In this issue of Blood, Davids et al1 demonstrate that chimeric antigen receptor (CAR) T-cell efficacy in chronic lymphocytic leukemia (CLL) is tightly constrained by tumor burden, with responses largely restricted to patients with low circulating disease.
In their phase 1 ZUMA-8 study, Davids et al evaluated brexucabtagene autoleucel, a CD19-directed CAR T-cell product, in patients with relapsed or refractory CLL. Manufacturing was consistently successful, and toxicity remained within acceptable limits. Clinical activity, however, was modest. The key signal came from patients with low tumor burden: all 3 in this subgroup responded, 2 with lasting remissions. By contrast, patients with high lymphocyte counts or recent ibrutinib exposure showed minimal expansion and few responses.1 These findings underscore that, in CLL, to a far greater extent than in other B-cell malignancies, tumor burden remains a central barrier to CAR T-cell efficacy. Expansion and clinical benefits appear to depend less on product characteristics than on the ability of CAR T cells, to interact effectively with tumor cells in vivo.
Patients with CLL who progress after both Bruton tyrosine kinase (BTK) and BCL2 inhibition, so-called double-refractory disease, have few remaining treatment options. A noncovalent BTK inhibitor has recently been approved, and several BTK degraders are in development. These agents rarely induce deep responses and are not expected to produce durable remissions. Median overall survival remains below 2 years, highlighting a critical unmet need in this population.2
The ZUMA-8 data closely mirror findings from the TRANSCEND CLL 004 trial of lisocabtagene maraleucel (liso-cel),3 currently the only approved CD19-directed CAR T-cell therapy in CLL. Due to the urgent need in double-refractory disease, the US Food and Drug Administration granted approval based on phase 2 data without requiring a phase 3 trial. In both studies, toxicity was manageable: grade 3 cytokine release syndrome (CRS) occurred in 9% of patients, with no grade 4 or 5 CRS. Grade 3 neurological events occurred in 18% of patients treated with liso-cel and 14% in ZUMA-8. These results should be interpreted with caution, as both trials enrolled selected patients, younger and fitter than the broader CLL population, which often includes older individuals with comorbidities. In real-world settings, the median age at diagnosis is around 70 years, and many patients have coexisting conditions such as cardiovascular disease, diabetes, chronic kidney disease, or pulmonary disorders. These comorbidities not only impact fitness for intensive cellular therapies but may also increase the risk of complications like infection or organ dysfunction following CAR T-cell infusion. Thus, the tolerability and applicability of these therapies in routine practice remain open questions, requiring prospective study in more representative patient cohorts.
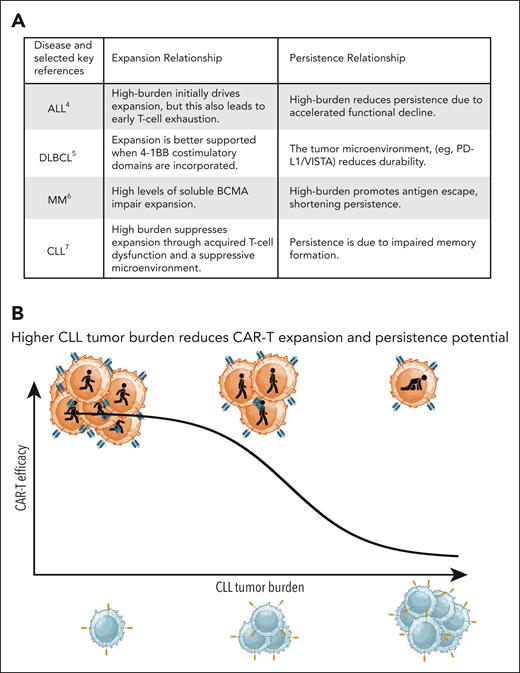
In both trials, durable clinical benefit was largely confined to patients with low tumor burden, though the mechanistic basis for this limitation varied across malignancies. Tumor burden exerts distinct, disease-specific effects on CAR T-cell kinetics in B-cell malignancies. In acute lymphoblastic leukemia (ALL), high tumor burden drives robust initial CAR T-cell expansion through abundant CD19 antigen exposure, but paradoxically accelerates functional exhaustion, leading to shorter persistence.4 Diffuse large B-cell lymphoma (DLBCL) and multiple myeloma (MM) exhibit intermediate profiles: in DLBCL, expansion depends on costimulatory domain design (eg, 4-1BB vs CD28) and tumor microenvironment (TME) factors,5 while in MM, CAR T-cell persistence correlates inversely with soluble B-cell maturation antigen (BCMA) levels, a surrogate for tumor burden.6 In stark contrast, CLL represents the extreme end of this spectrum, where high tumor burden creates a profoundly immunosuppressive niche, functionally impairing both CAR T-cell expansion and persistence.7 As summarized in figure panel A, tumor burden in CLL is not merely prognostic but directly inhibitory, necessitating disease-specific strategies such as T-cell fitness optimization prior to infusion.
Tumor burden as a key barrier to CAR T-cell efficacy in CLL. (A) In contrast to ALL,4 DLBCL,5 and MM,6 high tumor burden in CLL7 is not merely prognostic but directly impairs CAR T-cell expansion and persistence. Key mechanisms include chronic antigen exposure, metabolic dysfunction, and impaired immunological synapse formation, resulting in attenuated T-cell function. (B) Conceptual schematic illustrating the inverse relationship between CLL tumor burden and CAR T-cell expansion and persistence. In patients with low disease burden, CAR T cells remain, effector function is preserved and can mediate durable responses. In high-burden CLL, expansion is limited and clinical benefit is rarely achieved.
Tumor burden as a key barrier to CAR T-cell efficacy in CLL. (A) In contrast to ALL,4 DLBCL,5 and MM,6 high tumor burden in CLL7 is not merely prognostic but directly impairs CAR T-cell expansion and persistence. Key mechanisms include chronic antigen exposure, metabolic dysfunction, and impaired immunological synapse formation, resulting in attenuated T-cell function. (B) Conceptual schematic illustrating the inverse relationship between CLL tumor burden and CAR T-cell expansion and persistence. In patients with low disease burden, CAR T cells remain, effector function is preserved and can mediate durable responses. In high-burden CLL, expansion is limited and clinical benefit is rarely achieved.
Mechanistically, this disconnect from other malignancies stems from the distinctive immunobiology of CLL. Even when CAR T-cell products are phenotypically comparable to those used in ALL or mantle cell lymphoma, as in ZUMA-8, expansion and persistence falter. The CLL microenvironment fosters acquired T-cell dysfunction through inhibitory ligands, disrupted synapse formation, and metabolic derangement.8 These factors collectively contribute to altered activation patterns and distorted effector functions, such as impaired proliferation, and reduced cytotoxic capacity. Moreover, CLL T cells often display skewed differentiation with an accumulation of terminally differentiated effector cells and reduced pools of stem-like memory subsets, further limiting their capacity to respond to antigenic stimulation or persist after adoptive transfer.8
These insights inform clinical strategy. In ZUMA-8, durable responses were limited to patients with low circulating disease, highlighting tumor burden as a key determinant of CAR T-cell efficacy (see figure panel B). This supports a tumor debulking approach before leukapheresis, using BTK inhibitors, BCL2 antagonists, or anti-CD20-based therapy to reduce disease load and potentially improve the composition of T cells collected for manufacturing. Timing and sequencing of these agents remain areas of active investigation, especially considering the immunomodulatory effects some therapies exert on T-cell phenotype and function.
In ZUMA-8, short-term ibrutinib before apheresis did not enhance outcomes, suggesting that brief exposure may be insufficient to reverse chronic T-cell dysfunction. Prolonged BTK inhibition prior to leukapheresis, as explored in earlier studies, has been associated with partial restoration of immune synapse formation and reduced expression of inhibitory receptors. Alternatively, ex vivo modulation of CAR T-cell products during manufacturing offers a controlled setting to enhance functionality. Experimental strategies include phosphoinositide 3-kinase blockade9 to reduce exhaustion-associated transcriptional programs, metabolic reprogramming to bolster mitochondrial fitnes,9,10 and the development of engineered CAR constructs that incorporate signaling domains or transcriptional regulators designed to resist CLL-mediated reprogramming.8 Given the unique immune contexture of CLL, a tailored approach that simultaneously reduces tumor-induced suppression and reconditions autologous T cells, may be necessary to fully unlock the potential of CAR T-cell therapy in this disease.
In summary, CAR T-cell therapy holds promise in CLL, but its success depends on specific conditions. Durable responses require both low tumor burden and improved T-cell fitness. Overcoming the unique immunosuppressive landscape of CLL will demand integrated strategies that combine disease control, immune restoration, and refined CAR design. With a more tailored approach, consistent and lasting remissions may become attainable.
Conflict-of-interest disclosure: M.T. is an inventor in licensed patents and patent applications on CAR T-cell technologies. A.P.K. declares no competing financial interests.