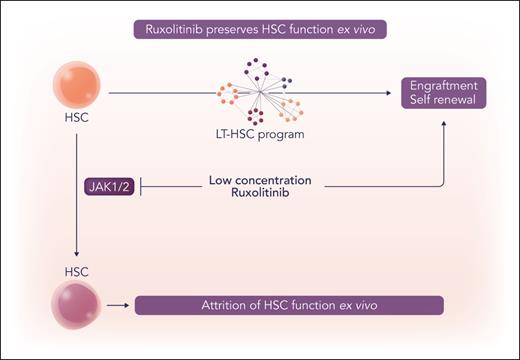
In this issue of Blood, Johnson et al1 elucidate a role for dose-dependent pharmacologic inhibition of JAK1/2 using ruxolitinib to preserve hematopoietic stem cell (HSC) function ex vivo.
The authors provide evidence that signaling via JAK1/JAK2 plays a role in modulating HSC potential in ex vivo culture systems and validated their findings using in vivo transplantation models. Using transcriptional and functional profiling, the authors show adaptation of human HSCs derived from primary cord blood or granulocyte colony-stimulating factor–mobilized adult peripheral blood in ex vivo culture systems occurred early, prior to first cell division, and was independent of cell proliferation and apoptosis. Among the metabolic and gene transcription changes that were observed, upregulation of JAK STAT signaling pathway genes was seen. The authors then showed the repopulation and self-renewal potential of human HSCs could be preserved (enriched approximately threefold) using ruxolitinib at concentrations lower than those that have been used for inhibition of canonical cytokine receptor signal transduction (see figure).
Ruxolitinib preserves HSC function ex vivo. Ruxolitinib at low concentrations maintains functional HSC potential in ex vivo culture conditions. Professional illustration by Somersault18:24.
Ruxolitinib preserves HSC function ex vivo. Ruxolitinib at low concentrations maintains functional HSC potential in ex vivo culture conditions. Professional illustration by Somersault18:24.
Following cloning of the JAK1 and JAK2 tyrosine kinases,2 the traditional canonical model for JAK phosphorylation of latent cytoplasmic STAT transcription factors following cytokine receptor signaling was developed from genetic complementation experiments using interferon signaling-resistant cell lines.3 STAT5, which is involved in prolactin signaling, was first cloned as a human STAT protein activated in response to interleukin-2.4 Cloning of STAT5B soon followed, identified by sequence homology.5 Since that time, extensive characterization of JAK and STAT family members in multiple cytokine receptor signaling pathways has been undertaken.6 Application of this knowledge has driven significant medical advances, including identification of mutations of several genes in these pathways that have been associated with oncogenesis. Understanding that excessive signaling activation was associated with specific human disease also led to the development of small-molecule inhibitors such as ruxolitinib, a relatively selective inhibitor of JAK1 and JAK2 kinases, for treatment of diseases such as myelofibrosis and polycythemia vera.7
It is fitting perhaps, that the use of ruxolitinib has now come full circle by allowing dose-dependent pharmacologic interrogation of the JAK and STAT pathways, which provide novel insights into ex vivo maintenance of HSC function that were not immediately extrapolatable from the HSC phenotypes arising from genetic loss of function models of Jak18 and Jak2.9 How ruxolitinib exerts an effect to guard the functional state of HSCs ex vivo at a molecular level was not fully elucidated by Johnson et al, as their therapeutic studies were principally focused on exploring the role of ruxolitinib and similar JAK inhibitors vs. other strategies to preserve ex vivo HSC potential in gene therapy culture conditions. Previous studies, however, have proposed a regulatory role for uSTAT5 that binds to specific regulatory genomic loci in a thrombopoietin-driven megakaryocyte differentiation model of HPC7 cells.10 This is an important area for future studies, including defining the direct molecular targets of JAK1/2 inhibition through examining the consequences of ruxolitinib treatment on chromatin accessibility that could be correlated with transcriptional changes in HSCs, and potentially investigating genomic targets of uSTAT5 vs phospho-STAT5 in ruxolitinib-treated and untreated HSCs.
Taken together, this article provides evidence that pharmacologic “fine-tuning” of JAK1/2 activity can preserve HSC potential ex vivo, a potentially impactful method by which HSC biology can be modified in a therapeutically advantageous way and one that is worthy of testing in clinical trials. With the caveat that the role of ruxolitinib and other similar JAK inhibitors may have broader effects than acting via a STAT5 mechanism, prior studies support the further exploration of a noncanonical role for uSTAT5 in genomic regulation.
Conflict-of-interest disclosure: A.P.N. declares no competing financial interests.