A “restrictive” red blood cell transfusion threshold, a hemoglobin concentration <7 to 8 g/dL, has long been recommended for most hospitalized patients including anemic patients with stable cardiovascular disease (CVD). Although no threshold recommendation is given for acute coronary syndromes (ACSs), recent evidence suggests that “liberal” rather than “restrictive” transfusion strategies are associated with significantly improved safety for hospitalized patients with stable CVD and/or ACS. This finding suggests that previously available data were misinterpreted. Conclusions drawn from earlier transfusion trigger trials have been confounded by unintentional trial design and analysis flaws that have contributed to erroneous recommendations regarding the safety of a restrictive threshold. Subsequently, these conclusions have been incorporated into widely accepted guidelines and clinical practice. Management with a restrictive vs liberal transfusion strategy (<10 g/dL) increases the risk of new-onset ACS in patients with CVD by ∼2%. We estimate that since 2019, using hospital databases and a recent meta-analysis, this practice may have resulted in ∼700 excess ACS events per year in orthopedic surgical patients. Given these findings, transfusion practices in other clinical conditions, particularly those derived from similar transfusion trigger trials, should be questioned. Restrictive and liberal transfusion policies merit a general reconsideration. Rather than a single numerical transfusion trigger, transfusion therapy should be personalized. Consideration of an individual patient’s age, clinical status, and comorbidities is integral to transfusing. To avoid making similar errors, future trials of transfusion therapy should determine common practices before study inception and incorporate them as a usual-care “control” comparator arm into the trial design. Such studies should more reliably improve current transfusion practice.
Anemia, CVD, and transfusion
Anemia has been well documented as a “powerful and independent predictor of major adverse cardiovascular events (MACE)” in patients with acute coronary syndromes (ACSs).1 In 20% to 25% of patients suffering a myocardial infarction (MI), hemoglobin (Hb) concentration is <10 g/dL. In part, this likely represents a marker of comorbidity. Surgical patients with cardiovascular disease (CVD) reportedly tolerate anemia less well than those without CVD. In a retrospective observational cohort study of 1958 surgical patients refusing red blood cell (RBC) transfusion for religious reasons, overall mortality increased with decreasing preoperative Hb concentration. At similar and even higher Hb levels, mortality increased for patients with ischemic heart disease.2 The myocardium appears more sensitive to anemia during ACS. In a retrospective observational study using Medicare billing data from 78 974 patients hospitalized for confirmed acute MI, older patients with acute MI and lower hematocrit had higher 30-day mortality.3
The management of patients with CVD and ACS has been controversial. RBC transfusion would seem to make physiologic sense by increasing the oxygen-carrying capacity of the blood, thereby potentially improving perfusion and oxygenation of at-risk cardiac tissue.3,4 In a study of 4470 critically ill patients in intensive care units (ICUs), those with cardiac disease trended toward higher mortality when Hb level fell below 9.5 g/dL (55% vs 42%; P = .09). However, anemic patients with Acute Physiology and Chronic Health Evaluation II (APACHE II) scores of >20 and a cardiac diagnosis had significantly improved survival when they received transfusion with 1 to 3 or 4 to 6 RBC units compared with those who did not receive transfusion (35% vs 32% vs 55%, respectively; P = .01).5 Other studies have provided contradictory results.6,7 A randomized, controlled study in 22 academic and 3 community ICUs across Canada raised the possibility that a restrictive transfusion strategy caused harm.7 However, wide variations in transfusion practice continued to be documented for patients with CVD and MIs.7,8
For over 2 decades, and following randomized transfusion trials, RBC transfusion guidelines have recommended a “restrictive” transfusion trigger strategy (Hb, 7 or 8 g/dL) for hospitalized anemic patients, including those with stable CVD.8,9 In 2012, a widely cited clinical practice guideline published by the AABB (formerly known as the American Association of Blood Banks) recommended a restrictive transfusion strategy for “hospitalized patients with preexisting CVD” but also advised “considering transfusion for patients with symptoms or a Hb level of ≤8 g/dL.”10 This guideline emphasized that no recommendation was being proposed for either a liberal or restrictive transfusion threshold for hemodynamically stable patients with ACS but made a “weak recommendation” that decisions be influenced by patient symptoms and Hb concentration. A Cochrane review in 2021 that analyzed 48 randomized trials, including 3 pediatric trials and 21 433 participants, similarly recommended a restrictive transfusion strategy (7.0-8.0 g/dL) for a broad range of clinical settings but concluded that data were insufficient to make recommendations for patients with ACS.11
Recent randomized trials of transfusion and CVD
Recently, 2 large, randomized trials have addressed this issue. The REALITY trial, an open-label, noninferiority, randomized trial conducted in 35 hospitals in France and Spain, enrolled 668 patients with acute MI and Hb concentration of 7 to 10 g/dL.12 The original protocol specified that, in the restrictive arm, a Hb transfusion “trigger” of 7 g/dL was to be used. However, this was subsequently changed to 8 g/dL due to concerns regarding investigator adherence to a Hb threshold of 7 g/dL. The liberal arm targeted a posttransfusion threshold of 11 g/dL and used a trigger of ≤10 g/dL as a threshold to transfuse. The primary clinical end point was a composite of MACE, which included all-cause death, stroke, recurrent MI, or emergency revascularization at 30 days. At 30 days, MACE occurred in 36 participants (11.0% [95% confidence interval (CI), 7.5-14.6]) in the restrictive group and in 45 participants (14.0% [95% CI, 10.0-17.9]) in the liberal group. The results met the noninferiority criteria for a restrictive compared with a liberal transfusion strategy. However, the study authors admitted that the prespecified noninferiority CI (1.25) was such that they could not rule out clinically relevant harm in the restrictive arm. Worrisome, at 1-year follow-up, the restrictive strategy was no longer noninferior.13 The authors concluded that “the late accrual of MACE in the restrictive group may reflect delayed harm related to persistent anemia, such as an increased risk of sudden death or arrhythmias in that group.”13
The second trial, the landmark Myocardial Ischemia and Transfusion (MINT) trial, randomized 3504 anemic participants with acute MI, at 144 sites in the United States, Canada, Brazil, France, Australia, and New Zealand, to a restrictive transfusion strategy (Hb, 7-8 g/dL) or a liberal strategy (Hb, <10 g/dL).14 The primary end point was a composite of new-onset MI or death at 30 days. Myocardial ischemia and transfusion trial was a pragmatic, intention-to-treat trial, in which clinicians and participants were unblinded. The trial was designed to have 80% power to detect a 20% relative reduction in the incidence of the primary outcome. The observed effect was a relative difference of ∼15%, favoring the liberal strategy. This result, coupled with a P value of .07, does not establish the superiority of a liberal transfusion strategy. However, neither does it exclude the potential harm of a restrictive transfusion strategy. Furthermore, the majority of the CIs consistently favored the liberal transfusion strategy as safer than the restrictive for the primary outcome, death, cardiac death, recurrent MI, and the composite of death, MI, ischemia-driven unscheduled coronary revascularization, or readmission to the hospital for an ischemic cardiac event (Figure 1). At 30 days, the risk of MI or death was 2.4% lower (95% CI, –0.1 to 4.7) in the liberal group than the restrictive group, and the risk of death was 1.6% lower (95% CI, –0.3 to 3.5). The apparent advantage of the liberal transfusion strategy was noted early after randomization and persisted over 30 days. Examining CIs and survival curves, the chance that blood transfused in the liberal arm would not improve patient outcome is very small, and there was no evidence that liberal transfusion caused harm. Together, these data suggest that a liberal RBC transfusion strategy is safer than a restrictive approach for these patients with ACS.
Cardiac outcomes reported in the MINT trial. In the MINT trial, the point estimates strongly favored the liberal transfusion strategy for the primary and secondary outcomes: death, cardiac death, recurrent MI, and the composite of death, MI, ischemia-driven unscheduled coronary revascularization, or readmission to the hospital for an ischemic cardiac event. Modified from Carson et al with permission.14
Cardiac outcomes reported in the MINT trial. In the MINT trial, the point estimates strongly favored the liberal transfusion strategy for the primary and secondary outcomes: death, cardiac death, recurrent MI, and the composite of death, MI, ischemia-driven unscheduled coronary revascularization, or readmission to the hospital for an ischemic cardiac event. Modified from Carson et al with permission.14
We performed a standard meta-analysis of 14 randomized RBC transfusion trials that enrolled noncardiac surgery participants with ACS or stable CVD (Figure 2).15 We found that a restrictive RBC transfusion strategy, compared with a liberal (≥10 g/dL), significantly increased the absolute risk of new-onset ACS by ∼2% (95% CI, 0.7-3.3). Using the PINC-AI (formerly Premier) database with a total of 10.6 million encounters between 2019 and 2023,16 we estimate that there are ∼11 250 orthopedic surgical patients with atherosclerotic CVD who have been discharged with a Hb <9 g/dL. Based on the total annual US hospitalization number of 33.7 million, there are ∼35 000 orthopedic surgical patients with atherosclerotic CVD discharged with a Hb <9 g/dL each year. If these anemic patients were managed with a restrictive strategy, we estimate that ∼700 excess ACS events (95% CI, 245-1155) may have occurred in anemic orthopedic patients each year.
Myocardial infarctions in patients with CVD enrolled in transfusion trigger trials. Relative risk of a MI (A) in individuals receiving a liberal vs restrictive transfusion trigger not undergoing cardiac surgery with preexisting CVD and hospitalized for ACS (top) or noncardiac reasons (bottom). Risk ratios of mortality and ACS were analyzed using random-effects models. All analyses were performed using R version 4.3.1 with package meta version 6.5-0. AMI, acute myocardial infarction; HCT, hematocrit; HgB, hemoglobin; PCI, percutaneous coronary intervention; RR, risk ratio. Adapted from Applefeld et al with permission.15
Myocardial infarctions in patients with CVD enrolled in transfusion trigger trials. Relative risk of a MI (A) in individuals receiving a liberal vs restrictive transfusion trigger not undergoing cardiac surgery with preexisting CVD and hospitalized for ACS (top) or noncardiac reasons (bottom). Risk ratios of mortality and ACS were analyzed using random-effects models. All analyses were performed using R version 4.3.1 with package meta version 6.5-0. AMI, acute myocardial infarction; HCT, hematocrit; HgB, hemoglobin; PCI, percutaneous coronary intervention; RR, risk ratio. Adapted from Applefeld et al with permission.15
Impact of trial design on results and recommendations
How did recommendations for a restrictive transfusion strategy persist for these patients? The conventional answer is that the lack of definitive guidelines for administering transfusions to patients with ACS results from an absence of clinical evidence. However, it is at least as likely that misinterpretation of data from early transfusion trials is to blame. In our view, 2 seminal transfusion trials that helped influence the guidelines for anemic patients with CVD suffered from fundamental design and analysis flaws.
The first trial, published in 1999 and widely known as the Transfusion Requirements In Critical Care (TRICC) trial, randomized 838 critically ill patients admitted to the ICU to either a “restrictive” RBC transfusion strategy (Hb ≤7 g/dL) or a “liberal” strategy (Hb <10 g/dL). In a prospective secondary end point analysis of in-hospital mortality, the liberal transfusion strategy was associated with an increased death rate compared with the restrictive strategy (P = .05). However, the TRICC trial failed to include a usual-care control group.17 Consensus conferences at that time had established that standard practice for RBC transfusion included titrated treatment based on such variables as age and comorbidities.18,19 Before the trial, the TRICC investigators confirmed this practice in Canada using a survey that canvassed their enrolling physicians.20 The authors wrote the following in their 1998 survey: “in summary, we believe this study (the survey) to be the first to demonstrate the importance of clinical characteristics [emphasis added] in influencing red cell transfusion decisions.” The authors continued, “respondents were more reluctant to administer red cells to the trauma patient (low-risk scenario) than … [to the] elderly patient with a … (high risk scenario).” In the TRICC trial, both study arms enrolled older participants with comorbidities and younger participants with fewer comorbidities. Because, in both arms, the trial design differed markedly from typical practice, the overall results of the trial should be viewed with caution. In a secondary analysis, we compared the outcome data for 2 subgroups from the TRICC trial.21 Across these 2 key subgroups, pursuing a restrictive vs liberal RBC transfusion strategy yielded significantly different (opposite) results regarding effects on mortality. For participants with CVD who tend to be older and at “higher risk,” the liberal strategy yielded improved survival rates compared with the restrictive strategy. For participants without CVD who tend to be younger and at “lower risk,” the liberal strategy had a higher mortality than the restrictive strategy. These divergent outcomes support the established practice of tailoring care at an individual patient level and using higher transfusion triggers in older participants with CVD and lower transfusion triggers in younger patients with fewer comorbidities. Because care administered in each arm of the TRICC trial was misaligned with common medical RBC transfusion practice in a way that increased the risk for different participants in each arm, it is ill advised to use the trial conclusions to establish transfusion policy.22-24 The investigators could have used their own survey data to develop inclusion and exclusion criteria bolstered by recommendations from the most recent expert guidelines to identify and characterize a usual-care group to serve as the control arm.23 A randomized trial with this well-defined usual care serving as a control arm could determine whether a restrictive, liberal, or other strategy is superior. We conclude that, because standard of care was determined but not studied, the proper interpretation of this trial should be “which of the 2 unusual RBC transfusion treatment programs presents more risk to patients?” The TRICC study design became a standard template for investigating transfusion practices, leading to a surge in trials of similar designs that failed to include usual-care RBC transfusion controls.
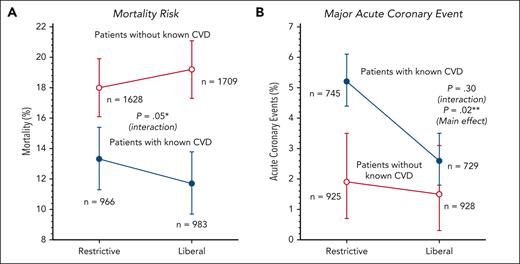
In 2018, we performed another meta-analysis of RBC transfusion trigger trials designed similar to the 1999 TRICC study.25 We again found that among 5385 participants across 9 trials separately reporting on mortality in participants with and without CVD, mortality was significantly different (opposite) with a restrictive vs liberal strategy, the same result in the identical 2 subgroups as in the 1999 TRICC trial (Figure 3). Furthermore, with an increased sample size, we found that those who received restrictive care suffered a significantly higher risk of MI (Figure 3). Our updated meta-analysis in 2024 showed that participants with CVD enrolled in a restrictive transfusion strategy bore a significantly increased risk of both MI (Figure 2) and death (Figure 4).15 The 2024 update to this 2018 meta-analysis added 3 transfusion trigger trials, cumulatively enrolling 4222 additional participants with CVD.
Comparison of outcomes in 9 transfusion trigger trials reporting myocardial infarction and death in patients with and without known CVD. (A) Mortality risk. ∗Restricitve vs liberal transfusion triggers had significantly different and opposite effect on mortality depending on the presence or absence of known CVD. In patients with known CVD, liberal strategies decreased mortality, and in patients without CVD, liberal strategies increased mortality. (B) Major acute coronary event. ∗∗In patients with known CVD as well as those without known CVD, there was a decrease in acute coronary events with a liberal transfusion strategy that overall was statistically significant. Adapted from Cortés-Puch et al with permission.25
Comparison of outcomes in 9 transfusion trigger trials reporting myocardial infarction and death in patients with and without known CVD. (A) Mortality risk. ∗Restricitve vs liberal transfusion triggers had significantly different and opposite effect on mortality depending on the presence or absence of known CVD. In patients with known CVD, liberal strategies decreased mortality, and in patients without CVD, liberal strategies increased mortality. (B) Major acute coronary event. ∗∗In patients with known CVD as well as those without known CVD, there was a decrease in acute coronary events with a liberal transfusion strategy that overall was statistically significant. Adapted from Cortés-Puch et al with permission.25
Death in patients with CVD enrolled in transfusion trigger trials. Relative risk of death in individuals receiving a liberal vs restrictive transfusion trigger not undergoing cardiac surgery with preexisting CVD and hospitalized for ACS (top) or noncardiac reasons (bottom). UGI bleed, upper gastrointestinal bleed. Adapted from Applefeld et al with permission.15
Death in patients with CVD enrolled in transfusion trigger trials. Relative risk of death in individuals receiving a liberal vs restrictive transfusion trigger not undergoing cardiac surgery with preexisting CVD and hospitalized for ACS (top) or noncardiac reasons (bottom). UGI bleed, upper gastrointestinal bleed. Adapted from Applefeld et al with permission.15
The second and arguably more influential transfusion study, the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) trial, was published in 2011.26 The original protocol restricted enrollment to participants with CVD aged ≥50 years who were scheduled to undergo hip surgery. Within a year, the enrollment criteria were amended to include participants with risk factors for CVD, presumably to increase enrollment. It is unclear which factors were considered specific for CVD risk in this trial; the protocol and methods include conventional CVD risk factors as well as renal failure; however, the manuscript seems to indicate that chronic lung disease, history of dementia or confusion, and history of cancer were considered to be CVD risk factors as well. Furthermore, the authors used a more stringent significance level of 0.01 for secondary and tertiary outcomes, presumably to (informally) account for multiple comparisons. The disadvantage of this approach is that it resulted in reduced statistical power and underreporting of possible harmful effects.
The FOCUS trial was designed to determine whether liberal and restrictive strategies are equally safe for anemic patients with CVD. If that were true, restrictive treatment could be widely applied with minimal risk and significant savings of volunteer blood and hospital costs.27 Unfortunately, the study did not report the frequency of MI suffered by older participants with CVD who underwent orthopedic surgery. We found subsequently that, in this “higher overall risk” population, 17 new-onset MIs occurred across 636 participants with CVD enrolled in the liberal arm but double that number of MIs in the restrictive arm: 32 new-onset MIs across 631 participants with CVD (P = .03; Figure 5).25 This result, had it been reported, would still not have met the higher threshold of 0.01 needed to be considered a statistically significant outcome in this trial. The authors did report the combined data, including odds ratios, for the enrolled participants with and without CVD: 23 new-onset MIs across 1005 participants in the liberal arm, compared with 38 new-onset MIs across 1008 participants in the restrictive arm (P = .055).21 This P value did not approach the significance threshold of .01 set for secondary outcomes in this trial. In keeping with the prospective analytical plan for the FOCUS trial, the analysis treated MIs only as one of many secondary/tertiary end points. The study did not treat these findings as key safety data of concern that need to be discussed.26 We believe that a trial of older participants with CVD undergoing major orthopedic surgery and, by randomization, having restricted RBC availability should have taken a more conservative approach and presented and discussed all the MI findings as safety data, regardless of significance levels.
Rate of myocardial infarctions almost doubled in the restrictive arm of the FOCUS trial. In the FOCUS trial,26 the number of new onset MIs doubled in participants with stable CVD having hip surgery and receiving a restrictive instead of a liberal transfusion strategy. This finding was not reported in the original publication,26 but the data were provided by the authors upon request and first published 7 years later in our 2018 meta-analysis.25 Adapted from Cortés-Puch et al with permission.25
Rate of myocardial infarctions almost doubled in the restrictive arm of the FOCUS trial. In the FOCUS trial,26 the number of new onset MIs doubled in participants with stable CVD having hip surgery and receiving a restrictive instead of a liberal transfusion strategy. This finding was not reported in the original publication,26 but the data were provided by the authors upon request and first published 7 years later in our 2018 meta-analysis.25 Adapted from Cortés-Puch et al with permission.25
Transfusion strategies for other patient subgroups
Unfortunately, flaws in trial design unforeseen before the trial began went unrecognized even after trial completion. Additionally, good faith attempts to reduce false positives led to the erroneous consideration of worrisome safety data as only one of many secondary/tertiary end points. These 2 inadvertent errors appear to have contributed to flawed RBC transfusion recommendations for patients with CVD. It would, therefore, not be unreasonable to ask whether recommendations for other patient subgroups drawn from studies using this trial design may also be faulty. This is not a question of whether liberal or restrictive is the preferred choice or whether equivalency is shown for these 2 strategies. The absence of usual practice controls calls into question the firmness of conclusions possible for defining RBC transfusion policies.
In our view, there are other problems with these trials. The definitions of “restrictive” and “liberal” transfusion triggers are arbitrary; various trials define both terms differently. The most frequent restrictive Hb value is 7 to 8 g/dL; however, in the recent Cochrane analysis, half of the trials defined restrictive as >8 g/dL and as high as 9.7 g/dL.11 Similarly, the definition of liberal usually ranges from 9 to 10 g/dL. However, “liberal” has been redefined in different trials as 11.3, 12.0, 11.5 to 13.1, and even 14.5 g/dL, albeit in markedly different clinical settings.28-32 This should not be surprising. Medical practitioners are unlikely to select the same single laboratory value for all patients in all circumstances. Furthermore, some trial designs allowed for sufficiently permissive RBC transfusion practices that the separation of Hb values between restrictive and liberal effectively vanished.33
The concept of “transfusion trigger” has similarly been altered over time. The original term, derived from a retrospective analysis of 535 031 surgical patients, described “the factor or constellation of factors which precipitates blood transfusion.”34 Hb concentration was only one of these factors. However, the clinical setting, signs and symptoms, and additional assays such as measures of oxygenation or acidosis could be better used, potentially more informative, produce better outcomes, as well as help conserve valuable resources if used as components of a transfusion trigger in clinical trials, in expert guidelines, or in practice. A single Hb concentration rather than some clinical algorithm emerged as the trigger, simplifying clinical trial design. After the TRICC trial, prospective observational studies surveying blood use in critically ill patients in Europe and the United States confirmed that “low Hb” was the most cited indication for transfusion.35,36 Additionally, in a review of records of 701 patients who underwent knee or hip arthroplasty in 3 hospitals in Montreal, 85% of postoperative transfusions could be predicted using only the low Hb value; adding clinical characteristics did not improve predictability.37 Physicians seemed to base their decision to transfuse solely on a single variable, the Hb concentration, and a restrictive strategy, even though conventional teaching advises that the decision to transfuse should be based on the patient's capacity to compensate for anemia and on the clinical setting.
Recent clinical practice guidelines seem more inclined to include clinical status in the decision to transfuse RBC. The 2023 Red Blood Cell AABB International Guidelines continue “to recommend restrictive transfusion strategies.”38 However, the guidelines now include a “Good Clinical Practice Statement” recommending that clinicians consider not only the Hb concentration but other laboratory data, clinical context, symptoms, and signs, which are described in detail. Similarly, although recommendations for patient blood management from the 2018 Frankfurt Consensus Conference still endorse restrictive RBC transfusion strategies for most surgeries, they note the “lack of agreement on the definition of Hb level for the diagnosis of preoperative anemia,” emphasize the “importance of individual patient clinical assessment,” and recognize that “measurement of Hb concentration alone cannot replace clinical evaluation.”39
Recently, another RBC transfusion trigger trial, this one for traumatic brain injury, using the same binary restrictive/liberal model, has been published.40 Although several patient-reported outcomes suggested better results with the liberal strategy, the results were not definitive, and the trial design was such that it could not exclude harm as a result of the restrictive strategy. Similar to many previous trials, the study was a logistical success but of uncertain clinical value. This does not denigrate the investigators’ intentions, efforts, or execution of the several large-scale, multicenter international trials. Trials such as these are difficult and expensive to perform. In our view, the conclusions remain controversial because of the trial design used. Results from the “gold standard” randomized clinical trials are often cited as evidence that protocol-based care for transfusion is demonstrably safer than personalized care, and the restrictive protocols are at least as safe and effective as titrated care. Neither hypothesis has been adequately tested. Controlled transfusion trials for critical care, acute MIs, cardiac surgery, and now traumatic brain injury are unlikely to be repeated. However, guidelines for RBC transfusion for anemic patients with hematologic malignancies, solid tumors, and bone marrow failure, as well as for other acute neurologic disorders, are still lacking.39,41 Future randomized controlled trials or comparative effectiveness studies should incorporate usual-care arms and not be limited to comparing Hb-based triggers.22-24
A path forward
Precision medicine is transforming the discipline of blood transfusion.42 Twenty-first century genomic sequencing technologies are used increasingly for RBC typing, RBC quality, and blood donor management and selection.43-45 The decision to transfuse RBC, in contrast, still relies primarily on an early 20th century laboratory determination, fixed targets, and rigid protocols.42 More sophisticated approaches using multiple biomarker patterns and genetic approaches are already being proposed, investigated, and applied to transfusion resuscitation strategies and management of coagulopathies.46,47 More effort should be devoted to developing sensitive measures of tissue hypoxia, multiple biomarkers, and other appropriate, physiologically relevant parameters for other indications for RBC transfusion.
We propose several short- and long-term measures to improve RBC transfusion therapy. First, medical practitioners who order blood should modify their practice to account for the mounting evidence that a more liberal, as opposed to a restrictive RBC transfusion strategy, lowers the risk of ACS and death in anemic patients with CVD. Furthermore, transfusion therapy should be personalized to account for an individual patient’s age, clinical status, and comorbidities. Second, weaknesses in randomized trial design can yield misleading results and contribute to flawed guidelines that adversely affect transfusion practice. We recommend that future trials try to understand, account for, and incorporate usual-care practices for the groups studied as control arm in the trial design. Third, investigators should report all harmful events in important subgroups that pertain to safety, regardless of P values. Failure to do so can mask possible safety signals. Fourth, the catchwords “liberal,” “restrictive,” and “transfusion trigger” are imprecise and no longer useful. These terms are obsolete and should be retired. Fifth, and finally, in the age of precision medicine, a single numerical Hb concentration seems inadequate as an indication for RBC transfusion therapy. A strategy designed for most patients may not benefit and may even harm a substantial minority. Additional effort should be devoted to developing other objective and physiologically relevant parameters, which might include noninvasive monitoring, imaging, biomarkers, and possibly genomics to enable more personalized patient management.
Acknowledgments
The authors acknowledge Kelly Byrne and Juli Maltagliati for their assistance with the manuscript, Junfeng Sun for statistical assistance, and Sameer Kadri and Alex Mancera for performing the PINC-AI (formerly Premier) database analysis.
This work was supported by National Institutes of Health (NIH) intramural funding from the NIH Clinical Center.
The work by the authors was conducted as part of US government funded research; however, the opinions expressed are not necessarily those of the NIH.
Authorship
Contribution: C.N., W.N.A., and H.G.K. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles Natanson, Critical Care Medicine Department, National Institutes of Health Clinical Center, 10 Center Dr, Room 2C145, Bethesda, MD 20892; email: cnatanson@cc.nih.gov.