In this issue of Blood, Zucca et al1 compare the outcome of patients with primary mediastinal B-cell lymphoma (PMBCL) treated with R-CHOP21 (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone given every 21 days) vs more intensive regimens as a post hoc analysis of the IELSG37 (NCT01599559) trial, the largest prospective trial in PMBCL to date. Although progression-free survival (PFS) and overall survival (OS) did not differ, patients treated with R-CHOP21 were more likely to have Deauville 5 (D5) positron emission tomography (PET) imaging at the end of therapy (EOT), suggesting an inferior metabolic response, which may be associated with reduced disease control and the need for additional therapy.
PMBCL is a subtype of non-Hodgkin lymphoma that occurs most commonly in young adults, especially women, and typically presents as a mediastinal mass. Although previously considered a subtype of diffuse large B-cell lymphoma (DLBCL), PMBCL is driven by distinct mechanisms, including dysregulated JAK-STAT signaling, NF-κB activity, and PD-L1 expression, and is now recognized as a distinct clinicopathologic entity.2
There is currently no universally accepted approach to initial therapy for PMBCL. Most centers use regimens that include rituximab and anthracyclines. R-CHOP21 with or without radiation therapy (RT), was traditionally considered the standard treatment for PMBCL. In an effort to mitigate toxicities and secondary malignancies associated with radiation exposure in a predominantly young, female patient population, dose-intensive treatment strategies such as DA-EPOCH-R, R-CHOP14, and R-VACOP/MACOP-B have become more common.3 These regimens are more frequently given without RT.
There is ongoing debate regarding the role of R-CHOP21 in PMBCL, with some studies demonstrating inferior outcomes compared to dose-intense regimens, and others reporting no difference. In the absence of prospective trials comparting the 2 approaches, the literature is composed of subgroup analyses of prospective trials and retrospective series. The analyses of the 2 largest subgroups (n = 50 and n = 131) of prospective trials both compared R-CHOP21 to R-CHOP14 and found no statistical difference in PFS or OS among patients with PMBCL.4,5 In contrast, a meta-analysis of >4000 patients with PMBCL, which included 11 prospective and 41 retrospective studies, reported inferior OS (80% vs 88%, P < .01) with R-CHOP21 vs dose-intense regimens and increased radiotherapy use (55% vs 22%, P < .01).6 Given the inherit limitations and mixed results of these studies, the optimal up-front treatment for PMBCL remains undefined.
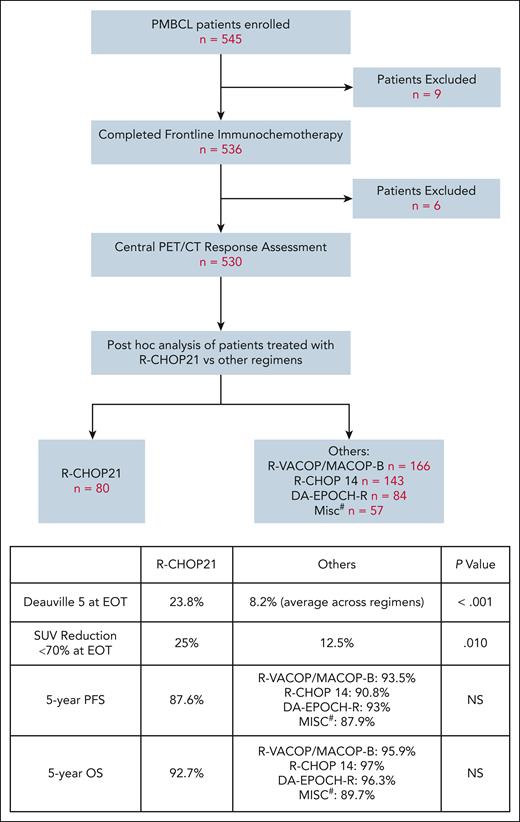
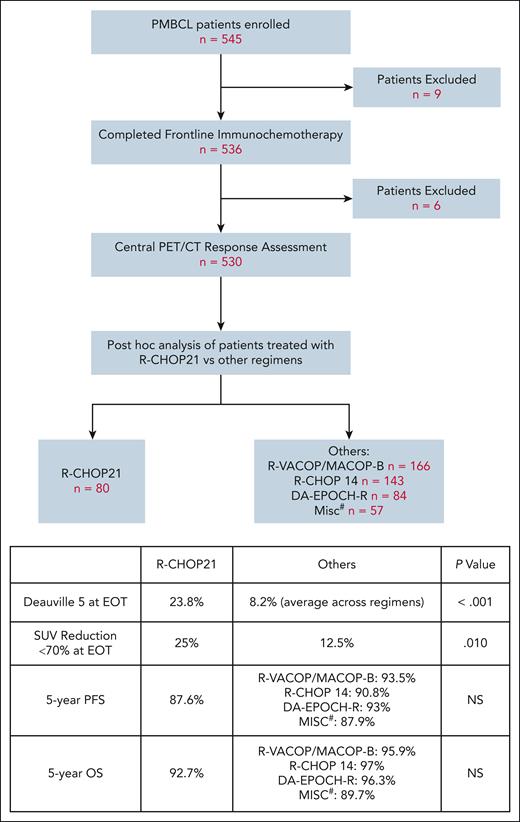
IELSG37 was a randomized phase 3 trial designed to determine if RT could be safely omitted in patients with PMBCL, who achieve a complete metabolic response (CMR) (D1-3) after chemoimmunotherapy. Patients aged ≥18 years with previously untreated PMBCL, who achieved a CMR after 6 cycles of chemotherapy, were randomized to RT vs no further therapy. Patients who were randomized to no further therapy, had comparable outcomes to those randomized to RT, demonstrating that RT can be safely omitted in this group.7 The chemoimmunotherapy backbone was chosen at the discretion of the treating physician and included R-CHOP21 and dose-intensive regimens. Although IELSG37 was not designed to compare chemotherapy regimens, this trial enrolled 545 patients, to our knowledge, the largest prospective series in PMBCL to date, and provides an unprecedented opportunity to evaluate R-CHOP21 in comparison to other regimens in a post hoc analysis.
Among 536 patients who completed frontline immunotherapy on IELSG37, 530 underwent central PET/CT response assessment. Of these, 80 patients received R-CHOP21, whereas 450 received other dose-intense regimens, including R-VACOP/MACOP-B, R-CHOP14, and DA-EPOCH-R. A review of EOT PET did not show differences in the rate of CMR in R-CHOP21 vs others. However, PET is known for a high false-positive rate in PMBCL. Many patients, especially those with D4 lesions at EOT, achieve long-term remission without additional intervention.8 Other PET measures that have been more predictive of poor outcome in PMBCL include D5 at EOT and SUVmax (maximum standardized uptake value) reduction of <70% at interim imaging.7,9 In this cohort, patients treated with R-CHOP21 exhibited a higher proportion of D5 at EOT compared to other regimens (23.8% vs 8.2%, P < .001). Additionally, at EOT, more patients in the R-CHOP21 group had <70% reduction in SUVmax (25% vs 12.5%, P = .010). Among all patients, those with D5 at EOT were more likely to receive further lines of systemic treatment compared to those with D1-4 (39.3% vs 4.6%, P < .001) (see figure).
Study schema and key findings. #, R-mega CHOP, R-COMP, R-CHOEP, G-MALL-B-ALL/NHL2000. DA-EPOCH-R, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab; G-MALL-B-ALL/NHL2000, German Multicenter Adult Acute lymphoblastic leukemia protocol; R-CHOEP, rituximab, cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone; R-COMP, rituximab, cyclophosphamide, vincristine, nonpegylated liposomal doxorubicin, prednisone; R-mega CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-VACOP/MACOP-B, rituximab, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisolone, bleomycin/methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisolone, bleomycin.
Study schema and key findings. #, R-mega CHOP, R-COMP, R-CHOEP, G-MALL-B-ALL/NHL2000. DA-EPOCH-R, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab; G-MALL-B-ALL/NHL2000, German Multicenter Adult Acute lymphoblastic leukemia protocol; R-CHOEP, rituximab, cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone; R-COMP, rituximab, cyclophosphamide, vincristine, nonpegylated liposomal doxorubicin, prednisone; R-mega CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-VACOP/MACOP-B, rituximab, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisolone, bleomycin/methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisolone, bleomycin.
A comparison of PFS and OS between R-CHOP21 and other regimens did not show statistically significant differences. There are a few potential explanations for this, including the possibility that R-CHOP21 is as effective as dose-intensive regimens in PMBCL. However, caution must be taken when interpreting these results because this study was not powered to detect differences in PFS/OS between treatment regimens. It is also possible that increased rates of additional therapy in patients with D5 lesions at EOT resulted in comparable outcomes across regimens despite inferior initial disease control.
This study benefits from a large, prospective cohort but is limited by its reliance on D5 as a surrogate marker for residual disease. Active disease was not systematically confirmed via biopsy, and treatment decisions after EOT imaging varied by institution. In addition, although the authors conclude that R-CHOP21 may be associated with increased long-term toxicity from additional therapy, the trial follow-up is too short to confirm such effects.
Taken together, the observations from this study provide some evidence that R-CHOP21 may result in inferior disease control and may require additional therapy to achieve outcomes comparable to intensive regimens. However, with no difference demonstrated in PFS or OS, this work does not resolve the debate on R-CHOP21 in PMBCL.
In the future, we should aim to define low-risk groups and/or novel treatment combinations, where lower intensity regimens may be sufficient and even preferable. For example, incorporating circulating tumor DNA as a biomarker of disease response, an approach that has demonstrated potential in DLBCL and Hodgkin lymphoma,10 may allow for selection of a group of patients at low risk of relapse. Furthermore, the addition of the immune checkpoint inhibitor nivolumab to standard chemoimmunotherapy in PMBCL is currently being studied in a randomized phase 3 trial (ANHL1931, NCT04759586). If successful, the addition of novel agents like nivolumab may help overcome the limitations of R-CHOP21 by enhancing efficacy, potentially redefining the standard of care.
Conflict-of-interest disclosure: L.G.-R. received consultancy fees from Merck, Roche, and Bristol Myers Squibb. N.G. declares no competing financial interests.