In this issue of Blood, Tatterton et al1 report how an alternative, antigen-independent way of signaling through the B-cell receptor (BCR) not only characterizes a specific subset of diffuse large B-cell lymphomas (DLBCL) of germinal center (GCB) type but might also serve as an independent indicator of poor prognosis. This is an important finding, which could improve our risk assessment in DLBCL and ultimately affect treatment choice.
The BCR is a crucial structure for growth and survival of normal B cells and most mature B-cell lymphomas, which usually retain BCR expression even if 1 of the 2 immunoglobulin heavy chain gene alleles is affected by a translocation. Binding of antigen to the BCR activates several downstream signaling cascades including the NF-κB pathway, which results in survival, proliferation, and differentiation of B cells. In B-cell lymphomas, BCR signaling can occur via antigen-dependent and antigen-independent routes. These include specific binding of foreign or autoantigens and mutations in BCR components and downstream signaling molecules resulting in autonomous or enhanced antigen-induced BCR signaling.
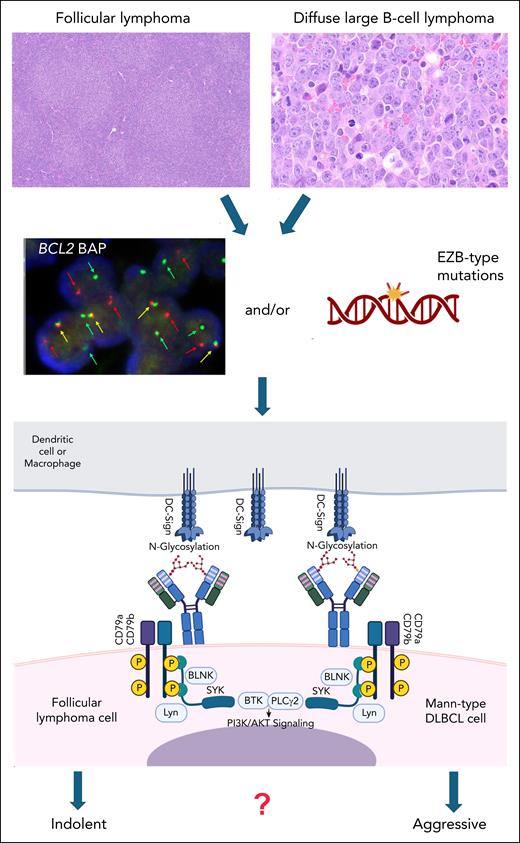
A specific form of BCR signaling, which is dependent on posttranslational modifications of the clonal immunoglobulin and bypasses cognate antigen binding has been described and elucidated in follicular lymphoma (FL).2-6 In most cases of FL, and in contrast to most other B-cell lymphoma subtypes, the complementarity determining regions (CDR) of the immunoglobulin, which are relevant for antigen binding contain immature, oligomannose-type (Mann-type) glycans. Further processing and maturation of these glycans is probably blocked by steric hindrance in the CDR. These Mann-type glycans bind to bacterial or microenvironmental lectins, especially DC-SIGN (dendritic cell–specific ICAM-3-grabbing nonintegrin), which is present on macrophages and dendritic cells in the tumor microenvironment. This interaction causes persistent low-level BCR signaling with activation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway, leading to survival and tissue retention of FL cells (see figure). The presence of Mann-type glycans on the antigen binding F(ab) part of the immunoglobulin is determined by specific acquired asparagine-X-serine/threonine amino acid sequence motifs serving as acceptor sites for N-addition of glycans in the CDR.6 These acquired N-glycosylation sites (AGS) are induced by somatic hypermutation in the GCB microenvironment. They are already present frequently in in situ follicular neoplasia, a precursor lesion of FL, and are positively selected and clonally retained throughout tumor evolution, indicating an important role in FL development.7,8
Schematic representation of the role of N-glycosylation in the CDR of the immunoglobulin heavy chain in FL and Mann-type DLBCL. Of note, immunoglobulin light chain CDRs can also be glycosylated in these lymphomas. Mann-type DLBCL (upper right) shares the presence of a BCL2 translocation and/or an EZB-type mutational profile (middle panel) with conventional FL (upper left). Oligomannoses in the CDR of the surface immunoglobulin as part of the BCR bind to DC-SIGN expressed by macrophages or dendritic cells. This results in continuous BCR signaling via CD79a/b with activation of the downstream PI3K/AKT pathway. The difference between the generally indolent behavior of FL and Mann-type DLBCL, especially in comparison with other DLBCL of GCB type, warrants further studies. The figure was created, in part, with BioRender.com. Montes I. (2025) https://BioRender.com/r2fnrs7.
Schematic representation of the role of N-glycosylation in the CDR of the immunoglobulin heavy chain in FL and Mann-type DLBCL. Of note, immunoglobulin light chain CDRs can also be glycosylated in these lymphomas. Mann-type DLBCL (upper right) shares the presence of a BCL2 translocation and/or an EZB-type mutational profile (middle panel) with conventional FL (upper left). Oligomannoses in the CDR of the surface immunoglobulin as part of the BCR bind to DC-SIGN expressed by macrophages or dendritic cells. This results in continuous BCR signaling via CD79a/b with activation of the downstream PI3K/AKT pathway. The difference between the generally indolent behavior of FL and Mann-type DLBCL, especially in comparison with other DLBCL of GCB type, warrants further studies. The figure was created, in part, with BioRender.com. Montes I. (2025) https://BioRender.com/r2fnrs7.
Not surprisingly, given the close relationship of FL and a subset of DLBCL, acquired N-glycosylation sites in the CDR are also present in ∼50% of GCB-type DLBCL.6,9 Their pathogenic role and potential clinical impact, however, has been much less investigated. In this study, building on previous work,9 Tatterton et al used RNA sequencing data of 2 large cohorts of DLBCL to search for N-glycosylation sites in the rearranged immunoglobulin sequences of different DLBCL subtypes. In addition, using recombinant F(ab)s derived from 30 DLBCLs expressed in HEK-293 cells and glycopeptide mass spectrometry, the authors determined the presence and type of glycans attached to the AGS. Ten DLBCL cases, and 12 FL cases analyzed in parallel as controls, carried Mann-type glycans in their F(ab)s, whereas in the remaining 20 DLBCL cases, the AGS were either unoccupied or carried complex glycans. Of note, all DLBCL samples with Mann-type glycans were either of EZH2 mutated B-cell lymphoma 2 (BCL2) translocation (EZB) molecular subtype with a mutational profile similar to that of FL or contained a BCL2 translocation. Overall, approximately two-thirds of FL-like DLBCLs belonging to the EZB type or carrying a BCL2 translocation contained AGS in the CDR, with much lower rates for other DLBCL of both GCB and activated B-cell types.
Most importantly, GCB DLBCL with Mann-type AGS had a worse progression-free and overall survival, comparable with activated B-cell DLBCL. In contrast, GCB-type DLBCL without this distinctiveness had a favorable prognosis. This prognostic difference persisted when the authors controlled for potentially confounding risk factors, including the presence of MYC rearrangements, double-hit status, and presence of the so-called dark-zone signature, a surrogate marker for high proliferation and poor prognosis.10 There was no association of these known poor prognostic features in EZB-type DLBCL with the presence of Mann-type AGS, and the worse prognosis of Mann-type DLBCL was observed for both dark-zone signature–positive and –negative cases. Mann-type status thus remained an independent prognostic factor for GCB-type DLBCL in the multivariate analysis in both studied cohorts.
This important study adds another layer of complexity to the molecular classification of DLBCL and might provide an answer for the heterogenous clinical behavior of GCB-type DLBCL beyond the known risk factors. As the authors point out, identification of Mann-type DLBCL requires detection of BCL2 translocations and/or EZB subtype and sequencing of the rearranged immunoglobulin heavy chain, techniques routinely carried out in many laboratories. If confirmed in other cohorts, the assessment of AGS may provide a relatively easy way to better define risk for GCB-type DLBCL and ultimately identify patients who could benefit from a direct targeting of the BCR–DC-SIGN interaction by specific antibodies or other approaches.
As for all studies breaking new ground, more questions arise. Why Mann-type DLBCL shows such an aggressive behavior compared with its low-grade relative, FL, remains a puzzling question. Due to the almost universal presence of Mann-type AGS in FL, to our knowledge, no published data comparing the clinical outcome of FL with and without AGS in their CDR exist. Functional studies in GCB-type DLBCL cell lines with and without Mann-type AGS and in vivo experiments will be required to show the functional impact of N-glycosylation in DLBCL and study the effect of blocking the BCR–DC-SIGN interaction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.