Abstract
We used the recently described sensitive and rapid detection assay called telomeric repeat amplification protocol (TRAP) to detect telomerase activity in lymphoblastoid (n = 5) and lymphoma cell lines (n = 7), hyperplastic lymph nodes (n = 6) and tonsils (n = 5), and tissues involved by non-Hodgkin's lymphoma (NHL) (n = 43) and Hodgkin's disease (HD) (n = 14). Clearly evident telomerase activity was found in all lymphoblastoid and lymphoma cell lines, and in 34 of 43 cases (80%) of NHL. These results were expected because of the proliferative and immortal nature of the cell lines and most malignant cells. However, positive results were obtained with the TRAP assay in all hyperplastic lymph nodes and tonsils, which raises the issue of derepression of telomerase activity during an immune response. Telomerase activity alone therefore does not distinguish malignant lymphoid proliferations from reactive states. Telomerase activity was detected in only 1 of 14 cases (7%) of lymphoid tissues involved by HD. Eight of the 13 negative cases were considered to be interpretable because of the lack (3 of 13 cases) or low level (5 of 13 cases) of telomerase inhibition. The five remaining cases could not be evaluated because of their telomerase inhibitor content. The findings imply either transient or very low levels of telomerase activity in HD or that HD for the greater part is a telomerase-independent neoplasm. Microdissection studies are needed to identify subsets of cells carrying telomerase activity in both reactive and neoplastic lymphoid tissues.
HUMAN TELOMERASE is a ribonucleoprotein that functions as a telomere terminal transferase by adding multiple repeats of the TTAGGG hexamer.1-3 Telomerase is active in embryonal or germ line cells but remains silent in terminally differentiated somatic tissues.4,5 Loss of telomerase activity has been correlated with cell senescence, whereas the preservation of activity by virtue of maintenance of the length of telomers prevents cells from cell-cycle exit.3-5 Telomerase activation has been shown to be an almost universal property of malignant tumors, evoking its role in the immortalization process.6,7 Terminal differentiation of immortal cells is said to repress telomerase activity.8 The reactivation of telomerase, which probably corresponds to a late event during the multi-stage tumorigenesis,9 may explain the stabilization of the telomeric sequences leading to immortalitity. Telomerase activity can be measured in protein extracts from cell lines or frozen tissues by the recently described telomeric repeat amplification protocol (TRAP).7,10 With this assay telomerase activity has been shown in various tumors and tumor-derived cell lines, including hematologic malignancies such as chronic lymphocytic leukemia, acute myeloblastic leukemia, and a few cases of non-Hodgkin's lymphomas (NHLs).7,11,12 Telomerase activity has been detected at low levels in peripheral blood lymphocytes11,13 and higher levels occur in activated B and T cells,13 proving its appearance in nonneoplastic cells other than germ line cells. Telomerase activity is said to be a better indicator of immortality than either the alterations of telomere length14 or the actual presence of telomerase RNA.15 We used the TRAP assay to study the telomerase activity in nonneoplastic lymphoid tissues and malignant lymphomas including Hodgkin's disease (HD).
MATERIALS AND METHODS
Cell Lines
Twelve cell lines were tested. Five lymphoblastoid cell lines and seven lymphoma cell lines were grown in Iscove or RPMI, respectively, supplemented with 5% to 10% of fetal calf serum (FCS). Lymphoblastoid cell lines were from our laboratory (Bever, Rosk, Ceb, Maco, Oru) and lymphoma cell lines corresponded to six B-cell lymphomas (Val, Rec-1, OCI LY8 C3, BL9, Raji, Deglis) and one T-cell leukemia CEM (T-ALL) (see Table 1 for details).16-19 Rec-1, Val, and OCI LY8 C3 were kindly provided by Drs R. Rimokh,18 Dr C. Bastard,19 and Dr N.L. Berinstein,17 respectively.
Cells, 4 × 106 were obtained and pelleted at 15,000g for 4 minutes at 4°C, resuspended in 200 μL of ice-cold lysis buffer (0.5% CHAPS, 10 mmol/L Tris-HCl [pH 7.5], 1 mmol/L EGTA, 0.1 mmol/L AEBSF, 1 mmol/L MgCl2 , 5 mmol/L β-mercaptoethanol) and kept on ice for 30 minutes.7 10 Then the protein extract was centrifuged for 15 minutes at 4°C at 20,000g, and 160 μL of the supernatant were collected and snap frozen in liquid nitrogen before being stored at −80°C.
Tissue Samples
Lymphomas.Frozen tissues from 14 cases of classical HD (nodular sclerosing subtype n = 8, mixed cellularity n = 6; 4 of 14 were Epstein-Barr virus [EBV]-positive) and 43 cases of NHLs were retrieved from our tissue bank. The NHL were classified according to the Revised European American Lymphoma (REAL) classification.20 All samples were processed as described by Kim et al7 and Piatyszek et al,10 with some modifications. Briefly, 40 cryostat sections of 5-μm thickness from each frozen block (corresponding to approximately 50 mg of tissue) were collected in Kontes homogenization tubes (Molecular Dynamics, Sunnyvale, CA).7,10 Cryostat blades were changed for each tissue sample. Tissue sections were suspended in 200 μL of ice-cold lysis buffer and were homogenized using matching disposable pestles rotated at 450 rpm by a drill.7 10 This step was repeated twice during the 30-minute incubation.
Protein concentrations were determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA) by using bovine serum albumin as internal control. The results were similar for cultured cell lines and for frozen tissue, and varied from 1.5 mg/mL to 8 mg/mL of proteins (Table 2). Samples were aliquoted at 1.5 and 3 mg/mL.
Nonneoplastic lymphoid tissues.Protein extracts obtained from six hyperplastic lymph nodes and five hyperplastic tonsils were tested in parallel (Table 2). The lymph nodes had no specific lesions but all displayed follicular hyperplasia.
Telomerase (TRAP) Assays
The procedure was similar to that described by Kim et al7 and Piatyszek et al.10 Step 1 consisted of extension of a telomerase substrate (TS) oligomer (5′ AATCCGTCGAGCAGAGTT-3′) and step 2 consisted of a hot-start polymerase chain reaction (PCR) amplification of the product using a reverse CX primer [5′-(CCCTTA)3CCCTAA-3′] that was sealed at the bottom of the PCR tube by a wax barrier (Ampliwax; Perkin Elmer, Norwalk, CT).
Two to 4 μL of CHAPS extracts (6 μg of protein) were tested in a PCR tube in 50 μL of a reaction buffer containing 68 mmol/L KCl, 1.5 mmol/L MgCl2 , 20 mmol/L Tris-HCl (pH 8.3), 1 mmol/L EGTA, 0.05% Tween 20, 0.5 μmol/L T4 gene 32 protein (Boehringer Mannheim, Meylan, France), 50 μmol/L of each dNTP (dATP, dTTP, dGTP, dCTP), 0.25 μL of (α-32P)dCTP, 0.25 μL of (α-32P)dTTP (10 μCi/μL each), 2.5 U of Taq DNA polymerase, 0.1 μg of primer TS, and 0.1 μg of primer CX.
The tube was incubated 30 minutes at 23°C to allow for the extension of the TS primer by the telomerase. After elongation, the sample was heated at 94°C for 3 minutes. This step inactivated the telomerase and released the CX primer after melting of the Ampliwax gem. The PCR assay consisted of 30 cycles of 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 45 seconds. Controls were included in each assay; positive control (one lymphoblastoid or lymphoma cell line) and negative controls: (1) predigestion of protein extracts from a positive control with RNase A (0.5 μg for 10 μL extract, 15 minutes at 37°C, (2) no protein, (3) no CX primer. To assess sensitivity of the TRAP assay, protein extracts from a positive control (Val lymphoma cell line) were diluted at 1/10, 1/100, 1/250, 1/500, 1/750, and 1/1,000 in protein extracts from negative cases. All dilution controls were run in duplicate.
TRAP Product Revelation
PCR amplification products were analyzed on 10% polyacrylamide gel (nondenaturing) in 1× Tris-borate-EDTA buffer at 50 V overnight. The gels were then fixed in 0.5 mol/L NaCl, 40 mmol/L sodium acetate (pH 4.2), 50% absolute ethanol for 45 minutes, and subsequently sealed in a plastic bag and exposed directly to an autoradiographic film (Kodak X-OMAT; Eastman Kodak, Rochester, NY) using intensifying screens. With the TRAP method, the amplification products resulted in a 6-bp ladder extending from 40 bp to the limit of gel resolution.7 10
RESULTS
Telomerase Activity in Lymphoblastoid and Lymphoma Cell Lines
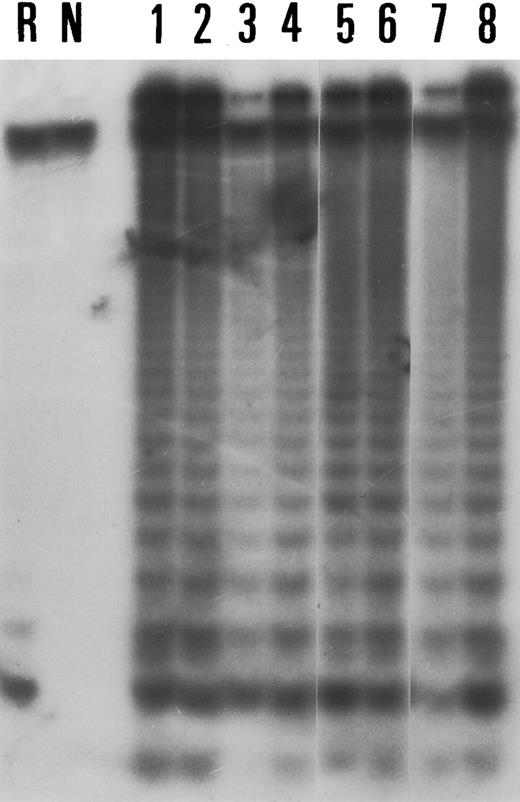
All five lymphoblastoid cell lines and all lymphoma cell lines tested displayed a telomerase activity (Table 1). There were small variations in the level of activity between cell lines (Fig 1) and in the level of telomerase expression in each cell line after dilutions of protein extracts until 1/50.
Results of the TRAP assay in four lymphoma cell lines (Lanes 1 through 4: Val, Deglis, Rec-1, BL9); four lymphoblastoid cell lines (lanes 5 through 8: Bever, maco, Ceb, Oru). Lane R corresponds to RNase A pretreated sample from the Val cell line and lane N corresponds to a negative control (without CHAPS extracts).
Results of the TRAP assay in four lymphoma cell lines (Lanes 1 through 4: Val, Deglis, Rec-1, BL9); four lymphoblastoid cell lines (lanes 5 through 8: Bever, maco, Ceb, Oru). Lane R corresponds to RNase A pretreated sample from the Val cell line and lane N corresponds to a negative control (without CHAPS extracts).
All controls that were run in parallel gave expected results. Thus, there was no amplification in positive controls without CX primer, in controls without CHAPS extracts, or in positive controls with previous incubation with RNase A (Fig 1).
Telomerase Activity in Nonneoplastic Lymphoid Tissues
We observed a clear telomerase activity in all cases (6 of 6) of nonneoplastic lymph nodes and in all cases (5 of 5) of hyperplastic tonsils (Table 2). The lymph nodes had no specific etiology but in all cases there were various numbers of hyperplastic follicles with conspicuous germinal centers by histopathologic examination. Hyperplastic follicles were more numerous in tonsils. The intensity of telomerase activity did not vary greatly from case to case but was similar to that observed in malignant lymphomas (Figs 2 and 3). The weak differences of intensity could result from differences in sensitivity of the assays because the volume of CHAPS extracts added in the tube varied from 2 to 4 μL to obtain the same amount of 6 μg of proteins. However, it has been shown that CHAPS extract in excess of 4 μL in each tube leads to the reduction of the sensitivity of the TRAP assay.10
Comparison of the results of the TRAP assay in six cases of NHLs (lanes 1 through 6) and in six reactive hyperplastic lymph nodes (lanes 7 through 12). Note that all cases have similar levels of telomerase activity. Lane T is a positive control (Val cell line). Lane R corresponds to RNase A pretreated sample from the Val cell line and lane N corresponds to a negative control (without CHAPS extracts).
Comparison of the results of the TRAP assay in six cases of NHLs (lanes 1 through 6) and in six reactive hyperplastic lymph nodes (lanes 7 through 12). Note that all cases have similar levels of telomerase activity. Lane T is a positive control (Val cell line). Lane R corresponds to RNase A pretreated sample from the Val cell line and lane N corresponds to a negative control (without CHAPS extracts).
Comparison of telomerase activity in hyperplastic tonsils (lanes 1 through 5), NHLs (lanes 6, 7, 10 through 12), and HD (lanes 8 and 9). The case of NHL (lane 11) and the two cases of HD (lanes 8 and 9) are clearly negative. Lane T corresponds to a positive control (Val cell line). Lane R corresponds to RNase A pretreated sample from the Val cell line.
Comparison of telomerase activity in hyperplastic tonsils (lanes 1 through 5), NHLs (lanes 6, 7, 10 through 12), and HD (lanes 8 and 9). The case of NHL (lane 11) and the two cases of HD (lanes 8 and 9) are clearly negative. Lane T corresponds to a positive control (Val cell line). Lane R corresponds to RNase A pretreated sample from the Val cell line.
Telomerase Activity in Lymphomas
Telomerase activity was observed in 34 of 43 cases (80%) of NHLs (Figs 2 and 3). There were differences in telomerase activity from case to case. The vast majority of B-cell proliferations was positive (29 of 34). The results were less clear with regard to T-cell lymphomas. The one case of T-ALL and 2 of 3 cases of anaplastic large cell lymphoma (ALC) of T-cell phenotype were TRAP-positive, but only 1 of the 4 cases of peripheral T-cell lymphomas had detectable telomerase activity. The negative cases of NHLs lymphomas had no display of telomerase inhibitors (not shown).
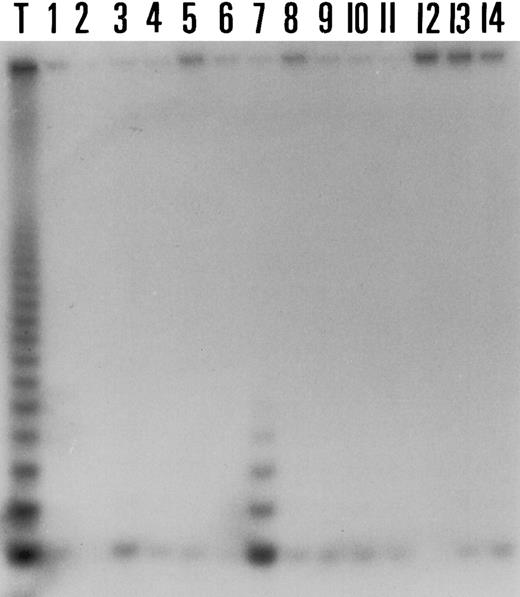
In HD telomerase activity was detectable at a weak level in only 1 of 14 cases (7%) (Fig 4). The signal remained negative even after a 10-day exposure to the film. Although the telomerase-positive case of HD was EBV+ , there were three EBV+ cases among HD with undetectable telomerase positivity. When slides were reviewed to compare the density of tumor cells in cases of HD, we observed that the positive case was abundant in tumor cells but five other telomerase-negative cases also contained Reed-Sternberg cells in similar numbers. Of interest, in two TRAP-negative cases there were remaining lymphoid follicles. To rule out the possibility of inhibitor of the Taq polymerase in the reaction, extracts were serially diluted in phosphate-buffered saline (PBS) as suggested by Piatyszek et al10 and remained TRAP-negative (not shown). However, the results obtained after dilutions of telomerase-positive extracts from Val cell line in protein extracts from the 13 negative cases of HD were heterogeneous. In the majority of cases (8 of 13) telomerase activity was detectable at 1/250 or below. As shown in Fig 5, in 3 of these 8 cases telomerase activity was still detected at 1/1,000 (equivalent to the activity of 10 telomerase positive cells in a background of 104 negative cells). In one additional case telomerase activity persisted at 1/100. In the four remaining cases telomerase activity was abolished at the dilution of 1/100, suggesting the presence of telomerase inhibitors in these cases.
Results of the telomerase activity in 14 cases of HD. Case 7 is weakly but undoubtedly positive. Lane T corresponds to the positive control (Val cell line).
Results of the telomerase activity in 14 cases of HD. Case 7 is weakly but undoubtedly positive. Lane T corresponds to the positive control (Val cell line).
Results of the TRAP assay in a telomerase-negative case of HD after serial dilutions of protein extracts from the Val lymphoma cell line. Line 1 corresponds to 6 μg of protein extracts from the Val cell line. Note that after serial dilutions the signal is still present at the dilution 1/1,000.
Results of the TRAP assay in a telomerase-negative case of HD after serial dilutions of protein extracts from the Val lymphoma cell line. Line 1 corresponds to 6 μg of protein extracts from the Val cell line. Note that after serial dilutions the signal is still present at the dilution 1/1,000.
DISCUSSION
Telomeres have important functions for chromosome integrity, and in somatic differentiated tissues they undergo a progressive shortening also referred to as “the end replication problem,” causing senescence and ultimately exit from the cell cycle.3,21 In most organisms telomere elongation involves an RNA-dependent DNA polymerase that adds repeated sequences at the 3′ ends of the chromosomes.1-5 Telomerase activity is detectable in germ line cells but has been shown to be absent in most normal somatic tissues where its reappearance is associated with the development of malignancy.6,7 Telomerase activity is present in most permanent in vitro cell lines derived from malignant tumors and in EBV-transformed lymphoblastoid cell lines.7,22 Rapid detection of telomerase activity is now possible due to the development of a reliable assay, TRAP.7,10 Weak but detectable telomerase activity has been observed in hematopoietic progenitors; normal leukocytes from blood, cord blood, and bone marrow; as well as in activated B or T cells.11-13
Our report confirms the activation of telomerase for growth and multiplication in lymphoma and lymphoblastoid cell lines. In the lymphoblastoid cell lines, EBV may be responsible for telomerase expression through a mechanism of cellular activation. As expected, telomerase activity was detected in the majority of NHLs. This finding has been previously reported in small series, but no simultaneous comparison was made with nonneoplastic lymphoid tissues.7
Most of the B-cell lymphomas had telomerase activity. Variable and less clear results were obtained in the T-cell lymphomas studied. Discrepancies of findings between peripheral T-cell lymphomas and ALC lymphomas of T-cell origin remain unexplained. Further studies are needed to confirm whether there is a relative absence of telomerase activity in different subsets of peripheral T-cell lymphomas.
The TRAP method was not used by Nilsson et al,23 and this may be the reason for the lack of detection of telomerase activity in acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), as well as in hyperplastic tonsils and peripheral blood lymphocytes. However, they did find telomerase activity in all (4 of 4) cases of high-grade lymphomas and in 3 of 5 cases of low-grade lymphomas.23 The positive telomerase activity in reactive lymph nodes and tonsils that we observed can be attributed either to activated lymphocytes or to germinal center cells. Telomerase activity in reactive lymphoid tissues and NHLs was detectable at similar levels. Telomerase activity was detected in only 1 of 14 cases (7%) of lymphoid tissues involved by HD. Despite the presence of telomerase inhibitors in a few cases, the presence of tumor cells in numbers below the detection threshold is an unlikely problem because of the sensitivity of the assay at dilutions of 1:250 (and below) of the protein extracts and the presence of greater than 1% tumor cells in many cases by semiquantitative estimates. Thus, 8 of the 13 negative cases were considered to be interpretable because of the lack (3 of 13 cases) or low level (5 of 13 cases) of telomerase inhibition. The five remaining cases could not be evaluated because of their telomerase inhibitor content. These results are intriguing because they would indicate the lack or low level of telomerase activity in both neoplastic and nonneoplastic components in lymph nodes involved by HD. What is the relation of telomerase activity to the difficulties of obtaining Reed-Sternberg cell lines and the lack of engraftment of Hodgkin's tumors in nude and severe combined immunodeficient mice, since it is relatively easier to graft NHLs?24 What is the relation of the presence or absence of telomerase activity to the outcome, because HD is curable in approximately 75% of cases? Because neuroblastomas with genetic changes such as N-myc amplification and unfavorable outcome have high levels of telomerase activity,25 it would be interesting to test this parameter in HD. However, the estimation of telomerase activity by current data cannot be applied for the distinction of a benign from a malignant lymphoproliferative process. Microdissection experiments are required for the localization of telomerase activity in subsets of cells in lymphoid tissues and to confirm the apparent lack of this activity in Reed-Sternberg cells of HD.
NOTE ADDED IN PROOF
During the revision of this manuscript, Norrback et al26 have reported the detection of telomerase activity in reactive lymphoid tissues and non-Hodgkin's lymphomas.
Supported by the Association pour la recherche sur le Cancer (ARC), the PHRC-Délégation à la Recherche Clinique, the Ligue Nationale Contre le Cancer (Comité de la Haute Garonne). S.C. was supported by the French Ministry of Education.
Address for reprint requests to Pierre Brousset, MD, Laboratoire du Groupe d'Etude des Lymphomes Malins (CNRS, CIGH), Centre Hospitalier Universitaire de Purpan, Place du Dr Baylac, 31059 Toulouse Cédex, France.