Key Points
Linear recruitment of enhancers to silenced globin genes overrides developmental silencing and reactivates gene expression.
Visual Abstract
The human genome contains regulatory DNA elements, known as enhancers, that can activate gene transcription over long chromosomal distances. Here, we showed that enhancer distance can be critical for gene silencing. We demonstrated that linear recruitment of the normally distal strong HBB enhancer to developmentally silenced embryonic HBE or fetal HBG promoters through deletion or inversion of intervening DNA sequences led to strongly reactivated expression in adult erythroid cells and ex vivo differentiated hematopoietic stem and progenitor cells. A similar observation was made for the HBA locus in which deletion-to-recruit of the distal enhancer strongly reactivated embryonic HBZ expression. Overall, our work assigned function to seemingly nonregulatory genomic segments; by providing linear separation, they may support genes to autonomously control their transcriptional response to distal enhancers.
Introduction
Most (98%) of the human genome consists of noncoding DNA sequences. They provide a home for more than a million transcriptional regulatory elements that collectively coordinate the cell-specific gene expression programs that drive cell differentiation, embryonic development, and organogenesis.1,2 Developmental gene activation depends on cis-acting DNA elements called enhancers. Enhancer action requires functional communication between transcription (co)factors recruited to the enhancer and the gene promoter. This functional communication is thought to occur through chromatin looping, which brings distant regulatory elements into close physical proximity to their target promoters.3,4 Endogenous enhancers can be located anywhere between 0 and 1500 kb away from their designated target gene,5 raising the question on whether linear distance has any functional implication. Random integration of a reporter gene in mice indicated that the likeliness of being expressed in a given tissue correlated with linear proximity to an enhancer active in that tissue.6 The transcript levels generally decreased with increasing enhancer-promoter distances.7-10 Artificial regulatory landscapes in which a reporter gene was activated by an enhancer at varying distances (0-400 kb) corroborated that enhancer distance was inversely correlated with transcriptional activity and stability; the more distal the enhancer was positioned, the higher the proportion of cells in which the reporter was silenced. Deletion of enhancer-promoter intervening sequences in the silenced cells led to strong transcriptional reactivation of the reporter.8 This suggested that by keeping enhancers at a distance, genes may gain control over their response to enhancers.8 Here, we aimed to study the role of genomic enhancer-promoter distance at the endogenous human α-globin (HBA) and β-globin (HBB) loci.
The HBA locus contains an embryonic gene, HBZ, and 2 adult genes, HBA1 and HBA2. Their expression is regulated by distal enhancers that are active in erythroid cells throughout development.11,12 The HBB locus consists of 5 β-like globin genes; the embryonic HBE gene precedes 2 fetal HBG genes, which are followed downstream by the adult HBD and HBB genes. Their activation and expression levels are controlled by the upstream locus control region (LCR),13 a strong enhancer that is active at all stages of erythroid development.
Silencing is intrinsically controlled by the promoter regions of the developmentally expressed globin genes.14 For instance, HBG inactivation is the consequence of repressor proteins recruited to the HBG gene promoters with BCL11A binding 115 bp upstream of the transcription start site (TSS) being the best characterized.15-17 Disruption of this binding site in the HBG gene promoter leads to HBG reactivation.18,19 Upstream of the HBG gene promoters, in the 25-kb interval that separates HBG from the LCR, there are no indications for regulatory sequences that prevent LCR-mediated activation of the HBG promoters in adult erythroid cells. Interestingly, HBG repression in adult red blood cell precursors can also be reversed by forced looping of the LCR to the HBG promoters.20 The current idea therefore is that stage-specific recruitment of repressor proteins to the HBG promoters prevents LCR engagement with the HBG genes, which leads to developmental silencing.
We hypothesized that enhancer remoteness may be required for the developmental silencing of globin genes. To test this, we designed a strategy called delete-to-recruit (Del2Rec), which forces linear recruitment of enhancers through CRISPR-Cas9–mediated deletion of intervening sequences. We showed that Del2Rec can induce strong reactivation of embryonic and fetal genes in adult erythroid cells, both in the HBB and HBA locus. We conclude that enhancer remoteness is required for developmental globin gene control.
Materials and methods
HUDEP-2 cell line
Cells were cultured in Cellquin proliferation medium, a fully defined, serum-free Iscove's modified Dulbecco's medium (IMDM) supplemented with components listed in supplemental Table 220 (available on the Blood website). HUDEP-2 cells were differentiated by switching to differentiation medium (supplemental Table 2); after 7 days, doxycycline was removed and cells were cultured for 3 more days. For the HBE experiments, the cells were directly placed in differentiation medium without doxycycline and cultured for 3 days.
SCD cell lines
Rotterdam erythroblast–sickle cell disease (SCD) cell lines were generated from peripheral blood mononuclear cells derived from patients with SCD (R. Majied, A. Rijneveld, M. Vasiliou, M. van Braem-Rest, N. Gillemans, K. de Koning, J. Lebbink, T.-W. Kan, B. Eussen, A. de Klein, L. Broer, J. van Meurs, E. van den Akker, M. von Lindern, Y. Nakamura, M. Rab, T. van Dijk and S. Philipsen, unpublished data, July 2023) as previously described for HUDEP-2 cells.21 The cells were cultured20 in IMDM with supplements (supplemental Table 3) and differentiated for 4 days in IMDM containing 3% Omniplasma, erythropoietin (EPO) (10 U/mL), transferrin (330 μg/mL), and heparin (3 U/mL). The medium was refreshed on day 2.
CD34+ HSPC culturing
Human material was obtained after informed consent. Mobilized peripheral blood was obtained from leukapheresis material (Sanquin, The Netherlands). CD34+ cells were isolated from fresh mobilized peripheral blood using a Miltenyi CD34 kit and cryopreserved using IMDM + 20% fetal calf serum and 5% final dimethyl sulfoxide concentration. The purity was determined by flow cytometry using the antibodies listed in supplemental Table 4. All the donors had >95% CD34+ hematopoietic stem and progenitor cells (HSPCs).
Cryopreserved CD34+ HSPCs were thawed and cultured in IMDM media (PAN biotech, P04-20251K), supplemented with a cytokine maintenance cocktail containing stem cell factor (SCF; 100 ng/mL), fms-related receptor tyrosine kinase 3 ligand (100 ng/mL), and thrombopoietin (10 ng/mL). All cells were precultured for 16 to 30 hours before nucleofection with Cas9 ribonucleoproteins (RNPs).
Cells were cultured with IMDM containing interleukin-3 (100 ng/mL), interleukin-6 (100 ng/mL), thrombopoietin (10 ng/mL), and SCF (100 ng/mL) at 37°C and 5% CO2 for 4 to 5 consecutive days. The cells were further cultured to pure pro-erythroblasts for 4 to 10 days in IMDM supplemented with 1 U/mL EPO, 100 ng/mL SCF, 1 μM dexamethasone, and 333 μg/mL holotransferrin.21 Differentiation of established pro-erythroblast cultures was initiated by culture in IMDM media supplemented with 5% human plasma, 10 U/mL EPO, and 1 mg/mL holotransferrin.21
RNP incubation and nucleofection in primary cells
For guide RNA (gRNA) screening, the ALT-R CRISPR-Cas9 CRISPR RNA system (IDT) was used. Equal amounts of CRISPR RNA and transactivating CRISPR RNA were incubated at 95°C for 5 minutes and then cooled to room temperature (RT). Nucleofections were performed as described.22
CRISPR editing in HUDEP-2 and Rotterdam erythroblast–SCD cell lines
Nucleofections were carried out with in-house purified 3xNLS-Cas923 using the Amaxa P3 Primary Cell 4D-Nucleofector X-Kit (EW-113). Single-cell clones were screened using polymerase chain reaction (PCR) assays, followed by Sanger sequencing.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The cells were homogenized in TRIzol (Life Technologies), and RNA was isolated using phenol-chloroform extraction or the Direct-zol RNA MiniPrep Kit (Zymo, catalog no. R2052). Complimentary DNA was synthesized using M-MLV reverse transcriptase H– enzyme (Promega).
Hemoglobin F (HbF) staining
The cells were fixed in 0.05% glutaraldehyde for 10 minutes at RT and then permeabilized with 0.1% Triton X-100 for 3 minutes. Staining was performed for 15 minutes in the dark. Flow cytometry was conducted using a Beckman Coulter Cytoflex S, and the data were collected using CytExpert 2.3.1.22 and analyzed in FlowJo 10.8.0 (supplemental Figure 7).
Lentivirus production and transduction
A founder cell line with Cas9-Blast (Addgene no. 52962) was maintained in medium with 1 μg/mL blasticidin (InvivoGen, ant-bl-05). Single-guide RNAs (supplemental Table 1) were cloned into a modified pU6-gRNA EF1α-puro-T2A–green fluorescent protein or blue fluorescent protein (BFP) lentiviral plasmid (Addgene no. 60955). The virus-containing medium was collected 48 hours after HEK293T transfection, filtered (0.45 μm), and concentrated 8× using Amicon Ultra 50 kDa filters (UFC905008). This was added to HUDEP-2 cells with 6 μg/mL Polybrene (Merck) and 1% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid). The cells were centrifuged for 90 minutes at 800g (RT) and refreshed after 24 hours.
Western blots
Whole-cell lysates were run on polyacrylamide gel and immunoblotted using standard procedures (antibodies in supplemental Table 4).
High-performance liquid chromatography (HPLC)
A total of 1 × 107 cells were collected and analyzed for hemoglobin expression using Waters Alliance 2690 equipment as described previously.22
Sickling assays
Sickling assays were performed at day 8 of differentiation. Approximately 2 × 106 cells were centrifuged at 1600 rpm for 5 minutes and resuspended in 10 μL phosphate-buffered saline. A total of 2 μL of cells was loaded onto a 35 mm glass bottom dish with 20 mm well size (D35-20-1.5-N, Cellvis), and 2 μL of 20 μg/mL sodium metabisulfite (Sigma-Aldrich) dissolved in phosphate-buffered saline was added to the sample. The sample was covered with a coverslip. Cells were monitored and imaged using differential interference contrast (DIC) microscopy for up to 30 minutes at RT on a Nikon Eclipse Ti inverted widefield microscope that was equipped with DIC optics (de Sénarmont DIC configuration) and a chromatic aberration free infinity Plan Fluor 40 × NA 1.3 oil objective and that was controlled by Metamorph software (version 7.8.0.0, Molecular Devices). The microscope was equipped with a CoolSNAP HQ2 CCD camera (Photometrics) with a 1392 × 1040 imaging array and a pixel size of 6.45 μm × 6.45 μm, and images were projected onto the camera at 161.25 nm per pixel.
ddPCR
The reaction mixtures (20 μL) contained 1× digital droplet PCR (ddPCR) Master Mix (Bio-Rad, catalog no. 12005909), the relevant primers and probe (400 nM primers, 100 nM probe), the telomerase reverse transcriptase reference (dHsaCP2500351), and 3 μL MseI-digested genomic DNA (20 ng). The droplet generation and PCR reactions followed published protocols24 and the manufacturer instructions. The rearrangement frequencies were calculated as fractions of the total.
ATAC-seq
Assay for transposase-accessible chromatin sequencing (ATAC-seq) was performed using the Omni-ATAC protocol on 200 000 cells, incubated with in-house–produced Tagment DNA Enzyme for 30 minutes at 37°C. Libraries were pooled and sequenced in single- or paired-end mode on a NextSeq 2000 with a P2 flow cell using Illumina-supplied kits. The reads were trimmed using cutadapt 4.4 (--minimum-length 10, adapter removal) and aligned with bowtie2 (--local --very-sensitive-local --no-unal --no-mixed --no-discordant --dovetail -X 1000). Nonprimary alignments, improperly paired reads (for paired-end), and alignments with mapping quality <15 were filtered out using samtools 1.17 (view -F 260) and were blacklisted using the ENCODE Unified GRCh38 Blacklist (ENCFF356LFX) via bedtools 2.31.0. Duplicates were retained because of the higher duplication likelihood of 51 bp single-end reads from distinct fragments.
For visualization, bigWigs were generated per the ENCODE ATAC-seq pipeline (https://github.com/ENCODE-DCC/atac-seq-pipeline) and displayed using rtracklayer 1.62.2 and GenomicRanges 1.54.1. Clone bigWigs were averaged using bigwigAverage (deepTools 3.5.2), and the final signal tracks were normalized to the average signal ±500 bp around TSS.
CUT&RUN
CUT&RUN was performed with the Cell Signaling CUT&RUN Kit (catalog no. 86652S) according to the manufacturer’s protocol and starting with 0.5 million cells. The libraries were prepared using the NEBNext Ultra II DNA Library Kit (catalog no. E7645) with 15 to 30 ng input DNA and were pooled and paired-end sequenced (2 × 50 bp) on an Illumina NextSeq 2000.
Results
Forced linear proximity of the HBB enhancer reactivates HBG
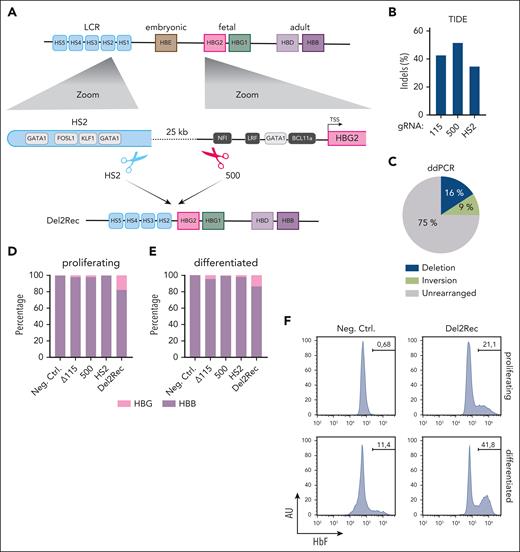
The HBB LCR spans nearly 20 kb and has 5 hypersensitive sites (HS1-5; Figure 1A) with different intrinsic enhancer capacities.25,26 HS2, -3, and -4 are potent individual enhancers, whereas HS1 and -5 lack strong intrinsic enhancer activity. To develop Del2Rec, we designed gRNAs close to HS2. The accompanying gRNA was placed upstream of the HBG2 promoter. Combined, they generated a 25-kb deletion, thereby placing HS5-4-3-2 immediately upstream of the HBG2 promoter and leaving the reported repressor protein binding sites intact (Figure 1A). Several gRNAs near HS2 and HBG2 were tested for efficiency. Based on the tracking of indels by decomposition (TIDE) analysis,24 we selected gRNA-HS2 (located inside HS2) and gRNA-500 (located 500 bp upstream of the HBG2 TSS); gRNA-500 is unique for the HBG2 promoter and leaves the HBG1 promoter intact.
Linear recruitment of the LCR reactivates fetal globin in HUDEP-2 cells. (A) A schematic representation of the HBB locus on chromosome 11 depicting the hypersensitive sites of the LCR (light blue), HBE (brown), HBG2 (pink), HBG1 (green), HBD (light purple), and HBB (dark purple) genes. Below (left) is a zoom-in on HS2 showing the most relevant transcription factor binding site (TF-BS) (gray) and the binding site of gRNA-HS2. On the right side, a zoom-in on the HBG2 promoter with the most relevant TF-BS (gray) and position of gRNA_500. At the bottom, the 25-kb deletion (Del2Rec) is shown that was generated by the combination of gRNA_500 and gRNA-HS2. (B) Frequency of indels as measured by the TIDE analysis for Δ115, 500, and HS2 gRNAs in single-gRNA transfected samples. (C) Frequency of the 25-kb deletion (blue), inversion (lime green), and the unmodified iteration (gray) as measured by ddPCR in the Del2Rec condition. (D) RT-qPCR analysis of the HBB and HBG messenger RNA (mRNA) levels in proliferating (day 0) cells. The HBB and HBG mRNA expression was normalized to actin mRNA and is displayed as a percentage of HBB plus HBG. (E) Same as in panel D but for cells differentiated for 10 days. (F) Flow cytometry plots showing the percentage of HbF-positive cells in proliferation (top row) and after 10 days of differentiation (bottom row). AU, arbitrary units; Ctrl., control; LRF, leukemia/lymphoma-related factor; Neg., negative; NFI, nuclear factor I.
Linear recruitment of the LCR reactivates fetal globin in HUDEP-2 cells. (A) A schematic representation of the HBB locus on chromosome 11 depicting the hypersensitive sites of the LCR (light blue), HBE (brown), HBG2 (pink), HBG1 (green), HBD (light purple), and HBB (dark purple) genes. Below (left) is a zoom-in on HS2 showing the most relevant transcription factor binding site (TF-BS) (gray) and the binding site of gRNA-HS2. On the right side, a zoom-in on the HBG2 promoter with the most relevant TF-BS (gray) and position of gRNA_500. At the bottom, the 25-kb deletion (Del2Rec) is shown that was generated by the combination of gRNA_500 and gRNA-HS2. (B) Frequency of indels as measured by the TIDE analysis for Δ115, 500, and HS2 gRNAs in single-gRNA transfected samples. (C) Frequency of the 25-kb deletion (blue), inversion (lime green), and the unmodified iteration (gray) as measured by ddPCR in the Del2Rec condition. (D) RT-qPCR analysis of the HBB and HBG messenger RNA (mRNA) levels in proliferating (day 0) cells. The HBB and HBG mRNA expression was normalized to actin mRNA and is displayed as a percentage of HBB plus HBG. (E) Same as in panel D but for cells differentiated for 10 days. (F) Flow cytometry plots showing the percentage of HbF-positive cells in proliferation (top row) and after 10 days of differentiation (bottom row). AU, arbitrary units; Ctrl., control; LRF, leukemia/lymphoma-related factor; Neg., negative; NFI, nuclear factor I.
We first assayed the transcriptional consequences of Del2Rec in HUDEP-2 cells, a model system for human adult erythroid cells.27 The TIDE analysis24 revealed that gRNA-HS2 and gRNA-500, when individually transfected as Cas9 RNPs into HUDEP-2 cells, reached local insertion/deletion (indel) rates of 35% and 52%, respectively (Figure 1B). This was similar to the 43% local indel rate measured for gRNA-11528 that served as a control. We applied ddPCR (supplemental Figure 1A) to quantify the 25-kb deletion, inversion, and unrearranged allele frequencies upon nucleofection of gRNA-HS2 + gRNA-500 RNPs. This showed that 16% of the alleles carried a deletion and 9% an inversion of the 25-kb fragment (Figure 1C).
We then tested whether Del2Rec caused HBG reactivation. We measured HBG and HBB expression levels using RT-qPCR. Although only 16% of the alleles carried the 25-kb deletion, we observed considerable HBG gene reactivation before and after differentiation (Figure 1D-E). Consistent with these data, flow cytometry revealed that Del2Rec increased the proportion of cells with high fetal hemoglobin levels (F-cells; Figure 1F). Replicate experiments confirmed that Del2Rec induced HBG reactivation (supplemental Figure 2). Together, these results indicate that forced linear proximity of the LCR can overwrite the promoter-encoded developmental silencing of the HBG genes in adult red blood cell precursors.
Del2Rec drives activation of the proximal HBG2 gene and suppression of the distal HBB gene
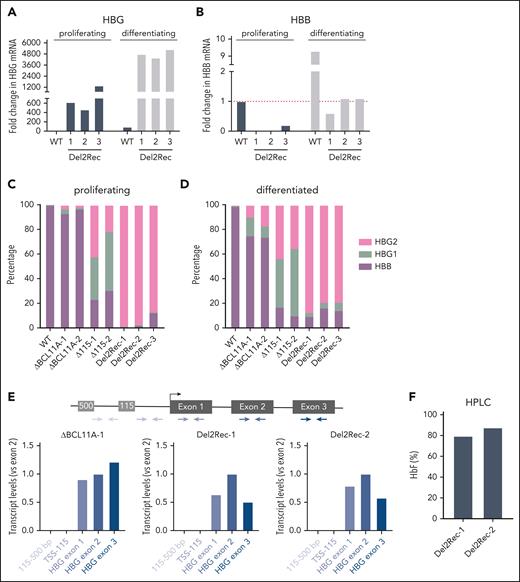
To obtain direct evidence that Del2Rec leads to HBG gene reactivation, we generated clonal HUDEP-2 cell lines that carried the intended 25-kb deletion on both alleles. These clonal cell lines, Del2Rec-1 to -3, carried alleles with slightly different deletion junctions. All alleles retained at least 493 bp of the HBG2 promoter and the complete HBG1 gene. For comparison, we applied gRNA161723 which targets the erythroid-specific enhancer of BCL11A (hereafter referred to as ΔBCL11A). In 2 clonal HUDEP-2 lines (ΔBCL11A-1 and ΔBCL11A-2) with indels on both alleles of the BCL11A enhancer, ATAC-seq showed that the BCL11A enhancer was no longer accessible and that BCL11A protein was no longer detectable by western blot (supplemental Figure 3A-B). In addition, we used gRNA-11528 to generate 2 HUDEP-2 clonal lines (Δ115-1 and Δ115-2) with homozygous disruption of the BCL11A binding sites in the HBG promoters (supplemental Figure 3C).
The Del2Rec lines showed strong HBG upregulation in proliferation conditions (Figure 2A). Upon differentiation, we found further increased HBG transcript levels (Figure 2A). Concomitantly, the HBB transcript levels increased but not to the control HUDEP-2 cells levels (Figure 2B), suggesting that the activated HBG genes compete with the more distal HBB genes for activation by the LCR.29-31 Next, we determined whether the proximal or distal HBG gene was activated by the LCR. Using a HBG gene-specific RT-qPCR strategy, we observed that the Del2Rec clones nearly exclusively reactivated the proximal HBG2 gene (Figure 2C-D). In contrast, the Δ115 and ΔBCL11A lines expressed HBG2 and HBG1 at similar levels (Figure 2C-D). Overall, the HBG expression levels in the Del2Rec lines were similar to those measured in the Δ115 lines and higher than found in the ΔBCL11A lines. We excluded that elevated HBG2 transcript levels were the consequence of LCR-initiated enhancer transcripts that read into HBG2 by analyzing the transcripts with different PCR amplicons covering the promoter area and the HBG2 TSS (Figure 2E).
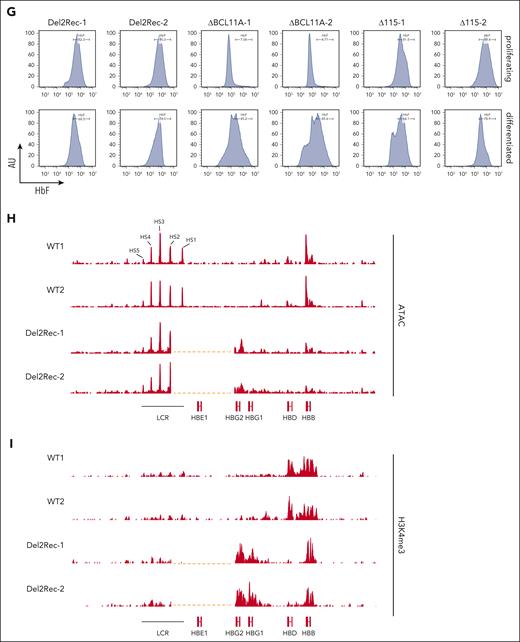
For the Del2Rec lines, HPLC showed that 80% to 90% of hemoglobin consisted of fetal hemoglobin (Figure 2F), and when flow cytometry was used, an almost pure population of F cells was observed (Figure 2G). This was comparable with HbF and F-cell levels observed in Δ115 cells. In the ΔBCL11A lines, high HbF and F-cell levels were only seen after differentiation. In the Del2Rec lines, ATAC-seq (Figure 2H) and CUT&RUN for H3K4me3 (Figure 2I) demonstrated that linear recruitment of the LCR led to chromatin opening and H3K4 trimethylation of the HBG2 promoter and partial reduction in accessibility of the more distal adult HBB gene (Figure 2H), likely as a consequence of Del2Rec-induced gene competition for activation by the LCR.
Finally, we investigated HBG transcript and protein levels in 2 clonal cell lines with a monoallelic 25-kb deletion and an intact wild-type allele. In these heterozygous Del2Rec lines, still 60% to 70% of the β-globin-like transcripts were of HBG origin (supplemental Figure 4A-B) and a high percentage of F cells was observed (supplemental Figure 4C). Thus, Del2Rec can support effective HBG2 reactivation upon monoallelic HBB locus rearrangement.
We conclude that Del2Rec causes strong reactivation of HBG2 expression in adult erythroid cells. We therefore propose that the linear distance between the LCR and the genes is essential for the HBG promoters to autonomously control their developmental silencing in adult erythroid cells.
Del2Rec overwrites HBG silencing in an SCD model and CD34+ HSPCs differentiated to the erythroid lineage
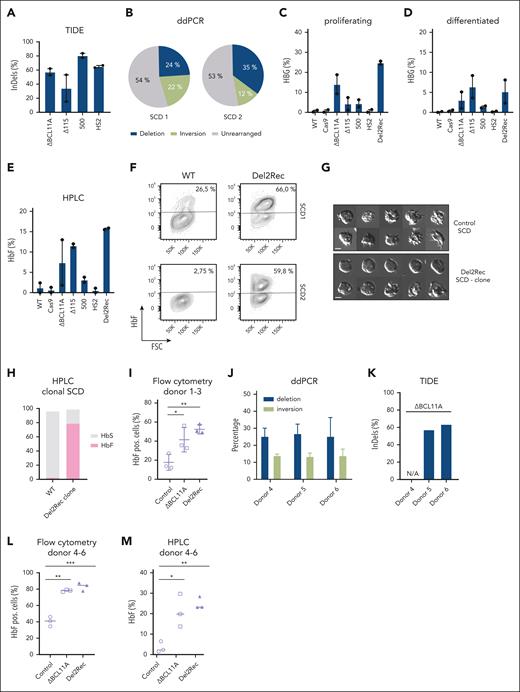
Partially restored HBG expression can ameliorate the symptoms of SCD and β-thalassemia,32-34 2 common, severe monogenic diseases caused by HBB variants. We therefore tested whether Del2Rec would also be effective in 2 erythroid progenitor cell lines (SCD1 and SCD2) that were derived from patients with SCD. The TIDE analysis showed that Del2Rec nucleofection was efficient (60%-80%) when compared with control nucleofections with gRNA-115 (15%-55%) and the BCL11A targeting gRNA1617 (55%-60%; Figure 3A). ddPCR showed that Del2Rec treatment led to 24% of the 22% (SCD1) and 35% of the 12% (SCD2) of alleles with the 25-kb deletion/inversion, respectively (Figure 3B).
Linear recruitment of the LCR reactivates fetal globin in immortalized cell lines derived from patients with SCD and HSPCs. (A) The frequency of indels, as measured by TIDE analysis, for ΔBCL11A (gRNA1617), Δ115, 500, and HS2 gRNAs in single-gRNA transfected samples in 2 SCD cell lines shown as average ± standard error of the mean (SEM). (B) Frequency of the 25-kb deletion (blue), inversion (lime green), and unmodified (gray) iteration as measured by ddPCR in Del2Rec cells. (C) RT-qPCR analysis of HBG mRNA levels in proliferating cells, displayed as the average ± SEM in percentage of the total HBB + HBG mRNA. (D) Same as in panel C but for cells differentiated for 4 days. (E) HbF of 2 Rotterdam erythroblast (REB)–SCD cell lines, as measured by HPLC, shown as the average ± SEM. It was calculated as the percentage of HbF over the total Hb tetramers. (F) Flow cytometry plots showing the percentage of HbF positive cells in 2 REB-SCD cell lines. Note that SCD1 shows relatively high baseline HbF levels. (G) Representative images of SCD cells under hypoxic conditions of a WT clone (top) and homozygous Del2Rec SCD clone (bottom). (H) HPLC analysis results of a homozygous SCD Del2Rec clone, calculated as the percentage of HbF (pink) and HbS (gray) over the total Hb tetramers. (I) Graph showing the percentage of HbF-positive cells of donors 1 to 3, as measured by flow cytometry. Error bars show the standard deviation (SD) of the triplicate samples. (J) Frequency of the 25-kb deletion (blue) and inversion (lime green) measured by ddPCR on Del2Rec transfected cells. The values were calculated as an average of 2 measurements from genomic DNA isolated from donors 4 to 6. The error bars represent the SD. (K) Frequency of indels, as measured by TIDE analysis, for ΔBCL11A (gRNA1617) of donors 4 to 6. (L) Percentage of F cells plotted as averages of 3 donors after 12 days of differentiation. A 1-way analysis of variance and Tukey’s post hoc test was used to calculated statistics. (M) HbF of donors 3 to 6 measured by HPLC 3 days after differentiation in control, ΔBCL11A, and Del2Rec conditions. We calculated the percentage of HbF over the total Hb tetramers. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant; WT, wild-type.
Linear recruitment of the LCR reactivates fetal globin in immortalized cell lines derived from patients with SCD and HSPCs. (A) The frequency of indels, as measured by TIDE analysis, for ΔBCL11A (gRNA1617), Δ115, 500, and HS2 gRNAs in single-gRNA transfected samples in 2 SCD cell lines shown as average ± standard error of the mean (SEM). (B) Frequency of the 25-kb deletion (blue), inversion (lime green), and unmodified (gray) iteration as measured by ddPCR in Del2Rec cells. (C) RT-qPCR analysis of HBG mRNA levels in proliferating cells, displayed as the average ± SEM in percentage of the total HBB + HBG mRNA. (D) Same as in panel C but for cells differentiated for 4 days. (E) HbF of 2 Rotterdam erythroblast (REB)–SCD cell lines, as measured by HPLC, shown as the average ± SEM. It was calculated as the percentage of HbF over the total Hb tetramers. (F) Flow cytometry plots showing the percentage of HbF positive cells in 2 REB-SCD cell lines. Note that SCD1 shows relatively high baseline HbF levels. (G) Representative images of SCD cells under hypoxic conditions of a WT clone (top) and homozygous Del2Rec SCD clone (bottom). (H) HPLC analysis results of a homozygous SCD Del2Rec clone, calculated as the percentage of HbF (pink) and HbS (gray) over the total Hb tetramers. (I) Graph showing the percentage of HbF-positive cells of donors 1 to 3, as measured by flow cytometry. Error bars show the standard deviation (SD) of the triplicate samples. (J) Frequency of the 25-kb deletion (blue) and inversion (lime green) measured by ddPCR on Del2Rec transfected cells. The values were calculated as an average of 2 measurements from genomic DNA isolated from donors 4 to 6. The error bars represent the SD. (K) Frequency of indels, as measured by TIDE analysis, for ΔBCL11A (gRNA1617) of donors 4 to 6. (L) Percentage of F cells plotted as averages of 3 donors after 12 days of differentiation. A 1-way analysis of variance and Tukey’s post hoc test was used to calculated statistics. (M) HbF of donors 3 to 6 measured by HPLC 3 days after differentiation in control, ΔBCL11A, and Del2Rec conditions. We calculated the percentage of HbF over the total Hb tetramers. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant; WT, wild-type.
With the RT-qPCR, we found that the individual gRNAs HS2 and -500 gave little HBG reactivation, but both cell lines strongly responded to Del2Rec (Figure 3C-D). This was confirmed at the protein level by HPLC (Figure 3E) and by flow cytometry for F cells (Figure 3F; supplemental Figure 5A). We conclude that Del2Rec also efficiently induced HBG reactivation in erythroid progenitor cell lines derived from patients with SCD (Figure 3C-F). To assess whether the Del2Rec deletion reduces sickling under hypoxic conditions, we analyzed a clonal cell line homozygous for the deletion. When compared with a wild-type clone, Del2Rec cells exhibited a markedly reduced sickling phenotype (Figure 3G). Because these are reticulocytes, they do not adopt the classical sickle morphology under chemically induced hypoxia; nonetheless, we observed a significant decrease in aberrant cell shapes associated with hypoxic stress. This was further supported by hemoglobin composition analysis via HPLC (Figure 3H).
Thus far, we induced deletions in cells already committed to the erythroid lineage, that is, those with an open HBB locus with an accessible LCR primed to engage with the downstream accessible β-like globin genes. Therefore, we next determined the effects of Del2Rec in primary CD34+ HSPCs. We edited CD34+ HSPCs that were isolated from 3 healthy donors (supplemental Figure 5B); gRNA161723 that targeted the BCL11A enhancer was used as a control. The proliferation, erythroid specification, and differentiation characteristics of Del2Rec-edited HSPCs were similar as those of control cells (supplemental Figure 5C-D). Importantly, Del2Rec led to high HBG activation at levels comparable with those obtained with the BCL11A enhancer gRNA (Figure 3I).
To further investigate this, we repeated the experiment with 3 new donors and used ddPCR to quantify the Del2Rec edits (Figure 3J). This revealed that Del2Rec induced deletions/inversions in, on average, 26% of the 14% of the alleles (Figure 3J). The TIDE analysis showed that gRNA1617 modified ∼60% of the BCL11A alleles (Figure 3K), as reported earlier.23 Upon differentiation, flow cytometry showed that both approaches yielded ∼80% of F cells when compared with 35% to 45% in the controls (Figure 3L). HPLC analysis revealed that cells expressed ∼20% to 25% of HbF with both approaches, but Del2Rec seemed to give less variable expression when compared with targeting the BCL11A enhancer (Figure 3M). Collectively, we conclude that Del2Rec can reactivate HBG expression in erythroid cells derived from CD34+ HSPCs.
Forced linear proximity through inversions also drives HBG reactivation
A relatively high proportion of alleles showed inversion of the 25-kb fragment upon Del2Rec. We sorted clones from the original Del2Rec (500-HS2) condition to obtain clones with at least 1 inverted allele, thereby placing the weak HS1 enhancer 4 kb upstream from HBG2 but leaving the strong elements HS2-3-4-5 at the endogenous 25-kb distance. Deletion clones all showed strong upregulation, but inversions had no effect on HBG expression (supplemental Figure 6A). We then designed 2 alternative editing strategies. In the first, gRNA-500 was used in combination with a novel gRNA-HS2/3 that targeted the region between HS2 and HS3. Together, they created deletions that placed HBG2 immediately downstream of HS5-4-3 and inversions that placed the gene downstream of the inverted HS2-1 (Figure 4A; supplemental Figure 6B). In bulk, this Del2Rec strategy induced high levels of HBG2 RNA and protein (Figure 4B-C). Isolated clonal cell lines (supplemental Figure 6C) confirmed that now both deletions and inversions contributed to HBG expression (Figure 4D-E). The HBG2 transcript and protein levels were higher than that of HBB, even in cells with only 1 rearranged allele, suggesting that a single reactivated HBG2 gene copy can produce more transcripts than 2 HBB gene copies. Thus, rearrangements that recruit a sufficiently strong enhancer, not by deleting but by inverting sequences, can also induce HBG gene reactivation. This is further evidence that developmental silencing of HBG expression is not controlled by sequences within the targeted 25-kb interval but by enhancer distance and promoter integrity.
Inversion of intervening sequence can also reactivate silenced genes. (A) A schematic representation of the HBB locus on chromosome 11 depicting the hypersensitive sites of the LCR (light blue), HBE (brown), HBG2 (pink), HBG1 (green), HBD (light purple), and HBB (dark purple) genes. Below (left) is a zoom-in on HS2 and HS3 showing the position of gRNA-HS2/3. On the right side, a zoom-in on the HBG2 promoter with the most relevant TF-BS (gray) and position of gRNA_500. At the bottom, the 27-kb deletion generated by the combination of gRNA_500 and gRNA-HS2/3. Below, a representation of the inversion generated by the same gRNAs. (B) RT-qPCR analysis of HBB, HBG1, and HBG2 mRNA levels in proliferating bulk cell populations. HBB, HBG1, and HBG2 mRNA expression was normalized to actin and is displayed as the percentage of HBB + HBG1 + HBG2. WT is the parental lentiviral Cas9-expressing cell line without gRNAs. (C) Flow cytometry plots showing the percentage of HbF-positive cells in proliferating bulk cell populations. (D) On the left side, RT-qPCR analysis of HBB, HBG1, and HBG2 mRNA levels in 2 proliferating heterozygous deletion clones. HBB, HBG1, and HBG2 mRNA expression was normalized to actin and displayed as a percentage of HBB + HBG1 + HBG2. On the right side, HbF of 2 clonal cell lines as measured by HPLC. Percentage of HbF calculated over the total Hb tetramers. (E) Same as panel D but for 3 HS2/3-500 inversion clones. (F) Same as panel A but a schematic representation of the gRNAs HS2 and HBG1/2, cutting between HBG1 and HBG2. (G) RT-qPCR analysis of the HBB, HBG1, and HBG2 mRNA levels in proliferating bulk cell populations. HBB, HBG1, and HBG2 mRNA expression was normalized to actin and displayed as a percentage of HBB + HBG1 + HBG2. (H) Flow cytometry plot showing the percentage of HbF-positive cells in proliferating bulk cell population.
Inversion of intervening sequence can also reactivate silenced genes. (A) A schematic representation of the HBB locus on chromosome 11 depicting the hypersensitive sites of the LCR (light blue), HBE (brown), HBG2 (pink), HBG1 (green), HBD (light purple), and HBB (dark purple) genes. Below (left) is a zoom-in on HS2 and HS3 showing the position of gRNA-HS2/3. On the right side, a zoom-in on the HBG2 promoter with the most relevant TF-BS (gray) and position of gRNA_500. At the bottom, the 27-kb deletion generated by the combination of gRNA_500 and gRNA-HS2/3. Below, a representation of the inversion generated by the same gRNAs. (B) RT-qPCR analysis of HBB, HBG1, and HBG2 mRNA levels in proliferating bulk cell populations. HBB, HBG1, and HBG2 mRNA expression was normalized to actin and is displayed as the percentage of HBB + HBG1 + HBG2. WT is the parental lentiviral Cas9-expressing cell line without gRNAs. (C) Flow cytometry plots showing the percentage of HbF-positive cells in proliferating bulk cell populations. (D) On the left side, RT-qPCR analysis of HBB, HBG1, and HBG2 mRNA levels in 2 proliferating heterozygous deletion clones. HBB, HBG1, and HBG2 mRNA expression was normalized to actin and displayed as a percentage of HBB + HBG1 + HBG2. On the right side, HbF of 2 clonal cell lines as measured by HPLC. Percentage of HbF calculated over the total Hb tetramers. (E) Same as panel D but for 3 HS2/3-500 inversion clones. (F) Same as panel A but a schematic representation of the gRNAs HS2 and HBG1/2, cutting between HBG1 and HBG2. (G) RT-qPCR analysis of the HBB, HBG1, and HBG2 mRNA levels in proliferating bulk cell populations. HBB, HBG1, and HBG2 mRNA expression was normalized to actin and displayed as a percentage of HBB + HBG1 + HBG2. (H) Flow cytometry plot showing the percentage of HbF-positive cells in proliferating bulk cell population.
To further substantiate this notion, we combined gRNA-HS2 with the new gRNA-HBG1/2 that targets the region between HBG2 and HBG1 (Figure 4F). In addition, this gRNA combination generated deletions and inversions (supplemental Figure 6B) and strongly reactivated HBG expression (Figure 4G-H), but now, transcripts of both HBG1 and HBG2 were observed. Because HBG2 is removed from the deleted alleles, its reactivated expression must come from inverted alleles that retained all the natural 25-kb upstream sequences of HBG2 but placed this gene proximal to HS2-3-4-5. Thus, linear separation enables the fetal HBG genes to negate the enhancers and execute their promoter-encoded developmental silencing program in adult erythroid cells.
Del2Rec reactivates the embryonic gene HBE in the HBB locus and HBZ gene in the HBA locus
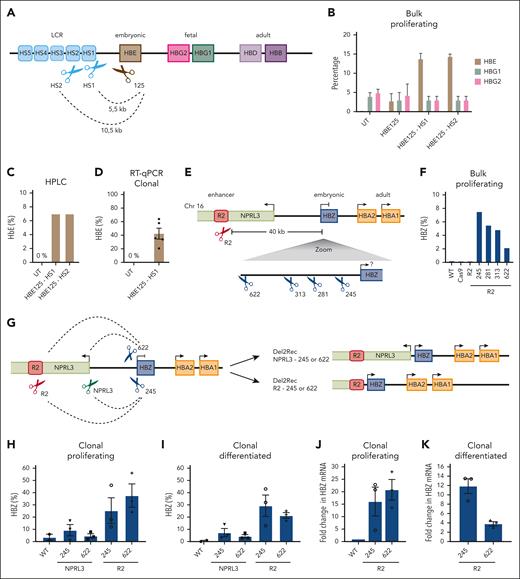
We next explored whether Del2Rec could also be applied to reactivate the embryonic HBE gene (Figure 5A). HBE is not expressed in wild-type HUDEP-2 cells (Figure 5B). We combined a gRNA that targeted the HBE promoter with a gRNA either at HS1 or at HS2. The HBE promoter gRNA itself already induced some HBE reactivation, measured by RT-qPCR, but this increased to much higher levels with both Del2Rec gRNA combinations (Figure 5B). The HBE protein formed functional complexes with HBA, as demonstrated by HPLC (Figure 5C). When measured across 5 heterozygous Del2Rec clonal lines, the HBE levels even reached an average of ∼40% of the total HBB transcript levels (Figure 5D; supplemental Figure 7A).
Linear recruitment enhancer reactivates embryonic genes in HUDEP-2 cells. (A) Schematic overview of the HBB locus on chromosome 11. The scissors indicate the binding site of the promoter that targeted gRNA (brown) with 125 bp distance to TSS and at HS1 or HS2 (light blue). (B) RT-qPCR analysis of HBE (brown), HBG1 (green), and HBG2 (pink) of transduced, bulk-proliferating cells. The mRNA levels were normalized to actin mRNA and were displayed as a percentage of HBB + HBE + HBG1/2. (C) HbE of bulk HBE125-HS1 or HS2 as measured by HPLC 3 days after differentiation. We calculated the percentage of HbF over the total Hb tetramers. (D) RT-qPCR analysis of HBE for untransduced (UT) and 5 average heterozygous HBE125-HS1 deletion clones. The mRNA levels were normalized to actin mRNA and displayed as a percentage (± SEM) of HBB + HBE + HBG1/2. (E) Schematic overview of the HBA locus on chromosome 16 depicting the R2 enhancer (red), HBZ (blue), HBA1/2 (orange), and NPRL3 (lime green) genes. The red scissors displays the gRNA at the R2 enhancer. Below that is a zoom-in on HBZ showing the binding sites of the 4 gRNAs (blue scissors) with the distance to the HBZ TSS. (F) RT-qPCR analysis of the HBZ mRNA levels in proliferating (day 0) bulk nucleofected HUDEP-2 cells that recruited R2 to different distances from the HBZ TSS. The HBZ mRNA expression was normalized to actin mRNA and is displayed as a percentage HBZ of HBA plus HBZ. (G) A schematic representation of deletions performed in the HBA locus. Two gRNAs at the HBZ promoter were either combined with a gRNA at the R2 enhancer or 490 bp upstream of the NPRL3 TSS. The dashed lines represent deletions obtained upon treatment with the 2 indicated gRNAs. (H) Same as panel F, but the RT-qPCR was performed in heterozygous-deleted HUDEP-2 lentivirus-transduced clonal cell lines. Average of 2× WT cell lines derived in the same experiment and clonal outgrowth that was genetically unrearranged. The percentages displayed as averages ± SEM of 2 to 3 clones. (I) Same as panel H but for clones differentiated for 3 days. (J) RT-qPCR results of R2-HBZ deletion clones, normalized to the HBZ levels in proliferating cells (± SEM). (K) Same as panel J but in differentiated cells, normalized to proliferating conditions.
Linear recruitment enhancer reactivates embryonic genes in HUDEP-2 cells. (A) Schematic overview of the HBB locus on chromosome 11. The scissors indicate the binding site of the promoter that targeted gRNA (brown) with 125 bp distance to TSS and at HS1 or HS2 (light blue). (B) RT-qPCR analysis of HBE (brown), HBG1 (green), and HBG2 (pink) of transduced, bulk-proliferating cells. The mRNA levels were normalized to actin mRNA and were displayed as a percentage of HBB + HBE + HBG1/2. (C) HbE of bulk HBE125-HS1 or HS2 as measured by HPLC 3 days after differentiation. We calculated the percentage of HbF over the total Hb tetramers. (D) RT-qPCR analysis of HBE for untransduced (UT) and 5 average heterozygous HBE125-HS1 deletion clones. The mRNA levels were normalized to actin mRNA and displayed as a percentage (± SEM) of HBB + HBE + HBG1/2. (E) Schematic overview of the HBA locus on chromosome 16 depicting the R2 enhancer (red), HBZ (blue), HBA1/2 (orange), and NPRL3 (lime green) genes. The red scissors displays the gRNA at the R2 enhancer. Below that is a zoom-in on HBZ showing the binding sites of the 4 gRNAs (blue scissors) with the distance to the HBZ TSS. (F) RT-qPCR analysis of the HBZ mRNA levels in proliferating (day 0) bulk nucleofected HUDEP-2 cells that recruited R2 to different distances from the HBZ TSS. The HBZ mRNA expression was normalized to actin mRNA and is displayed as a percentage HBZ of HBA plus HBZ. (G) A schematic representation of deletions performed in the HBA locus. Two gRNAs at the HBZ promoter were either combined with a gRNA at the R2 enhancer or 490 bp upstream of the NPRL3 TSS. The dashed lines represent deletions obtained upon treatment with the 2 indicated gRNAs. (H) Same as panel F, but the RT-qPCR was performed in heterozygous-deleted HUDEP-2 lentivirus-transduced clonal cell lines. Average of 2× WT cell lines derived in the same experiment and clonal outgrowth that was genetically unrearranged. The percentages displayed as averages ± SEM of 2 to 3 clones. (I) Same as panel H but for clones differentiated for 3 days. (J) RT-qPCR results of R2-HBZ deletion clones, normalized to the HBZ levels in proliferating cells (± SEM). (K) Same as panel J but in differentiated cells, normalized to proliferating conditions.
Finally, we examined whether reactivating developmentally silenced genes by Del2Rec could be extended to other loci. The HBA locus on chromosome 16 (Figure 5E) also contains an embryonic gene, HBZ, that is completely silenced (Figure 5F), along with 2 active adult globin genes (HBA1 and HBA2), in HUDEP-2 cells. Their main enhancer, R2, is located ∼40 kb upstream of HBZ.11 We selected 4 gRNAs that targeted sites between 245 and 622 base pairs upstream of the HBZ TSS35 and combined each with a gRNA directly downstream of enhancer R2.36 All 4 gRNA combinations led to the expected deletions (supplemental Figure 7B) that linearly recruited R2 to the HBZ promoter, and in all cases, this was accompanied by an increase in HBZ expression (Figure 5F).
We isolated clonal lines with heterozygous deletions that brought the R2 enhancer either 622 bp or 245 bp upstream of the HBZ TSS. We also generated heterozygous clonal lines with smaller deletions of ∼15 kb that linked the HBZ promoter to the NPRL3 promoter. NPRL3 is a gene that harbors R2 and is transcribed away from the HBA genes (Figure 5G; supplemental Figure 7C-D). The recruitment of the NPRL3 promoter did not lead to reactivation of HBZ expression (Figure 5H-I). In contrast, the recruitment of enhancer R2 to either location in the HBZ promoter both led to strong reactivation of HBZ expression (Figure 5H-K), whereas HBA expression seemed to be unchanged (supplemental Figure 7E). Similar to Del2Rec in the HBB locus, increased HBZ transcript levels were the consequence of regained promoter activity and not of enhancer transcripts that extend into the HBZ gene (supplemental Figure 7F). Collectively, we conclude that Del2Rec can be applied to (re)activate the expression of developmentally silenced genes in both the HBB and HBA locus.
Discussion
Our demonstration that Del2Rec can reactivate the HBG genes seems surprising in light of a previous study in which transgenes with the human LCR juxtaposed to an HBG gene were shown to silence the gene in adult mice.14 In that study, the degree of silencing was dependent on the genomic integration site of the transgenic construct, and the distance between the HBG and LCR was bigger than in our configuration, and both may explain why HBG gene silencing was observed in these transgenic mice. Our results align well with the previous remarkable observation that forced looping of the LCR to the HBG genes reactivates their expression in adult red blood cells.20,37 In that experimental setup, but not in ours, some bystander activation of other nearby genes was observed. We propose that this may be explained by gene competition and differences in contact dynamics; in Del2Rec, the enhancer is permanently associated with and distracted by its most proximal gene, making it very difficult for more distal genes to also productively engage in enhancer interactions. Recruitment by looping, however, will be dynamic, and when dissociating, there is a chance that the now available LCR also contacts nearby genes. Regardless, together with our work, this study shows that to establish and maintain the HBG promoters in their silenced state in adult erythroid cells, the LCR needs to be kept away from these promoters. We showed that the same principle is also operational at the HBA locus.
Chromosomal rearrangements that cause enhancer hijacking and ectopic gene activation have been extensively documented, because they can drive cancer and cause developmental diseases. These hijacking events either involve trans-rearrangements that fuse different chromosomes to recruit enhancers to genes they would normally never engage with or cis-rearrangements that eliminate the insulating boundaries of topologically associating domains that serve to prevent undesired interactions between enhancers and nontarget genes.38,39 In this study, we showed that forced recruitment or enhancer hijacking can also be accomplished by the deletion or inversion of a genomic stretch without intrinsic insulating capacity that lies in between an enhancer and gene and thus creates linear distance between these elements. Genes require this chromosomal distance to negate activation by the enhancer, thus enabling execution of the promoter-encoded developmental silencing program. This work therefore assigns a regulatory function to some of the intrinsically nonfunctional stretches of genomic DNA.
Besides promoter integrity and LCR distance, gene competition and locus insulation have been implicated in controlling HBG gene silencing. They may explain why downstream deletions in the HBB locus that leave the HBG promoters and LCR distance intact but that delete the adult HBB gene and/or the more downstream CCCTC-binding factor (CTCF) binding site in 3'HS1 are associated with a condition called hereditary persistence of fetal hemoglobin, which is hallmarked by continued HBG expression during adulthood. The lack of gene competition (with an active HBB gene) and/or the lack of an insulating CTCF boundary that enable other enhancers to interfere with normal globin gene regulation40 may drive HBG gene reactivation in these individuals.
Understanding the contribution of the enhancer distance to gene regulation provides valuable insights into therapeutic opportunities for globin gene reactivation. For α-thalassemia, restoring the HBZ levels could be a promising approach; however, bringing R2 near the HBZ promoter disrupts the essential NPRL3 gene.12 In the HBB locus, reactivation of HBG, for example, by targeting BCL11A, has already shown therapeutic success in SCD and β-thalassemia.32 Although targeting the BCL11A binding site at position −115 with a single gRNA seems to be a promising approach, our Del2Rec strategy seems to yield higher HBG expression levels. Del2Rec as a method also offers notable advantages in terms of flexibility. Rather than being restricted to a specific regulatory element, Del2Rec allows for optimization of target sites based on efficiency and off-target considerations. Although using 2 gRNAs may raise concerns about potential unwanted rearrangements, the ability to fine-tune target selection provides an opportunity to maximize editing efficiency while minimizing undesired effects. However, whether recruitment of the LCR to HBG may offer an orthogonal therapeutic approach requires further investigation.
Acknowledgments
The authors thank the members of their laboratories for discussions and feedback, M. Bauer for help with setting up the ATAC-seq, C. Valdes-Quezada for input on the digital droplet PCR, and A. Didriksen for providing Cas9 protein. They thank the protein facility of the Netherlands Cancer Institute for providing the Tn5 protein, and acknowledge the Utrecht Sequencing Facility (USEQ) for providing sequencing service and data.
Research in the laboratory of W.d.L. was financially supported by the European Union (EU) Horizon 2020-funded Innovative Training Network “Molecular Basis of Human Enhanceropathies” (Enhpathy, www.enhpathy.eu), under Marie Sklodowska-Curie grant agreement no. 860002; a Netherlands Organisation for Scientific Research (NWO) GROOT grant (2019.012) from the NWO; and Oncode Institute Base Funding. Work in the laboratory of S.P. was supported by Top Consortium for Knowledge and Innovation Health Holland (grants EMCLSH20006 and EMCLSH20025), Zorgonderzoek Nederland en Medische Wetenschappen Pluripotent Stem cells for Inherited Diseases and Embryonic Research (PSIDER) consortium TRACER (grant 10250022110001), EU Horizon Europe Pathfinder EdiGenT (grant 101070903), and NWO Applied and Engineering Sciences Open Technology Programme (grant 18947). Work in the laboratory of E.v.d.A. was supported by Sanquin research fund l2842 and Sanquin Blood Supply grant PPODR21-07. USEQ is subsidized by the University Medical Center Utrecht and The Netherlands X-omics Initiative (NWO project 184.034.019).
Authorship
Contribution: A.-K.F., P.H.L.K., and W.d.L. conceived of and supervised the study; R. Majied, T.C.J.V., J.v.H., and S.P. designed and executed the experiments in sickle cell disease cell lines; H.J.M.P.V. and E.v.d.A. designed and executed the experiments in CD34+ HSPCs and performed HPLC analysis; S.J.D.T. designed and performed the HBA experiments with help from A.-K.F. and M.J.A.M.V.; A.-K.F. performed the TIDE and digital droplet PCR analyses, and also performed the experiments in HUDEP-2 cells with help from M.J.A.M.V. and R. Mohnani; R.G. analyzed the Cut&Run and ATAC-seq data; and A.-K.F. and W.d.L. wrote the manuscript with help from S.J.D.T., R. Majied, S.P., H.J.M.P.V., and E.v.d.A.
Conflict-of-interest disclosure: W.d.L. reports having filed patent applications PCT/EP2022/053341 and PCT/EP2023/070536, which describe the Del2Rec methodology. A.-K.F. and P.H.L.K. report having filed patent application PCT/EP2023/070536, which describes the Del2Rec methodology. The remaining authors declare no competing financial interests.
Correspondence: Sjaak Philipsen, Erasmus MC-Erasmus Universitair Medisch Centrum Rotterdam, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands; email: j.philipsen@erasmusmc.nl; Emile van den Akker, Sanquin Blood Supply Foundation, Plesmanlaan 125, 1066CX, Amsterdam, The Netherlands; email: e.vandenakker@sanquin.nl; and Wouter de Laat, Hubrecht Institute, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands; email: w.l.delaat@umcutrecht.nl.
References
Author notes
A.-K.F., S.J.D.T., H.J.M.P.V., and R. Majied contributed equally to this study.
The H3K4me3 CUT&RUN and ATAC-seq data sets have been deposited in the Gene Expression Omnibus database (accession codes GSE274030 and GSE274029, respectively).
The data are available on request from the corresponding authors, Sjaak Philipsen (j.philipsen@erasmusmc.nl), Emile van den Akker (e.vandenakker@sanquin.nl), and Wouter de Laat (w.l.delaat@umcutrecht.nl).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.