Abstract
Forty-two patients with cutaneous T-cell lymphoma, including 31 with exfoliative erythroderma or Sezary syndrome and 11 with mycosis fungoides, were studied for the occurrence of staphylococcal infection. Thirty-two of 42 (76%) had a positive staphylococcal culture from skin or blood. One half of the patients with positive cultures grew Staphylococcus aureus. This group included 11 with Sezary syndrome and 5 with rapidly enlarging mycosis fungoides plaques or tumors. All of the S aureus carried enterotoxin genes. Surprisingly, 6 of 16 strains were the same toxic shock toxin-1 (TSST-1)-positive clone, designated electrophoretic type (ET)-41. Analysis of the T-cell receptor Vβ repertoire in 14 CTCL patients found that only 4 had the expected monoclonal expansion of a specific Vβ gene, whereas 10 had oligoclonal or polyclonal expansion of several Vβ families. All patients with TSST-1+S aureus had overexpansion of Vβ 2 in blood and/or skin lesions. These studies show that S aureus containing superantigen enterotoxins are commonly found in patients with CTCL, especially individuals with erythroderma where they could exacerbate and/or perpetuate stimulate chronic T-cell expansion and cutaneous inflammation. Attention to toxigenic S aureus in CTCL patients would be expected to improve the quality of care and outcome of this patient population.
CUTANEOUS T-cell lymphoma (CTCL) is an indolent, epidermotrophic CD4+CD3+CD45Ro+ T-cell lymphoma characterized by eczematous or psoriasiform skin lesions (mycosis fungoides [MF]) or by diffuse erythroderma and circulating atypical lymphocytes (Sezary syndrome [SS]).1,2 Alibert first described MF in 1806 and Sezary Bouvrain recognized the association between erythroderma and abnormal circulating lymphocytes. This condition has also been called the “Red Man Syndrome” or “L'Homme Rouge” by Besnier and was recognized as a helper T-cell leukemia by Lutzner in 1975.3 The cause of erythroderma in the SS is unknown. Systemic administration of interleukin-2 (IL-2), a T-cell growth factor, produces erythroderma and scaling.4 Exfoliative erythroderma also accompanies atopic dermatitis, drug reactions, psoriasis, and toxic shock syndromes caused by Streptococcus pyogenes and Staphylococcus aureus, including Kawasaki's disease.5-9 These bacteria contain genes encoding toxins and related molecules that are capable of causing erythroderma and stimulating helper T cells as superantigens.10-14
Our clinical experience has taught us that patients with SS who have indwelling catheters virtually always get staphylococcal sepsis accompanied by worsening erythroderma, not uncommonly in the absence of significant fever.15 In some of our patients, systemic antibiotic therapy may be associated with decreased erythroderma and tumor size, in the absence of other therapy. Tokura et al16 reported two erythrodermic Sezary patients responding to topical treatment of staphylococcal colonization. In as much as erythroderma can accompany infection or disease progression and is a clinical parameter for determining the outcome of therapy in CTCL patients, we studied the occurrence of staphylococcal infections in our patient population and characterized the strain subtype, superantigen gene presence, and T-cell receptor Vβ gene usage.
MATERIALS AND METHODS
Patient Population and Staging
Patients diagnosed with cutaneous T-cell lymphoma over the course of 2 years were prospectively studied if they had erythroderma or skin plaques/tumors that were rapidly increasing in size. All patients had a complete physical examination and staging work-up including a blood smear for Sezary cells, skin biopsy for histology and T-cell immunophenotyping, flow cytometry, computed tomography (CT) scan, and bacterial cultures of their skin by swab and/or blood. Sixteen patients had S aureus and 14 patients gave consent to have T-cell Vβ analysis performed by reverse transcriptase-polymerase chain reaction (RT-PCR) in skin and blood samples (Table 1). Controls included six healthy donors who consented to give peripheral blood samples or skin biopsy samples.
Staphylococcus Subtyping and Identification of Superantigen Genes
Cultures.Swabs from skin lesions were immediately inoculated into transport media and seeded onto nutrient agar and mannitol salt agar. Blood was drawn from the antecubital fossa using sterile technique into broth containing vacuum tubes.
Multilocus enzyme electrophoretic analysis of bacterial strains.Each isolate was grown in 150 mL of tryptic soy broth (Life Technologies, GIBCO-BRL, Grand Island, NY) overnight at 37°C on an orbital shaker and the cells and were obtained by centrifugation. The bacterial pellet was resuspended in 2 mL of lysis buffer (50 mmol/L Tris-HCL, pH 7.5; 5 mmol/L EDTA) containing 100 μg/mL lysostaphin (Sigma, St Louis, MO) and incubated at 37°C for 45 minutes. The suspension was then sonicated for 30 seconds at 50% output with a Branson model 200-sonicator (VWR Scientific, Sugarland, TX) with ice-bath cooling, and centrifuged at 20,000g for 20 minutes. The enzyme-containing supernatants were either immediately analyzed by multilocus enzyme electrophoresis or stored at −80°C. After electrophoresis on starch gels, selective histochemical staining for 20 metabolic enzymes was conducted.17 Distinctive mobility variants of each enzyme were numbered in order of decreasing anodal migration, and equated with alleles at the corresponding chromosomal structural gene locus. Each isolate was characterized by its combination of alleles at 20 enzyme loci, and unique combinations of electromorphs were designated as electrophoretic types (ETs).18
Detection of genes encoding enterotoxins and toxic shock syndrome toxin-1 (TSST-1). S aureus strains isolated from 16 CTCL patients were analyzed for the presence of genes encoding SEA, SEB, SED and SEE, and TSST-1 (Table 1). Briefly, polymerase chain amplification was performed using oligonucleotide primers and reaction conditions as described.12,18,19 Bacterial cell lysates generated by the lysostaphin procedure were treated with RNAse and genomic DNA was purified using the method of Vannufel et al.19 Distinctive PCR products for each toxin were detected by agarose gel electrophoresis.
T-Cell Receptor Vβ Gene Analysis
RNA isolation.RNA was isolated from peripheral blood mononuclear cells and simultaneously from skin biopsy specimens when possible. Mononuclear cells were separated by gradient centrifugation of the peripheral blood with Ficoll-Paque (Pharmacia, Piscataway, NJ). Four-millimeter skin specimens were snap frozen, and 30 sections of 20-μm thickness were cut with a cryostat and homogenized by several passages through 16- to 23-gauge needles. Total RNA was extracted from cells and tissue using TRIzol reagent (Life Technologies, GIBCO-BRL) according to the manufacturer's protocol. After RNA isolation, the dried pellets were resuspended in RNAse-free treated water (diethylpyrocarbonate) and the RNA concentration was measured using a spectrophotometer (Gene Quant, RNA/DNA calculator; Pharmacia).20 To avoid secondary structure and optimize the synthesis of the cDNA, the RNA was incubated at 70°C for 10 minutes.
cDNA synthesis.Three micrograms of total RNA was used as a template for cDNA synthesis at 42°C for 1 hour in a 120-μL reaction using the GeneAmp RNA PCR kit (Cetus-Perkin-Elmer, Emeryville, CA) and protocol with M-MLV reverse transcriptase and random hexamers.
PCR amplification and detection.Twenty-five specific primers for the T-cell receptor variable or Vβ 5′ families, a constant Cβ 3′ primer, and primers for the constant Cα region, as a control, were purchased from Clontech Laboratories (Palo Alto, CA). Two hundred nanograms of cDNA was amplified in a 50-μL reaction using AmpliTaq DNA polymerase (Cetus-Perkin-Elmer) by following the manufacturer's recommended buffers and instructions. All the components were added in a master mix and aliquoted to each tube and 0.5 μL of each of the 25 Vβ 5′ primers was incorporated individually. A positive control was prepared with the Cα controls primers as well as a negative control. PCR reactions were performed on an MJ thermocycler (MJ Research, Inc, Watertown, MA) at 95°C for 1 minute, 55°C for 1 minute, 72°C for 1 minute × 35 cycles, followed by a 72°C extension for 7 minutes.
Southern blot hybridization.PCR products were detected on a 1.8% agarose/0.5 Tris-Borate-EDTA electrophoresis gel following ethidium bromide staining by UV light visualization. The DNA was transferred to Zetabind nylon membranes (Cuno Inc, Meriden, CT) and hybridized to an end-labeled [γ-32P]-ATP 3′ Cβ control probe (Figs 1 and 2). The membranes were directly counted on an Instant Imager (Packard, Meriden, CT), exposed overnight on film at −70°C (Hyperfilm; Amersham, Arlington Heights, IL).20 Each Vβ signal was expressed as a percentage of total signal after background subtraction with expression at a value of 6% or more of the total value considered significant overexpression of a TCR Vβ gene (Tables 2 and 3).
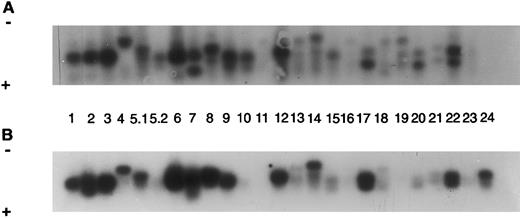
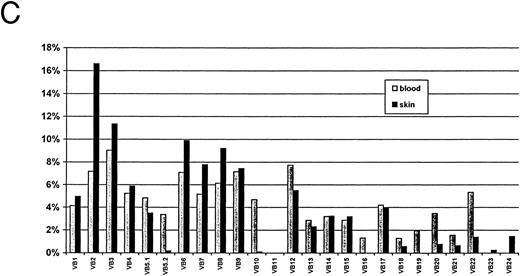
Autoradiograms and histogram for TCR Vβ study from SS patient 6 (SS-6, Table 2). The signals were detected by Southern blot hybridized to a labeled constant primer Cβ and quantitated on a Instant Imager. The blots were then exposed to film overnight and the signals for each receptor gene studied (numbers) are shown for blood (A) and for skin (B). A histogram with the relative amounts of each signal are shown as a percentage of the total counts for blood and for skin (C).
Autoradiograms and histogram for TCR Vβ study from SS patient 6 (SS-6, Table 2). The signals were detected by Southern blot hybridized to a labeled constant primer Cβ and quantitated on a Instant Imager. The blots were then exposed to film overnight and the signals for each receptor gene studied (numbers) are shown for blood (A) and for skin (B). A histogram with the relative amounts of each signal are shown as a percentage of the total counts for blood and for skin (C).
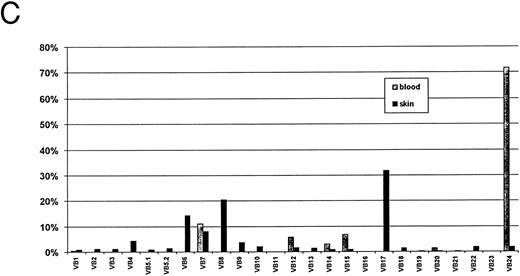
Autoradiograms and histogram from SS-1 patient, Table 2. Signals from blood (A) and from skin (B) were counted and the percentages of the total for both blood and skin are shown in the histogram (C).
Autoradiograms and histogram from SS-1 patient, Table 2. Signals from blood (A) and from skin (B) were counted and the percentages of the total for both blood and skin are shown in the histogram (C).
RESULTS
Enterotoxin Genes, Especially TSST-1 ET-41, Are Present in CTCL S aureus Isolates
We next characterized the enterotoxin genes present in the 16 S aureus isolates from our CTCL patients (Tables 1 and 2). A total of 10 distinct electrophoretic types (ETs), marking S aureus clones, were identified in the sample of 16 isolates. However, 6 patients had the identical ET (ET-41) that is characterized by the presence of the TSST-1 gene. One of these patients (no. 7) developed SS after breast implants21 and had the same strain of S aureus with TSST-1, ET-41, cultured from skin lesions, breast implants at removal, blood, and vagina over the course of 6 months. A complete and durable remission of SS has occurred after treatment with prolonged systemic antibiotics as well as photopheresis, α-interferon, and gammaglobulin therapy. Five of the S aureus isolates contained other toxin genes: SEA, SEB, SEC, or SED. Nine patients' S aureus isolates were found to have two toxin genes, including 3 with both TSST-1 ET-41 and SEA. Four patients had SEC in combination with SEB and SED, and 1 patient's strain had SEB with SED.
Staphylococci Are Frequently Isolated From CTCL Patients
Forty-two CTCL patients were enrolled in the study over the course of the 2.5 years including 31 with erythroderma or the SS and 11 with MF. On enrollment, 32 (76%) had a skin or blood culture positive for staphylococcus. Coagulase negative staphylococci or S epidermidis were cultured from one half of the 32 patients. The other 16 patients had cultures positive for S aureus, including 11 patients with erthroderma or SS and 5 patients with rapidly enlarging MF lesions (Table 1).
Oligoclonal Expansion of the T-Cell Vβ Receptor Is Frequently Observed in CTCL
Because staphylococcal enterotoxins function as superantigens to amplify T cells with specific Vβ receptors, we next determined the T-cell receptor (TCR) Vβ repertoire use in paired skin lesion and peripheral blood samples in 14 consenting CTCL patients. These included 9 patients from whom S aureus enterotoxins were determined (Table 2). Only 4 of 14 CTCL patients had a predominant T-cell clone identified. Two had a single TCR Vβ gene that was expressed at a level of more than 25% in skin and blood and 2 had a major TCR clone in their skin. The other 10 patients had three or more TCR Vβ genes expressed at greater than 6% of the total, which represented oligoclonal or polyclonal expansion. The TCR Vβ usage was variable, both within blood and skin specimens from the same patient and among the different individual patients studied.
Of the 25 T-cell Vβ families studied by RT-PCR, we found that TCR genes Vβ 6, 7, 8, and 12 were most often amplified in the skin and blood of CTCL patients and controls. TCR Vβ 6 was increased to greater than 6% of the total in 10 of 11 skin samples and 8 of 11 blood lymphocyte samples from CTCL patients compared with 3 of 6 or 4 of 6 control samples, respectively. In contrast, the TCR Vβ 5.2 and Vβ 11, which are not known to be recognized by staphylococcal toxins,5,9,10 22 were rarely amplified to greater than 6% predominance.
T-Cell Vβ2 Expansion Is Seen in CTCL Patients in Association With TSST-1
It was previously reported that the staphylococcal toxin TSST-1 is associated with amplification of TCR Vβ2.5 10 Therefore, we examined its frequency among 11 CTCL patients who had both the TCR Vβ repertoire and staph toxin typing performed. Figure 1 shows the polyclonal TCR expansion including Vβ2 in skin and blood of SS patient 6 with TSST-1. Five of 11 patients, including 4 with SS, had TSST-1 present in their staphylococci, and all had TCR Vβ 2 expanded in either skin or blood (Table 2 and Fig 1). Four overexpressed TCR Vβ 2 in blood (6.2% to 16%), 3 in skin only (8% to 17%), and 2 in both skin and blood. This is compared with 2 of 6 control bloods and 1 of 6 control skin specimens (Table 3).
In the remaining CTCL patients with S aureus, only 1 SS patient with SEB toxin had overexpression of Vβ 2 in blood. Figure 2 shows the TCR Vβ repertoire of a patient whose S aureus contained SED enterotoxin. Vβ2 was not amplified in blood but was weakly amplified in skin. However, Vβ 5 and 12, associated with this toxin, were also amplified weakly in skin. TCR Vβ 2 gene was not over-expressed in 3 CTCL patients who were culture-negative for S aureus.
DISCUSSION
Bacterial superantigens, which stimulate clonal expansion of T cells by mechanisms involving specific HLA molecules,10,13,23-28 have also been hypothesized to cause inflammatory skin diseases.7-9,22,29-31 The evidence to support this mechanism for cutaneous T-cell lymphoma is limited to in vitro stimulation of T cells by superantigens32 and to report of two patients treated for staphylococcal colonization.16
This is the first report of the occurrence of staphylococcus and its enterotoxins in a large, prospective cohort of CTCL patients. We found that 32 of 42 consecutive CTCL patients had a staphylococcal culture positive from either skin or blood and that coagulase positive S aureus was present in 16 patients. In a smaller subset of 14 patients studied for T-cell receptor Vβ expansion, 78.6% had S aureus–containing toxins. Unexpectedly, a specific S aureus strain (ET-41) was present in 1 of 3 MF patients and in 4 of 11 Sezary patients whose cultures were positive for S aureus. This toxic shock toxin-1 strain occurred in association with oligoclonal expansion of several Vβ genes, including the expected TCR Vβ 2. Our data support the role of S aureus enterotoxins in the pathogenesis of CTCL and raise the question “To what extent do superantigens contribute to the features of this disease?”
In cutaneous T-cell lymphomas there is a “malignant” proliferation of atypical CD4+CD45Ro+ T cells that is thought to initially expand in skin and later invade lymph nodes and other organs, including blood (SS).2,33,34 Although Tan et al35 first suggested that CTCL arises from persistent antigenic stimulation of T cells, the identity of a putative antigen and its ability, if any, to stimulate T cells is unknown and speculative.21 36-38 If CTCL results from antigen persistence, then colonizing skin flora including staphylococci or streptococci could provide the necessary antigens, and superantigens, for initiation and perpetuation of T-cell proliferation in some, if not all, patients with this disease.
Clonal expansion of TCR Vβ genes has been shown for CTCL, as well as for a number of other autoimmune diseases (Table 4), but no single TCR variable gene has been identified. This has been interpreted to imply lack of a common antigen (or superantigen). Our study was surprising in that it suggested that oligoclonal or polyclonal (rather than monoclonal) expansion of T-cell Vβ genes is more common in CTCL patients' skin and blood, as detected by RT-PCR analysis with the limitations of this technique and the availability of primer sequences. Secondly, CTCL patients with TSST-1+S aureus had the expected expansion of the Vβ2 gene. In addition, most patients' TCR Vβ repertoires included expansion of TCR Vβ 6 and 8, frequently cited in other studies.39-44 Although Vβ 6 and 8 genes are also expanded in normal cutaneous T cells relative to blood, this is hypothesized to be the result of their reactivity to common skin flora.45 Epidermotropic T cells of CTCL lymphoma may be similar.
We hypothesize that like other T-cell–mediated skin diseases, CTCL occurs in the setting of a genetically determined host (HLA determinants), a trigger (antigens or superantigens), and an immune response with cytokine and chemokine production. In CTCL, T cells are attracted into the epidermis and they may be unable to limit their proliferation (absent apoptosis). Staphylococcal superantigen, presented either by Langerhans cells or by class II–bearing keratinocytes, results in cytokines that stimulate T cells.35 It is reasonable that persistent colonization with toxigenic bacteria would expand the population of epidermotropic T cells and elicit production of T-cell–activating cytokine/chemokines, such as interferon-inducible protein 10, capable of perpetuating the reaction and recruiting additional cells.46
In T-cell–mediated autoimmune diseases (Table 4), genetic susceptibility may be conferred to some degree by major histocompatibility locus antigens.47 We confirmed that CTCL patients have an association with HLA-DR5 (DR11),48 and found stronger associations with HLA-DQ*03 alleles and with HLA-DQ*0502 in SS (odd's ratio [OR] = 7.75).49 HLA class II antigens are clearly known to affect binding of bacterial superantigens SEA, SEB, and TSST-1 and to present processed antigen to CD4+ T cells.13,23,26-28,47 There may be an HLA-mediated sensitivity to superantigens as suggested by the data that fibroblasts transfected with HLA-DR5β3 are more highly stimulated with SEA superantigen than those with other HLA chains.50 HLA type may influence nasal colonization with S aureus, specifically HLA-DR3, in combination with HLA-DR5.51 Thus, HLA-DR5 presence may play a role in susceptibility to colonization and to sensitivity to superantigen stimulation once colonization occurs.
The boundary between normal T-cell proliferation to antigen stimulation and “malignant” clonal expansion of T cells is not always clear, especially with the finding that psoriatic lesions may have clones of T cells with specific Vβ restriction.52 Early “CTCL/mycosis fungoides and pre-Sezary” are notoriously difficult to diagnose, sharing clinical and histologic features with “benign” dermatitis/eczema and psoriasis including a high incidence of S aureus colonization and a similar response profile to therapy.7,29 53 The design of our study underestimated the prevalence of staphylococcus and toxigenic staphylocci in CTCL patients and it did not address the difficult question of whether S aureus is a primary cause or a secondary effect of the “disease.” If the propensity for establishing staphylococcal colonization and ability to tolerate enterotoxins without fever or hypotension is the host-related integral part of this disease, then the question of which came first may be unanswerable.
The dogmatic view is that staphylococcal colonization is secondary and the result of profound immunosuppression.54 In this sense, CTCL patients resemble patients with acquired immunodeficiency syndrome who cannot clear the skin of staphylococcus and have protracted pruritus and erythrodermic psoriasis.55-57 Regardless of etiology, clinical management of CTCL patients would improve by recognizing that TSST-1 S aureus may be found in CTCL and cause bacteremia or sepsis, sometimes in the absence of fever. Disease evaluation is generally measured by degree of skin erythroderma and scale58 so that reactive superantigen erythema may be interpreted as “disease progression” and result in change to more aggressive therapy. Rapid clinical improvement within days has occurred with administration of systemic antibiotics in our erythrodermic and MF patients, and administration of intravenous gammaglobulin, containing antibodies inhibitory to superantigen activation,59 may also benefit the hypogammaglobulinemic patient. Erythroderma skin score has improved with photopheresis as serum Ig levels are restored to normal.15
The association between staphylococcal infections and the erythrodermic form of CTCL deserves further attention and study. Genetic susceptibility and disease initiation or persistence may involve the ability to be colonized and/or relative sensitivity to superantigens in common skin flora. Because allelic variation in bacterial superantigens can significantly alter T-cell interactions, it will be critical to more precisely characterize the enterotoxin genes found in the S aureus isolates cultured from our patients. Attention to the infectious component of CTCL would be expected to greatly impact the outcome of care, improve prognosis and mortality, and lead to novel forms of therapy and disease prevention.
ACKNOWLEDGMENT
We thank Drs Gerald Bodey, MDACC, and Doug Grossman, MD, PhD, for helpful ideas and encouragement; Barbara LeBlanc for specimen collection; and X. Pan for technical assistance.
Funded through a Physician's Referral Grant, MD Anderson Cancer Center, NIAMSD Grant No. AR39915 (M.D.), and fellowship support to C.M.J. provided by the Ladies Leukemia League, New Orleans, LA.
Presented in part at the Society for Investigative Dermatology, Washington DC, May 5-8, 1996.
Address reprint requests to Madeleine Duvic, MD, Section of Dermatology, Division of Medicine, MD Anderson Cancer Center, Box 47, 1515 Holcombe Blvd, Houston, TX 77030.