@nhs.net.
References
Author notes
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report, statistical analysis plan, and data set specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further information related to Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.