Necessity is the mother of invention, but serendipity is often the driving force behind scientific innovation. In this issue of Blood, Sun et al leverage an incidental observation to advance the treatment of immune thrombocytopenia (ITP).1
ITP reflects a combination of accelerated platelet destruction and inappropriately low platelet production, the latter attributed to the impact of autoantibodies and cytotoxic lymphocytes on megakaryocytes.2 Therapies such as glucocorticoids, intravenous immunoglobulin G, rituximab, and splenectomy decrease the destruction of antibody-coated platelets by macrophages, whereas thrombopoietin receptor agonists (TPO-RAs) such as eltrombopag, romiplostim, and avatrombopag enhance platelet production by the bone marrow. TPO-RAs have revolutionized the care of patients with ITP and have become a preferred second-line therapy for this condition.3-6 Unfortunately, a significant fraction of patients fail to respond to TPO-RAs, so strategies to improve efficacy are needed.
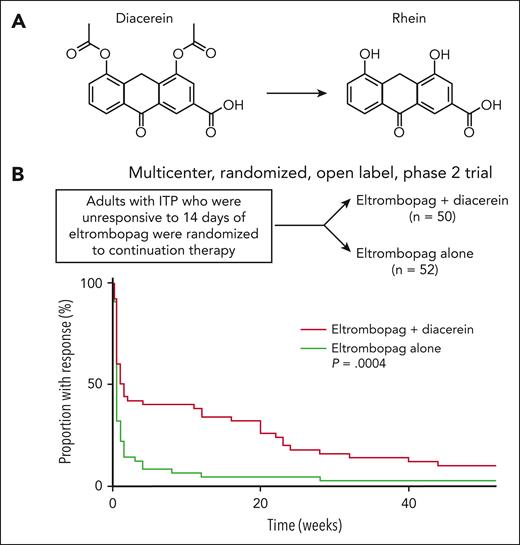
In preliminary studies, Sun et al7 insightfully noted that 3 adults with ITP and low platelet levels (30 to 50 × 109/L) during eltrombopag treatment achieved a significant platelet response (≥100 × 109/L) after concurrently taking diacerein, an agent classified as a symptomatic slow-acting drug in osteoarthritis that has anti-inflammatory and anticatabolic properties on cartilage and synovium.8 Subsequent in vitro studies showed that rhein, the active metabolite of diacerein (see figure) and a major ingredient in several traditional Chinese medicines,9 potentiates the effects of eltrombopag on megakaryocyte polyploidization and platelet formation in ITP.7 In a mouse model of ITP, the combination of rhein + romiplostim alleviated thrombocytopenia better than romiplostim alone.7
Diacerein augments the effects of eltrombopag in ITP. (A) Diacerein is an anthraquinone derivative used to treat osteoarthritis. After oral administration, diacerein is converted into its active metabolite, rhein. (B) Adults with ITP who were unresponsive to 14 days of eltrombopag monotherapy were randomly assigned to the 2 treatment arms. Kaplan-Meier analysis confirmed a longer duration of response in the eltrombopag + diacerein group than in the eltrombopag group (P = .0004). ITP, immune thrombocytopenia. The graph in panel B is adapted from Figure 2 in the article by Sun et al that begins on page 1791.
Diacerein augments the effects of eltrombopag in ITP. (A) Diacerein is an anthraquinone derivative used to treat osteoarthritis. After oral administration, diacerein is converted into its active metabolite, rhein. (B) Adults with ITP who were unresponsive to 14 days of eltrombopag monotherapy were randomly assigned to the 2 treatment arms. Kaplan-Meier analysis confirmed a longer duration of response in the eltrombopag + diacerein group than in the eltrombopag group (P = .0004). ITP, immune thrombocytopenia. The graph in panel B is adapted from Figure 2 in the article by Sun et al that begins on page 1791.
To validate the synergistic effect between TPO-RAs and diacerein, Sun et al conducted a multicenter, open-label, phase 2 trial of eltrombopag + diacerein (n = 50) vs eltrombopag alone (n = 52) in adults with ITP. Patients who were unresponsive to 14 days of eltrombopag monotherapy were randomly assigned to the 2 treatment arms. The overall response rate, defined as a platelet count ≥30 × 109/L, at least doubling of the baseline platelet count, and no bleeding, was reached in 44% of patients in the eltrombopag + diacerein group compared with 13% in the eltrombopag group at day 15 (P = .0009). The addition of diacerein to eltrombopag also led to a longer duration of response (see figure). The incidence of adverse events was similar in the 2 groups. The authors concluded that eltrombopag + diacerein is a safe and effective salvage therapy for patients with ITP who were unresponsive to 14 days of eltrombopag monotherapy. These results have the potential to enhance the care of patients treated with eltrombopag or other TPO-RAs.
What is the mechanistic basis for this synergy? Rhein has complex pharmacological properties and is known to modulate multiple signaling pathways, including the MAPK, phosphatidylinositol 3-kinase/AKT, and transforming growth factor-β pathways.8,9 Rhein may augment the effects of TPO-RAs by enhancing phosphatidylinositol 3-kinase phosphorylation during megakaryocyte maturation and platelet formation or by decreasing proinflammatory cytokines such as interleukin (IL)-1 and IL-17.7 Sun et al found that the addition of diacerein to eltrombopag significantly elevated transforming growth factor-β1 and decreased IL-17 in eltrombopag-refractory patients with ITP.
There are noteworthy limitations to the study. First, the response to eltrombopag + diacerein wanes with time (see figure). By 12 months, only 5 patients (10%) on this combination therapy were classified as ongoing responders (with a median platelet count of approximately 60 × 109/L). Thus, this combination therapy may be more of a bridge than a long-term solution. Second, diacerein is licensed in some Asian and European countries but is not available worldwide. Physicians in other countries, including the United States and Canada, will not be able to exploit these results immediately. Third, diacerein has dose- and age-dependent side effects; owing to the risk of severe diarrhea, diacerein is no longer recommended by the European Medicines Agency for patients ≥65 years of age. These drawbacks notwithstanding, the findings by Sun et al should spur additional research aimed at optimizing the efficacy of TPO-RAs.
Conflict-of-interest disclosure: D.B.W. declares no competing financial interests.