Abstract
Herpes simplex viruses (HSVs) would offer numerous advantages as vectors for gene transfer, but as yet they have not proved capable of transducing hematopoietic cells. Using a genetically inactivated form of HSV that is restricted to a single cycle of replication (disabled single-cycle virus, [DISC-HSV]), we have transduced normal human hematopoietic progenitor cells and primary leukemia blasts with efficiencies ranging from 80% to 100%, in the absence of growth factors or stromal support. Toxicity was low, with 70% to 100% of cells surviving the transduction process. Peak expression of transferred genes occurred at 24 to 48 hours after transduction with the DISC-HSV vector, declining to near background levels by 14 days. Despite this limitation, sufficient protein is produced by the inserted gene to permit consideration of the vector for applications in which transient expression is adequate. One example is the transfer of immunostimulatory genes, to generate leukemia immunogens. Thus, murine A20 leukemia cells transduced with a DISC-HSV vector encoding granulocyte-macrophage colony-stimulating factor were able to stimulate a potent antitumor response in mice, even against pre-existing leukemia. The exceptional transducing ability of the DISC-HSV vector should therefore facilitate genetic manipulation of normal and malignant human hematopoietic cells for biological and clinical investigation.
MANY APPLICATIONS of gene transfer to normal and malignant human hematopoietic cells require vectors, such as retroviruses, that integrate the host cell DNA and produce long-term expression of the transgene in the host cell and all of its progeny.1 In other applications, however, the only requirement is that gene transfer be produced rapidly and with high efficiency. These applications include the modification of malignant hematopoietic cells to generate tumor immunogens,2-4 or the introduction of putative regulatory genes to study proliferative and developmental events in normal and malignant cells.5-8 In these settings, it would also be preferable if high-efficiency transfer could be produced without additional manipulations of the target cells that might modify their immunogenicity9 or perturb responses to the introduced genes.5-8 At present there is a dearth of vectors possessing all of these characteristics.10
It has been recognized for some time that vectors derived from herpes simplex viruses can be used to transduce neuronal cells in a variety of preclinical models.11-15 It is also evident that herpes vectors can be used to transduce certain rodent tumor cell lines.12 Such vectors have a number of potential advantages over alternative systems in terms of their titer, the size of the gene insert, and their safety,13,15 but they have not proved capable of transducing hematopoietic cells. We recently developed a genetically inactivated form of herpes simplex virus as a vaccine candidate against herpes simplex virus (HSV) disease in humans.13,15 This virus lacks the gene for the essential glycoprotein H (gH), but can be grown to high titer in a complementing cell line expressing gH. In noncomplementing cell lines that are permissive for herpesvirus growth, it is restricted to a single cycle of replication, leading to the release of noninfectious virus.16,17 In animal models this disabled infectious single-cycle herpes simplex virus (DISC-HSV) has been safe to administer.16 17
We show here that our DISC-HSV vector rapidly transduces normal and malignant human hematopoietic progenitor cells with efficiencies ranging from 60% to 100%, even in the absence of growth factors or other stimulatory agents. Although expression of the transferred gene is transient, sufficient material is produced for functional effects in vivo, since murine A20 leukemia cells transduced with a DISC-HSV granulocyte-macrophage colony-stimulating factor (GM-CSF ) vector can act as a potent tumor immunogen.
MATERIALS AND METHODS
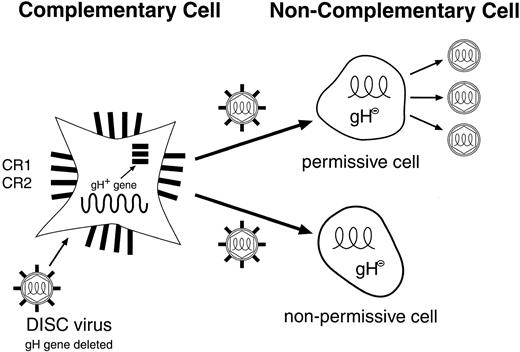
Preparation of DISC herpes virus vector.HSV-2 strain HG52 was obtained from the Institute of Virology (Glasgow, Scotland) and propagated and titrated on Vero cells. Glycoprotein H-deleted (DISC) HSV-2 viruses were purified, propagated and titrated either on gH expressing Vero cells (CR1) or gH expressing thymidine-kinase negative (TK−) baby hamster kidney cells. The β-galactosidase (β-GAL) or GM-CSF genes were inserted into the genome of HSV-2 strain HG52 in place of the gH gene using the recombination vectors pIMMB56 and PIMR3, respectively. In the case of the β-GAL gene, part of the TK gene was also deleted. To construct these plasmids, two regions of the HSV2 flanking sequences corresponding to the TK gene and a region downstream of gH were cloned by polymerase chain reaction from the HSV-2 genome, and inserted into pUC119. A functional copy of the β-galactosidase or GM-CSF genes under control of the SV40 or CMV early promoters respectively were then inserted between the two HSV-2 sequences. Ten micrograms of sodium iodide purified viral DNA and 0.5 μg of plasmid DNA were transfected into gH expressing CR1 or CR2 cells using standard calcium phosphate transfection. After recombination, the viral stocks were prepared as previously described16 17 with use of the gH expressing CR1 cells. The multiplicities of infection (MOI) for the experiments described here were based on plaque-forming units (pfu) assayed on CR1 cells. The production and life cycle of the DISC-HSV vector is shown in Fig 1.
Life cycle of the herpes simplex disabled infectious single-cycle (DISC) vector. The HSV-DISC vector lacks the glycoprotein-H gene, an essential component for the infectivity of HSV. The vector particles are made in a complementary cell (CR1 or CR2), which supplies the gH gene product in trans. The vector generated may have any gene of interest substituted for the missing gH gene. On entry into a permissive cell, herpes virus and inserted gene products are made, but only noninfectious (gH negative) virus is made. In a nonpermissive cell, an abortive infection may result, with limited expression of herpes genes.
Life cycle of the herpes simplex disabled infectious single-cycle (DISC) vector. The HSV-DISC vector lacks the glycoprotein-H gene, an essential component for the infectivity of HSV. The vector particles are made in a complementary cell (CR1 or CR2), which supplies the gH gene product in trans. The vector generated may have any gene of interest substituted for the missing gH gene. On entry into a permissive cell, herpes virus and inserted gene products are made, but only noninfectious (gH negative) virus is made. In a nonpermissive cell, an abortive infection may result, with limited expression of herpes genes.
Transduction with DISC-HSV β-GAL and DISC-HSV-GM-CSF vectors. Hematopoietic cells were transduced with DISC-HSV carrying either the β-GAL or the murine GM-CSF gene at various MOI for 2 hours at 37°C in an atmosphere of 5% CO2 . At various times after transduction, cells were assessed for expression of β-GAL or for GM-CSF production, as described below.
Normal CD34+ progenitor cells.Mononuclear cells prepared by Ficoll sedimentation of normal donor marrow were separated on an anti-CD34 column (CellPro, Seattle, WA) to enrich the CD34+ progenitor population. The cells were then exposed to the DISC-HSV- β-GAL vector at MOIs ranging from 0.5 to 20 pfu/cell. After 2 hours of exposure, the cells were grown on stromal support in liquid media supplemented with fetal bovine serum.
Stromal support cultures.Stromal support cultures with 8 × 105/cm2 of surface area were established in Fisher's medium (GIBCO Life Technologies, Grand Island, NY) with 15% horse serum and 5% fetal calf serum (FCS; Summit Biotechnology, Ft. Collins, CO), 1 × 10−6 mol/L hydrocortisone (Abbott, Chicago, IL), 10−4 mol/L mercaptoethanol (Sigma, St Louis, MO) and 400 μL/mL transferrin (Life Technologies). Cells were cultured in 25-mL tissue culture flasks (Nunc, Roskilde, Denmark) at 37°C; every 2 weeks half of the spent medium was replaced with fresh medium until the stromal layer was fully established. Stromal cells were then employed as feeder layers and reseeded with transduced CD34+ cells obtained as described above.
Leukemic blast cells.Leukemic cells were isolated from patients with >80% blast cells by Ficoll sedimentation of peripheral blood or bone marrow mononuclear cells. Myeloblasts were maintained in liquid culture in RPMI supplemented with 10% FCS (Biowhittaker, Walkersville, MD), 100 IU/mL penicillin and 100 μg/mL of streptomycin (Biowhittaker), and 2 mmol/L L-glutamine. Lymphoblasts were maintained in liquid culture or, when necessary, on allogeneic skin fibroblasts as stromal support.18
Leukemic cell lines.The AD and RS human pre-B–leukemic cell lines were established at this institution from clinical samples and cultured in RPMI 1640 (Biowhittaker) supplemented as described above. The A20 murine pre-B–lymphoblastoid cell line19 was obtained from the American Type Culture Collection (Rockville, MD), and grown in supplemented Dulbecco's modified Eagle's medium (Biowhittaker).
Detection of β-GAL activity.The glycoside hydrolase β-galactosidase, encoded by the lacZ gene, can be detected by FACS analysis with the FluoReporter lacZ flow cytometry kit (Molecular Probes, Eugene, OR).20 21 After 2, 7, 14, and 21 days, cells from the stromal support cultures were analyzed by dual staining with FDG and a fluorescent anti-CD34 antibody (Becton Dickinson, San Jose, CA) to determine β-GAL expression in CD34+ hematopoietic progenitor cells. Myeloid and lymphoid CD34+ subpopulations were defined by forward and side scatter. Similarly, appropriate gating by forward-versus-side scatter was used to determine β-GAL expression in leukemic blast cell populations.
Detection of GM-CSF production.Leukemic blasts were also transduced with the DISC-HSV GM-CSF vectors. Supernatant was collected for 24 hours at various times posttransduction, and its GM-CSF content subsequently analyzed by enzyme-linked immunosorbent assay (ELISA; R & D, Minneapolis, MN) and standardized to production/106 cells/mL.
Tumor growth in vivo.Female BALB/CBYJ mice aged 12 to 18 weeks (The Jackson Laboratory, Bar Harbor, ME) were used to study the effects of gene transfer on tumor cell proliferation in a syngeneic system, using the A20 (BALB/C derived) cell line. Before injection, A20 cells were transduced with DISC-HSV carrying either the β-GAL gene or the murine GM-CSF gene. Transduction was performed as described above at an MOI of 30 pfu/cell. Two hours later, cells were washed three times in phosphate-buffered saline to remove residual virus. Trypan blue-negative cells were adjusted to the desired concentrations in a total volume of 200 μL. Mice were inoculated subcutaneously with 1 × 105 live A20 cells on day 1, followed by three subcutaneous injections on days 4, 11, and 18 with 1 × 105 A20 tumor cells transduced with either the β-GAL gene (control) or murine GM-CSF. Immediately before these injections, the immunizing cells were irradiated to 1,000 cGy.
Tumor volumes were determined by measuring the largest diameter and the respective perpendicular diameter. Animals bearing a tumor larger than 10 mm in diameter were considered positive. Animals were killed when tumor growth resulted in an increase of more than 30% body weight, in ulceration or in distress. Tumor volumes are reported as means ± one standard error (SE). Statistical significance was calculated by the Wilcoxon test.
RESULTS
To assess the ability of the DISC-HSV vector to transduce normal hematopoietic cells, we exposed CD34+ marrow cells to DISC-HSV β-GAL for 2 hours at 0.5 to 20 pfu/cell. Figure 2 shows the results of a detailed FACS analysis of CD34+ cells 48 hours after transduction. As the MOI increased, there was a progressive increase in the proportion of cells positive for the marker gene. At 2 pfu/cell, almost 100% of the CD34+ cells were marker positive. Only scant evidence of β-GAL gene expression could be detected over the first 6 hours after exposure to the vector, but the level of positivity increased markedly thereafter, reaching a peak by 24 to 48 hours (Fig 3). We do not believe that our favorable results reflect the uptake of β-GAL protein by nontransduced cells after its release by a minority population of transduced cells, since addition of supernatants from highly positive cultures had no effect on the fluorescence of control CD34+ cells (Fig 4). Hence, this vector yields highly efficient transfer and expression of the β-GAL marker gene in normal hematopoietic progenitor cells, in the absence of any growth stimulatory signals.
The DISC-HSV β-GAL vector transduces CD34+ cells with high efficiency. The x-axis denotes the intensity of β-GAL staining 48 hours after transduction with the DISC-HSV vector at various pfu/cell. The myeloid and lymphoid subpopulations were first gated by forward-versus-side scatter (FSC v SSC) (inset fig), with β-GAL expression in the CD34+ subset displayed separately for each population. *pfu/cell:- for details of titering see Materials and Methods section.
The DISC-HSV β-GAL vector transduces CD34+ cells with high efficiency. The x-axis denotes the intensity of β-GAL staining 48 hours after transduction with the DISC-HSV vector at various pfu/cell. The myeloid and lymphoid subpopulations were first gated by forward-versus-side scatter (FSC v SSC) (inset fig), with β-GAL expression in the CD34+ subset displayed separately for each population. *pfu/cell:- for details of titering see Materials and Methods section.
β-GAL expression is low for up to 6 hours after transduction of CD34+ cells with the DISC-HSV β-GAL vector. The inset figs show the gating used, whereas the x-axis denotes the intensity of β-GAL staining of CD34+ cells at various times posttransduction at 2 pfu/cell.
β-GAL expression is low for up to 6 hours after transduction of CD34+ cells with the DISC-HSV β-GAL vector. The inset figs show the gating used, whereas the x-axis denotes the intensity of β-GAL staining of CD34+ cells at various times posttransduction at 2 pfu/cell.
The supernatant of transduced CD34+ cells taken 48 hours after exposure to 20 pfu of vector/cell does not affect β-GAL expression by nontransduced CD34+ cells (NT + supernatant) measured 48 hours after exposure. β-GAL expression by transduced and nontransduced hematopoietic progenitors 48 hours after transduction at the same MOI is shown for comparison.
The supernatant of transduced CD34+ cells taken 48 hours after exposure to 20 pfu of vector/cell does not affect β-GAL expression by nontransduced CD34+ cells (NT + supernatant) measured 48 hours after exposure. β-GAL expression by transduced and nontransduced hematopoietic progenitors 48 hours after transduction at the same MOI is shown for comparison.
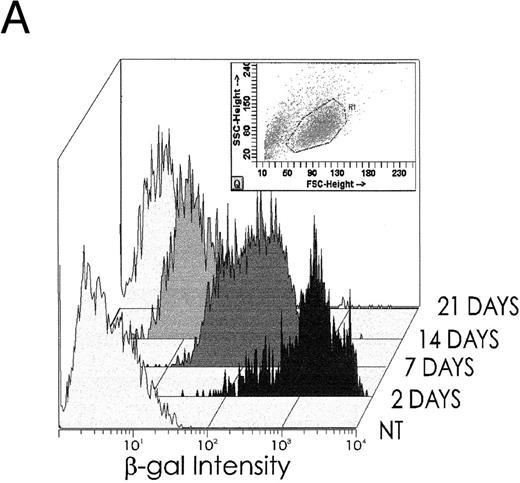
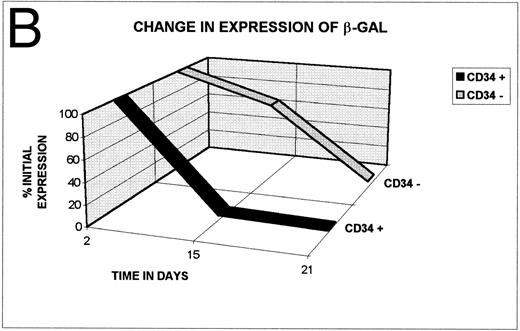
Although gene transfer was highly efficient, the lack of a latent origin of replication in vectors derived from HSV22 means that the transferred DNA is progressively lost from dividing cells. As anticipated, the number of cells that were positive for the β-GAL marker gene progressively declined in stromal support cultures (Fig 5A). On day 14, 27.5% of the cultured cells remained marker positive, whereas on day 21, only 1% to 2% of the cells were marker gene positive. Although CD34+ cells divide in cultures of the type used here, they also mature to their CD34− derivatives.23 Hence, it is also possible that loss of marker signal occurs because DISC- β-GAL expression is restricted to an early stage in hematopoietic cell differentiation and is lost as maturation occurs in culture. The behavior of the marker gene was therefore analyzed in both the CD34+ population and the maturing CD34− subset. The results do not support the concept of maturation-stage dependent expression, since both CD34+ and CD34− hematopoietic cells are positive at the beginning of culture (Fig 5B). Moreover, CD34− cells remain positive longer than CD34+ cells, consistent with the input of marker positive cells from the maturing CD34+ subset (Fig 5B). Similarly, there are no data to suggest that loss of signal is caused by a progressive destruction of transduced cells. Although the number of positive cells decreases by 0% to 30% (median, 12%) within the first 4 to 6 hours after exposure to the vector, once the cultures of transduced and nontransduced cells were adjusted to the same cell concentrations, the viable cell count in each population was the same. Instead, the rate and pattern of loss is consistent with that expected when a nonreplicating vector transduces dividing cells: by 14 days, total cell numbers have increased more than eightfold,23 which can fully account for the decline in β-GAL positivity (Fig 5A and B).
(A) After transduction with 2 pfu/cell, β-GAL expression in hematopoietic progenitor cell cultures declines progressively with time of culture. (B) β-GAL expression declines in both CD34+ and CD34− subsets of hematopoietic cells. To determine whether the loss of β-GAL expression was confined to the CD34+ population, we examined the proportion of cells that were marker positive in both the CD34+ and CD34− marrow population, at different times after transduction with the HSV-DISC vector at 2 pfu/cell. Both CD34+ and CD34− populations showed a decline in positivity, and the rate of decline was greater in the CD34+ cells.
(A) After transduction with 2 pfu/cell, β-GAL expression in hematopoietic progenitor cell cultures declines progressively with time of culture. (B) β-GAL expression declines in both CD34+ and CD34− subsets of hematopoietic cells. To determine whether the loss of β-GAL expression was confined to the CD34+ population, we examined the proportion of cells that were marker positive in both the CD34+ and CD34− marrow population, at different times after transduction with the HSV-DISC vector at 2 pfu/cell. Both CD34+ and CD34− populations showed a decline in positivity, and the rate of decline was greater in the CD34+ cells.
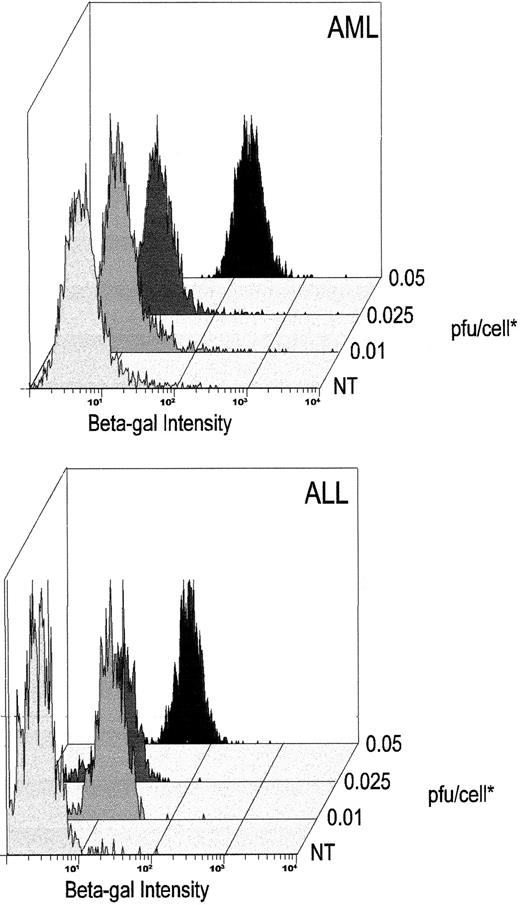
Despite its transiency, the high-level gene transfer and expression, we have demonstrated would have practical value in primary malignant hematopoietic cells, for example, in the generation of tumor immunogens or in efforts to transfer lethal or corrective genes to these cells. To discover whether malignant hematopoietic cells were transduced as readily as their normal counterparts, we exposed fresh blast cells from patients with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) to the DISC-HSV vector at various MOIs (Fig 6 and Table 1). All cells were consistently positive for β-GAL when they were exposed to the vector at 0.05 pfu/cell (Fig 6). As shown in Table 1, this high level of transduction was found consistently even in blast cells that we found to be highly resistant to transfer with retroviral or adenoviral vectors. The DISC-HSV vector regularly transduced 80% to 100% of exposed cells from all AML and ALL patients studied.
High-efficiency transduction of myeloid and lymphoid leukemic cells with the DISC-HSV β-GAL vector. The x-axis denotes the intensity of β-GAL staining 48 hours after transduction with the DISC-HSV vector at various pfu/cell. Blast cell populations are defined by appropriate gating using forward-versus-side scatter. Representative results of one AML and one ALL patient are shown from the patients presented in Table 1. *pfu/cell:- for details of titering see Materials and Methods.
High-efficiency transduction of myeloid and lymphoid leukemic cells with the DISC-HSV β-GAL vector. The x-axis denotes the intensity of β-GAL staining 48 hours after transduction with the DISC-HSV vector at various pfu/cell. Blast cell populations are defined by appropriate gating using forward-versus-side scatter. Representative results of one AML and one ALL patient are shown from the patients presented in Table 1. *pfu/cell:- for details of titering see Materials and Methods.
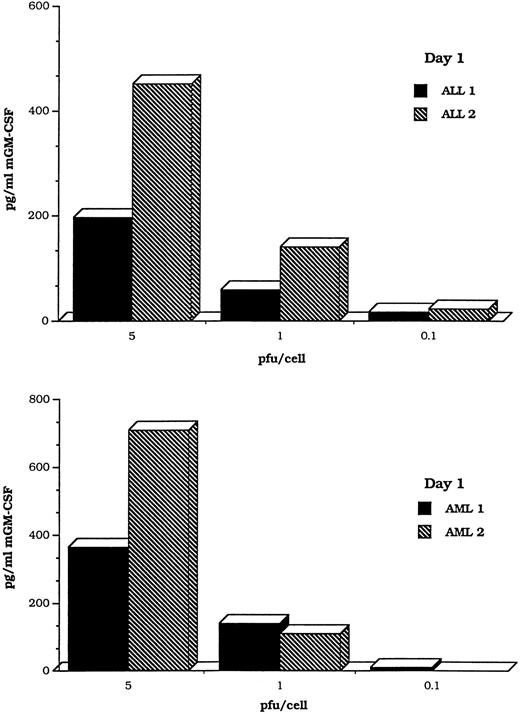
To determine whether the transduced cells were able to secrete transgene products, we prepared a DISC-HSV vector containing a murine GM-CSF cDNA and used it to transfect human myeloid and lymphoblastic leukemia cells. Figure 7 shows MOI-dependent production of GM-CSF after transduction of blast cells from four patients, two with AML and two with ALL at MOIs ranging from 0.1 to 5 pfu/cell. A time course study was performed on an additional two blast cell samples (AML and ALL). GM-CSF was detected in a commercial ELISA assay, for periods ranging from 4 to 7 days (Fig 8).
Myeloid and lymphoid leukemic cells transduced with the DISC-HSV GM-CSF vector produce GM-CSF in a MOI-dependent manner. The bars represent findings in samples from four different patients, two with AML and two with ALL.
Myeloid and lymphoid leukemic cells transduced with the DISC-HSV GM-CSF vector produce GM-CSF in a MOI-dependent manner. The bars represent findings in samples from four different patients, two with AML and two with ALL.
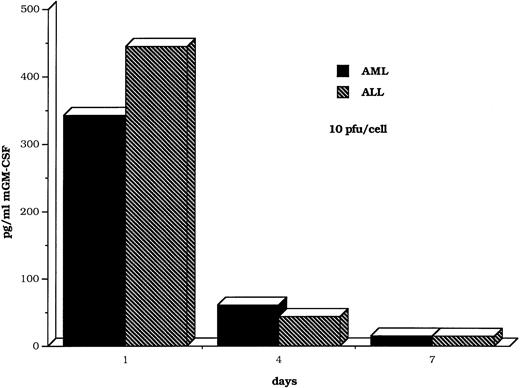
GM-CSF production by myeloid and lymphoid leukemic cells is maintained for several days after transduction with the DISC-HSV GM-CSF vector. The bars represent findings in clinical samples from single patients with AML or ALL. The multiplicity of infection was 10 pfu/cell.
GM-CSF production by myeloid and lymphoid leukemic cells is maintained for several days after transduction with the DISC-HSV GM-CSF vector. The bars represent findings in clinical samples from single patients with AML or ALL. The multiplicity of infection was 10 pfu/cell.
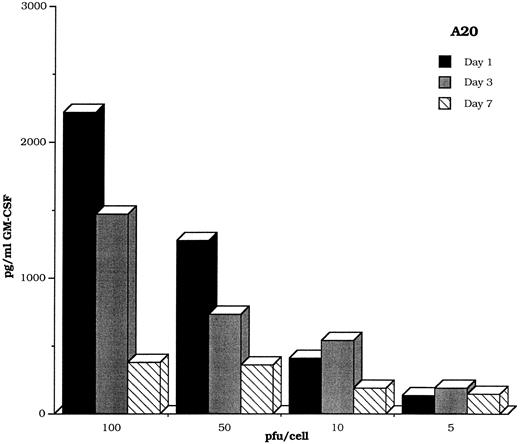
Malignant cells transduced with vectors carrying GM-CSF are highly immunogenic in a variety of murine models. The transduced cells induce an immune response that not only protects against tumor challenge, but also eradicates pre-existing tumor.24 To ensure that GM-CSF transferred by the DISC-HSV vector elicited the same reactions, we transduced the A20 murine lymphoid leukemia cell line with the murine DISC-HSV GM-CSF vector at varying pfu/cell. A time course study showed that these tumor cells produced GM-CSF for at least 7 days (Fig 9). Injection of 1 × 105 wild-type tumor cells into one flank of mice, followed 3 days later by injections of 1 × 105 irradiated DISC-HSV β-GAL or GM-CSF–transduced tumor resulted in significantly decreased tumor growth in the latter group (P < .001; Fig 10).
GM-CSF production by the murine A20 lymphoblastic cell line is maintained for at least 7 days after transduction with the DISC-HSV GM-CSF vector.
GM-CSF production by the murine A20 lymphoblastic cell line is maintained for at least 7 days after transduction with the DISC-HSV GM-CSF vector.
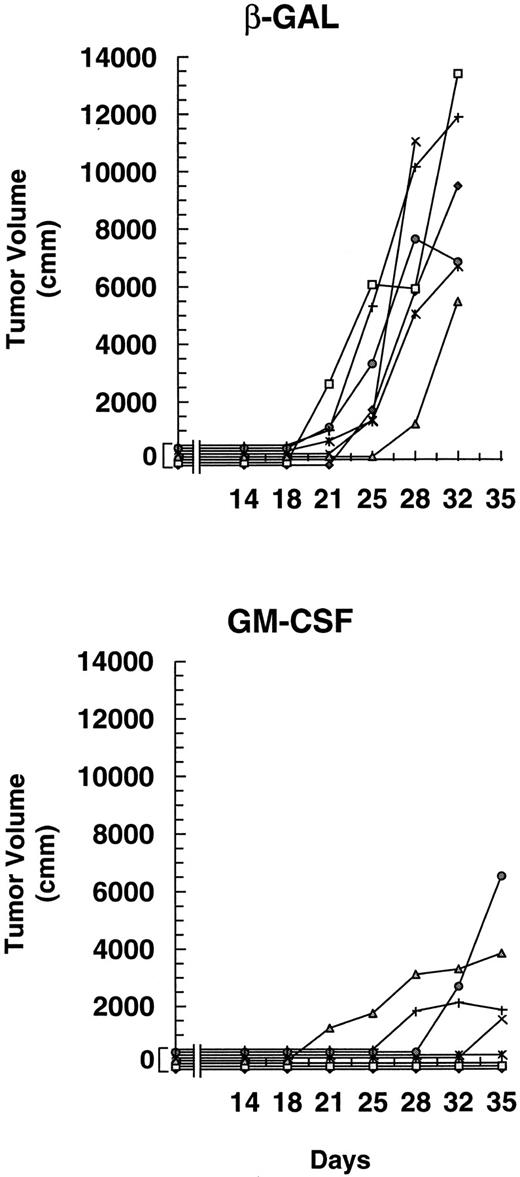
Injection of mice with irradiated A20 cells secreting GM-CSF inhibits the growth of preexisting tumor. Two groups of mice (seven per group) were challenged with 1 × 105 A20 cells on day 1, followed on days 4, 11, and 18 by subcutaneous injection of 1 × 105 irradiated (1,000 cGy) A20 cells that had been transduced with the DISC-HSV GM-CSF vector or the control DISC-HSV β-GAL vector at 30 pfu/cell. The data are reported as mean tumor volume (mm3) ± SE. The difference in tumor growth patterns in the presence of GM-CSF or β-GAL was highly significant (P < .005 by the Wilcoxon test).
Injection of mice with irradiated A20 cells secreting GM-CSF inhibits the growth of preexisting tumor. Two groups of mice (seven per group) were challenged with 1 × 105 A20 cells on day 1, followed on days 4, 11, and 18 by subcutaneous injection of 1 × 105 irradiated (1,000 cGy) A20 cells that had been transduced with the DISC-HSV GM-CSF vector or the control DISC-HSV β-GAL vector at 30 pfu/cell. The data are reported as mean tumor volume (mm3) ± SE. The difference in tumor growth patterns in the presence of GM-CSF or β-GAL was highly significant (P < .005 by the Wilcoxon test).
DISCUSSION
We have described a novel vector based on the human herpes simplex-2 virus that is able to transduce normal and malignant hematopoietic cells with extremely high efficiency. At present, retroviral vectors are the most widely used for the transfer of genes to hematopoietic progenitor cells.1 Their major advantage is the ability to stably integrate the host cell genome, so that they persist in the progeny of all the transduced cells.1 Although important for many applications of hematopoietic stem cell (HSC) transduction,25 this feature is offset by the inability of retroviral vectors to integrate or be expressed in nondividing cells, limiting their overall value when used with HSC targets.26-28 For similar reasons, they are rather ineffective at transducing primary cells from human hematopoietic malignancies.28-30 Extensive efforts have been made to increase the efficiency of retrovirus-mediated gene transfer, particularly with use of hematopoietic growth factors to induce the HSCs into cycle.31-33 Unfortunately, in many instances, the cytokines appear to have induced differentiation with loss of the desired self-renewal capacity.28,32 Because of these limitations, adeno-associated viral vectors, which may integrate the genomes of both dividing and nondividing cells,34 have been proposed as an alternative means to achieve efficient transduction of HSCs. However, current evidence increasingly suggests that the efficiency of integration of such vectors is low.34 Efforts aimed at transient infection of hematopoietic progenitor cells have been more successful. For instance, recent experience with adenoviral vectors suggests that they may infect approximately 50% of hematopoietic cells,10 35 although gene expression is often delayed for several days and there are no reports of successful infection of the cells of primary hematopoietic malignancies.
The DISC-HSV system we describe offers considerable potential as a gene delivery vehicle. It can infect cells efficiently, and its genome is expressed within the cell nucleus, yet the virus cannot spread from cell to cell. Heterologous genes can be readily inserted into the DISC-HSV genome at the site from which gH has been deleted, thereby minimizing the risk of gene transfer to replication-competent virus by homologous recombination. Moreover, there is no direct homology between the HSV-2 genome and coding sequences in the producer cell. Thus, the probability of generating a replication-competent virus by recombination between the virus and the cell genome should be extremely low. Indeed, we have been unable to detect such viruses in DISC-HSV preparations containing as many as 1 × 109 pfu (Boursnell et al, unpublished data, March 1995).
The data presented here show that the DISC herpesvirus system is a highly effective means of transducing normal and malignant hematopoietic cells. The DISC-HSV β-GAL vector yields marker gene positivity in 80% to 100% of CD34+ cells, as well as AML and ALL blasts, within 24 hours after exposure to the vector. The ability to obtain high levels of transduction at extremely low MOIs based on pfu per cell indicates that the true multiplicity of infection (vector particles per cell) is substantially higher than the pfu level would suggest. In fact, electron microscopy shows up to 100-fold differences between pfu titers on CR1 cells and viral particle counts.
A related DISC-HSV GM-CSF vector shows that human primary leukemic cells will release the product of the transduced gene for at least 7 days, at a level that is proportional to the MOI. This is of potential clinical interest since there are extensive data from a number of murine models that tumor cells expressing immunostimulatory molecules such as GM-CSF may be highly immunogenic, leading not only to rejection of the genetically modified tumor cells, but also to rejection of unmodified cells.24,36 Until now, the logistic problems of transducing many types of primary human leukemia cells has impeded the production of human leukemia immunogens that could be tested for their ability to induce antileukemic activity. Since GM-CSF transferred into murine leukemia cells by the DISC-HSV vector functions well as a tumor immunogen, and since GM-CSF is readily expressed in primary leukemia cells, the DISC-HSV vector may be useful for the generation of tumor immunogens derived from primary human leukemic blasts. Although it may be possible to obtain equivalent or even higher levels of cytokine production with retroviral or adenoviral vectors in susceptible cells, only the DISC-HSV vector consistently transduces high proportions of leukemic blasts from all patients, providing a considerable logistic advantage in clinical studies. Moreover, because the efficiency of transfer is so high, these vectors may also be useful in studies requiring insertion of corrective or lethal genes to destroy malignant blast cells.8 37-39
A major limitation of the DISC-HSV vector is that the transferred gene is expressed for only a limited time. We believe this outcome more likely reflects failure of the vector to integrate (with consequent loss as the cells divide) than the lytic activity of herpes virus genes. Lymphoid cells are generally nonpermissive for expression of the late lytic genes of herpes simplex virus,40 and at the MOIs used here, toxic effects are only seen in a minority of cells (0% to 30%), and only in the immediate posttransfection period. Hematopoietic cells, like lymphocytes,40 may therefore either fail to express the cytotoxic herpes late-gene products or are resistant to their activities. These possibilities are now being investigated. We suggest that the observed transience of expression is due to the replicative characteristics of the herpes simplex virus from which the vector derives.13 HSV lacks a latent cycle origin of replication, and appears to replicate only during lytic cycle.22 Because the HSV-DISC vector exists in latent form extrachromosomally, it will be unable to divide as the cells multiply in the stromal cultures, and will become progressively diluted.
Another limitation of the DISC-HSV vector is that it was originally designed to be used as a vaccine to induce anti-herpes responses.17 Hence, transduced cells may generate an anti-herpesvirus response in vivo, leading to immune destruction of the transduced cells. This phenomenon has already been described in studies testing adenoviral vectors in vivo,41,42 and may underlie some of the reported examples of central nervous system toxicity attributed to herpes virus vectors used in animal studies.43,44 Does this potential immunogenicity necessarily preclude clinical use of the DISC-HSV vector for tumor transduction with agents such as GM-CSF? The generation of an anti-herpesvirus response following injection of tumor cells modified to express immunomodulatory genes might have two consequences. First, the response could prove to be immunodominant, leading to destruction of the virus antigen expressing cells before adequate recruitment of the more exiguous anti-tumor precursor cells. This alternative would in turn lead to a failure to generate a response capable of recognizing nontransduced tumor cells. Second, the anti-virus response could act as an adjuvant to the antitumor immune response, increasing the number of immunocytes reacting with the malignant cells and amplifying production of lymphotactic and immunostimulatory molecules, thereby creating an environment favorable to an immune response. Certainly, the deliberate introduction into tumor cells of a gene encoding a foreign major histocompatibility complex (MHC) molecule (HLA-B7) with the intent to generate an adjuvant anti-allo MHC response is one of the approaches currently used to generate an antitumor immune response in human trials.4 Because we have shown that murine leukemia cells transduced with the DISC-HSV GM-CSF vector can induce an effective response to parental tumor cells in an animal model (whereas DISC-HSV β-GAL does not), we suggest that the immunogenic effects of the DISC-HSV vector itself will be neutral or beneficial, at least in the clinical settings we propose.
The limitations described above might be overcome by the production of herpes amplicons, vectors that use a packaging system which incorporates multiple plasmids into an otherwise empty herpes simplex shell.44,45 Immunogenicity due to expressed herpes genes would thereby be reduced. Moreover, even if these plasmids individually integrate at a low rate, the delivery of a high multiplicity of genes encapsulated in a highly efficient transducing platform, may produce a significant rate of stable transductants with minimal toxicity. Alternatively, it may be possible to use the amplicon to introduce plasmids that themselves have a high rate of integration.45
We have described a herpes simplex-based DISC-HSV vector that produces high-level transduction and gene expression in both normal and malignant primary human hematopoietic cells. Despite its transience of expression and possible immunogenicity in vivo, we believe that it and its subsequent derivatives will find a number of applications in biologic and clinical studies of gene transfer to hematopoietic cells. In particular, it is possible to modify the DISC-HSV vector to generate DISC-HSV amplicons, in which plasmids are packaged as large tandemly repeated DNA in place of the HSV genome. In this way, it should be possible to retain the exceptional transducing abilities of the original vector while reducing the risk of immunization and increasing the probability of generating stable transfectants of normal and malignant hematopoietic cells.
Supported in part by National Institutes of Health Grants CA 20180 and CA 21765, Cancer Center Support CORE Grant, by the American Lebanese Syrian Associated Charities (ALSAC), and by the German Research Foundation (DFG) Di 583/1-1.
Address reprint requests to Malcolm Brenner, MD, PhD, FRCP, MRCPath, Division of Bone Marrow Transplantation, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105.