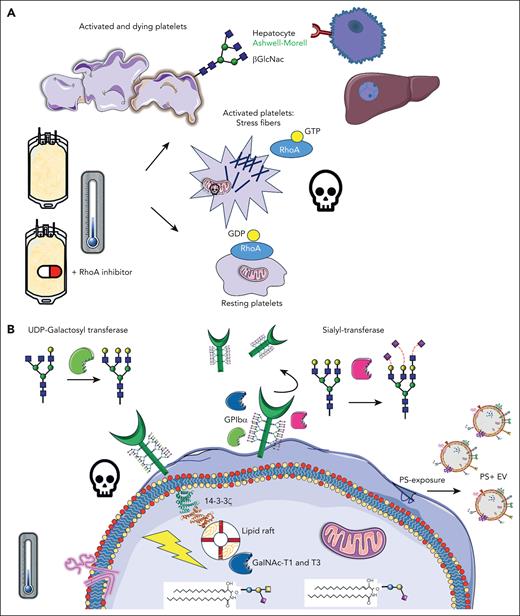
In this issue of Blood, Hegde et al1 used 2 RhoA guanosine triphosphatase (GTPase) inhibitors to prevent key activation and cell death processes and clearance in cold-stored platelets in elegant thrombocytopenic mouse and human in vitro clearance models. They have linked years of fundamental research by their own laboratory and others, resulting in the demonstration of novel mechanisms involved in the clearance of cold-stored platelets. They showed that RhoA GTPase inhibitors are not toxic and are successful when added to actual platelet concentrates. Transfusion of refrigerated platelets in RhoA-deficient mice showed an improved half-life.
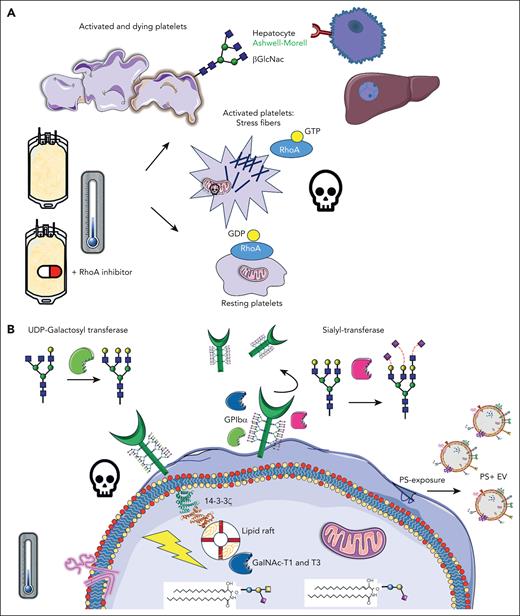
Hegde et al are the first to show, to our knowledge, how RhoA inhibition prevents clearance of human refrigerated platelets by β-N-acetylglucosamine (GlcNAc) recognizing Ashwell-Morell receptors, present on hepatocytes in the liver. Several previously unexplored, novel glycan-modulating enzymes were found in the cell signaling platforms, specifically, the lipid rafts after cold storage: uridine diphosphate (UDP) N-acetyl-D-galactosamine:polypeptide N-acetyl-galactosaminyltransferases T1 and T3, UDP-galactosyl transferase, and sialyltransferase (see figure).
Inhibition of RhoA activity in cold-stored platelets improve their function and half-life. (A) Upon cold storage of platelet concentrates, platelets are activated, and stress fibers are formed, mediated by RhoA activity, leading to consequent downstream signaling, apoptosis, and clearance. β-GlcNAc glycan residues become exposed on the platelet surface, acting as an “eat-me” signal, recognized by Ashwell-Morell receptors on hepatocytes in the liver. The addition of Rho inhibitors prevents stress fiber formation and all major activation processes, as per panel B, preventing rapid platelet clearance. (B) After cold-storage of platelet concentrates, GPIbα reorganization within the membrane leads to 14-3-3ζ recruitment, release of procoagulant phosphatidylserine (PS)+ extracellular vesicles (EVs), mitochondrial depolarization, and death. In addition, several glycan-modulating enzymes are translocated to signaling platforms, specifically the lipid rafts, including UDP N-acetyl-D-galactosamine (GalNAc):polypeptide N-acetyl-galactosaminyltransferases T1 and T3 (GalNAc-T1 and -T3), sialyltransferase, and UDP-galactosyltransferase. GDP, guanosine diphosphate. Claire Linnane (Australian Red Cross Lifeblood) provided some of the building blocks of this cartoon.
Inhibition of RhoA activity in cold-stored platelets improve their function and half-life. (A) Upon cold storage of platelet concentrates, platelets are activated, and stress fibers are formed, mediated by RhoA activity, leading to consequent downstream signaling, apoptosis, and clearance. β-GlcNAc glycan residues become exposed on the platelet surface, acting as an “eat-me” signal, recognized by Ashwell-Morell receptors on hepatocytes in the liver. The addition of Rho inhibitors prevents stress fiber formation and all major activation processes, as per panel B, preventing rapid platelet clearance. (B) After cold-storage of platelet concentrates, GPIbα reorganization within the membrane leads to 14-3-3ζ recruitment, release of procoagulant phosphatidylserine (PS)+ extracellular vesicles (EVs), mitochondrial depolarization, and death. In addition, several glycan-modulating enzymes are translocated to signaling platforms, specifically the lipid rafts, including UDP N-acetyl-D-galactosamine (GalNAc):polypeptide N-acetyl-galactosaminyltransferases T1 and T3 (GalNAc-T1 and -T3), sialyltransferase, and UDP-galactosyltransferase. GDP, guanosine diphosphate. Claire Linnane (Australian Red Cross Lifeblood) provided some of the building blocks of this cartoon.
RhoA GTPase, which is active when bound to GTP, is crucial for dynamic cytoskeletal reorganization in cells. In platelets, they are crucial for shape change during their activation, for example, the development of stress fibers, such as filopodia and lamellipodia reorganization. RhoAs play roles in platelet secretion of α and dense granules downstream of Gα13 and Gαq proteins,2 clot retraction, and αIIbβ3-integrin activation through GTPase Rap1. RhoA inhibition previously slowed down cold-stored platelet storage lesions, and Marini et al showed this conserved δ-granule release, platelet aggregation, adhesion, and stable clots.3
In blood banks globally, platelet components are a life-saving product. They are essential for surgery, oncology, and cardiac patients. However, they have a short shelf life of only 5 to 7 days at 22° to 24°C and their function deteriorates gradually. In the late 1960s, platelets were refrigerated to reduce cytokine production, febrile reactions, and the risk of bacterial contamination. However, transfusion of these products was soon abandoned due to the rapid clearance of cold-stored platelets. Years later, Hoffmeister et al4 provided key insights into this mechanism. One of the main platelet adhesion receptors, glycoprotein (GP) Ibα, contains many carbohydrates on its surface (glycans) and cold-induced removal of either sialic acid or underlying β-GlcNAc residues, reorganizing the receptor into the cell membrane. The term “clustering” was coined and shown using electron microscopy. Many years later, these and other research groups further explored these mechanisms, aiming to improve their reduced half-life. Initially, many basic studies were performed in typical laboratory settings, for example, washed platelets in Tyrode buffer stored at 0° to 7°C, at low concentrations in test tubes.5 Although very interesting changes were found after refrigeration only, many studies did not rewarm the platelets after cold storage, which is needed to mimic transfusion and might alter their phenotype. We showed earlier that cold storage induced GPIbα signaling, for example, binding to lipid rafts, scaffolding 14-3-3ζ, complexes with BCL-2 family proteins, and initiation of apoptosis downstream,5 changes that were confirmed by this study using actual platelet concentrates.1 The released arachidonic acid contributed to apoptosis and mitochondrial dysfunction. Depleting platelets with this lipid improves circulation significantly in mice.5 It became clear that reorganizational changes in GPIbα within the membrane and/or deglycosylation induced downstream activation/clearance signals and vice versa.
To date, there is still no good laboratory platelet quality marker available to guide us toward better and safer transfusion practices with improved platelet responses after transfusion. During the cold storage of actual platelet concentrates, platelets undergo many other changes. Not only surface GPIbα is removed, also GPVI and GPIIb (αIIb), increased integrin, and activation/apoptosis markers (CD62P, CD63, and phosphatidylserine exposure) were found.6 Notably, refrigeration altered several cytoskeletal proteins and resulted in an increase in actin filamentation. Compared with ∼7 days after room temperature (RT) storage, cold-stored platelets circulate only ∼3 days in mice. Although surface platelet desialylation and degalactosylation are key in their clearance, modulating glycans in cold-stored platelet components unfortunately did not improve the circulation of human platelets after transfusion, despite being successful in a mouse model.7 Hegde et al confirmed that although glycan changes are closely linked to activation during cold, targeting RhoA would be a better pharmacological target.
Different platelet components are manufactured globally. Some are donated by a single donor using apheresis and stored in 30% to 100% autologous plasma and a variety of platelet additive solutions (PASs). Pooled platelet products derived from multiple donors are also used and stored in 30% to 40% ABO-matched plasma and PAS. The mandatory pathogen inactivation process, using methods like gamma or X-ray irradiation, can further change the platelets. The percentage of plasma, PAS type, and lack of consistency in manufacturing lead to platelets with variable activation levels.
Conventionally, RT-stored apheresis platelets show a high degree of variability in both in vitro platelet activation markers and surface glycans (n = 50).8 Larger studies using original-sized storage bags are needed to assess variability in refrigerated platelets. The efficacy of RhoA inhibitors on RhoA activity might be different for different platelet components, and therefore influencing apoptosis and clearance. It would be interesting to investigate products that are refrigerated after RT storage to further replenish the platelet stock.
A pilot trial showed that cold-stored platelets transfused in cardiac patients were noninferior to conventional products.9 Future clinical trials in humans will need to be performed with different platelet components (apheresis platelets in 30%-100% plasma and pooled platelets). Platelets will need to be stored at RT vs cold at various time points and compared with matched controls, with and without RhoA inhibitors.
Although improving the circulation time of platelets sounds most sensible and the authors have moved this field forward immensely, it is unclear whether a longer circulation time is beneficial in actively bleeding patients. For example, as RhoA-inhibited platelets do circulate longer, is there a potential risk of thrombotic complications if this inhibition is reversed? Because transfused platelets are rapidly incorporating into the thrombus when needed, this seems unlikely.10 More in vivo clearance studies of human platelets in mice are needed, using not yet commercially available micro storage bags, before eventual testing in different human patients. To assess the potential harm vs benefit, various transfusion regimens and different doses will need to be tested, along with assessing the ideal circulation time of transfused platelets.
Conflict-of-interest disclosure: D.E.v.d.W. declares no competing financial interests.