In this issue of Blood, Xu et al1 identify a new regulatory axis of hematopoietic stem cells (HSCs) senescence that links the ubiquitin-proteasomal system (UPS) to HSCs polarity to provide novel therapeutic targets to combat aging of HSCs.
Changes in the function of HSCs upon aging are thought to be central mechanisms of aging-associated remodeling of the immune system and the initiation of leukemia.2 Aging of HSCs might even affect aging of other organ systems, such as the heart, or limit life span.3 The identification of the mechanisms of aging of HSC is a prerequisite for the identification of novel targets to ameliorate these unwanted effects of aging of HSCs. Mechanisms of aging of HSCs still remain poorly understood and are often seen as being exclusive. Additional understanding of their interactions will likely be needed to advance the field.4
The authors initiated their examination of novel regulators of HSC aging by looking at components of the UPS. Proteostasis decline has already been proposed to be involved in aging of HSCs.5 Using a combination of genetic and highly sophisticated omics tools, the authors identified a large set of straight-forward western blots and single-cell immunofluorescent approaches, a not yet recognized DCFA8-DOCK11-CDC42 axis in regulating senescence of HSCs. Senescence is one cellular aspect in aging of HSCs.4 DDB1- and CUL4-associated factor 8 (DCAF8) functions as a substrate-recognition receptor for CUL4-DDB1 E3 ubiquitin ligase and is thus, an integral part of the UPS.6 The CUL4-DDB1 ubiquitin ligase performs targeted ubiquitination of specific substrates. Dedicator of cytokinesis (DOCK) family guanine nucleotide exchange factors are specific activators of small Rho GTPases through catalyzing the exchange of guanosine diphosphate for guanosine triphosphate. DOCK11 is in this respect, specific for the small Rho GTPase CDC42.7
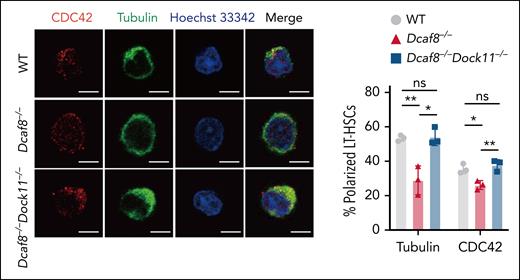
DCFA8 is a protein that is highly expressed in HSCs, as compared to hematopoietic progenitor cells, and whose expression is significantly reduced with aging. DCFA8 plays an important role in the ubiquitin-proteasome system in general, but for the stability of the DOCK11 protein. The authors show, primarily via genetic means, that DOCK11 becomes more active if DCFA8 levels are low. A more active DOCK11 upon aging in turn confers a higher level of activity on CDC42, which turns HSC apolar (see figure). Elevated activity of CDC42 and HSC apolarity have been shown to be causes of and consequences of aging of HSCs.8A DOCK11 knockout in HSCs reduces genomic instability in DCAF8-deficient HSCs, underscoring the importance of the DCAF8-DOCK11–regulatory axis for HSCs biology. Interestingly though, DOCK11 knockout mice themselves show only a very-mild phenotype with respect to changes in HSCs.
Aged HSCs show low levels of DCFA8. In the absence of DCAF8, HSCs become apolar, which can be rescued by knockout of DOCK11. Polarity of HSCs is determined by the level of activity of the small RhoGTPase CDC42. See Figure 6 in the article by Xu et al that begins on page 1462.
Aged HSCs show low levels of DCFA8. In the absence of DCAF8, HSCs become apolar, which can be rescued by knockout of DOCK11. Polarity of HSCs is determined by the level of activity of the small RhoGTPase CDC42. See Figure 6 in the article by Xu et al that begins on page 1462.
Although a general role for DOCK11 linked to the activity of CDC42 has been previously established in senescent-like immune disorders,9 they have not been mechanistically linked before to HSC senescence, and have not been connected to the level of DCFA8 and the UPS system as the causal initiator of this cascade. So, there is more to the UPS system in aging than simply regulating the amount and quality of proteins within a cell. These novel connections are very interesting but also surprising aspects of the regulation of HSCs senescence. This work will provide a set of novel targets like DOCK11 to attempt to attenuate phenotypes of aged HSCs, like senescence.
Of course, questions remain that will require additional exploration.
The phenytope conferred by the signaling axis in this work is primarily senescence of HSCs (also known as, segmental aging), but not full-fledged aging of HSCs. Which signaling component might be missing? DOCK11 is central to this axis, but only upon aging. Is CDC42 the only target of DOCK11 in that system? Besides DCAF8, are there other pathways that affect DOCK11 upon aging? Is regulation of DOCK11 a very dynamic system in HSCs with multiple players involved and in which levels of activity of proteins might have more regulatory power than those shown upon genetic deletion? Interestingly, drug-like inhibitors of DOCK11 activity are already available,10 so the most pressing question might be answered soon: do these findings translate to aging-associated senescence of human HSCs?
Conflict-of-interest disclosure: The author declares no competing financial interests.