To the Editor:
Despite B-cell counts usually normalized by 1 year, humoral immunity and the incidence of bacterial infections continue to be abnormal even after 1 year following bone marrow transplantation (BMT). In a recent issue of Blood, Suzuki et al1 reported a possible explanation: even at 1 year after grafting, the bulk of the B cells are naive (without somatically mutated Ig variable chain genes), whereas memory (somatically mutated) B cells are lacking. Compared to naive B cells, memory B cells are capable of brisker differentiation into plasma cells that produce higher-affinity antibodies, which should result in more efficient opsonization of phagocytes and neutralization of pathogens.2 Because the DNA method used by Suzuki et al is laborious, only 4 patients were studied. Here we report a confirmatory study using a simple flow cytometric technique and a sample size of 59 patients. Naive B cells were defined as mIgD+ and memory B cells as mIgD− since B cells without somatic mutations are primarily found in the mIgD+ subset and those with somatic mutations in the mIgD− subset of circulating B cells.1 3
Blood from 59 marrow transplant recipients, 14 normal neonates, 2 normal 4-year-old children, and 24 normal adults was examined. Demographic and clinical characteristics of 53 of the 59 patients were reported in our corresponding study on T-cell reconstitution4 and characteristics of the remaining 6 patients were similar. B cells were defined as mononuclear cells expressing CD19 and/or CD20 and not expressing markers of T cells, natural killer cells, or monocytes; details of the three-color flow cytometric method were described.5 The difference in B-cell subset counts between subject groups was tested by Mann-Whitney-Wilcoxon rank-sum test.
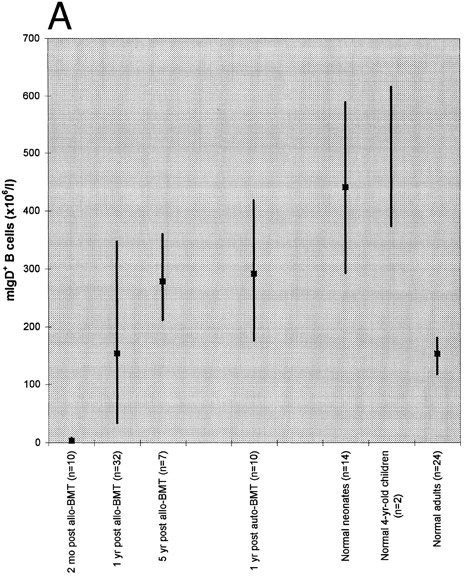
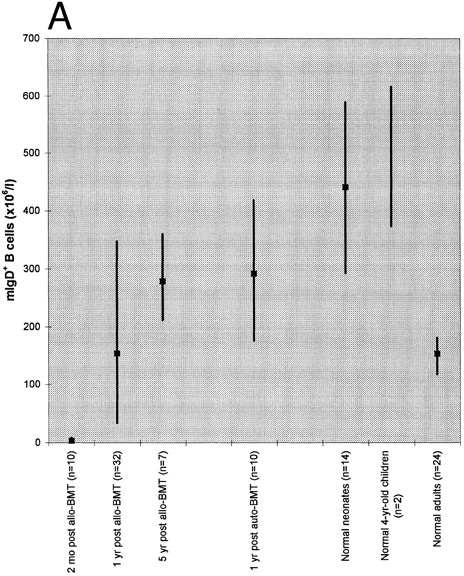
Absolute blood counts of mIgD+ (A) and mIgD− (B) B cells. Data are expressed as medians (squares) and 25th to 75th percentiles (vertical lines), with the exception of the two 4-year-old children in which case the vertical line represents the range. The differences between each of the groups of patients or normal children and the reference group of normal adults were significant (P ≤ .05), except for the group of patients at 1 year after allogeneic BMT in case of mIgD+ B cells and the group of patients at 5 years after allogeneic BMT in case of mIgD− B cells.
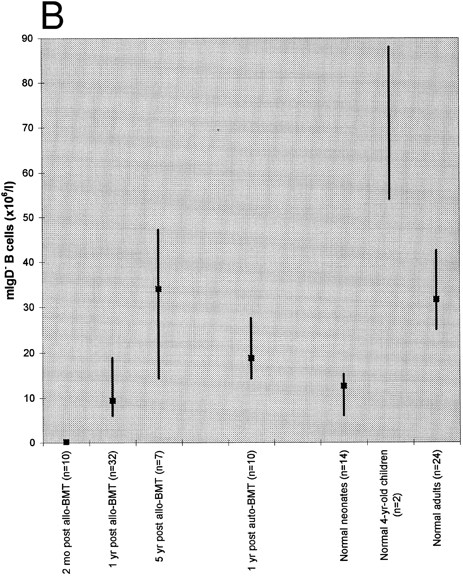
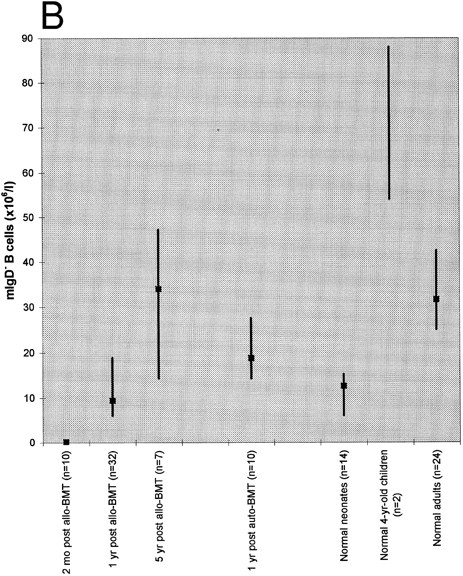
Absolute blood counts of mIgD+ (A) and mIgD− (B) B cells. Data are expressed as medians (squares) and 25th to 75th percentiles (vertical lines), with the exception of the two 4-year-old children in which case the vertical line represents the range. The differences between each of the groups of patients or normal children and the reference group of normal adults were significant (P ≤ .05), except for the group of patients at 1 year after allogeneic BMT in case of mIgD+ B cells and the group of patients at 5 years after allogeneic BMT in case of mIgD− B cells.
As shown in Fig 1, both mIgD+ and mIgD− B cells were barely detectable at 2 months after allogeneic BMT. Although the median mIgD+ B-cell count became normal by 1 year and supranormal by 5 years after allogeneic BMT, the median mIgD− B-cell count lagged behind: it was still subnormal at 1 year and became normal by 5 years after allogeneic BMT. (Subnormal/supranormal denotes higher/lower cell numbers than those of the normal adults, P ≤ .05). After autologous BMT, the reconstitution of both naive and memory B cells appeared to proceed faster than after allogeneic BMT; nevertheless, the lagging of mIgD− B cells was also apparent: at 1 year after grafting the median mIgD− B-cell count was subnormal whereas the median mIgD+ B-cell count was supranormal.
Both the tendency to develop supranormal B-cell counts as well as the lag of mIgD− behind mIgD+ B-cell reconstitution posttransplant were reminiscent of B-cell development in early life: children had supranormal median mIgD+ B cell counts both at birth and at the age of 4 years; their median mIgD− B cell counts were subnormal at birth and supranormal at the age of 4 years.
Together with the results of Suzuki et al our findings suggest that the prolonged deficiency of humoral immunity after transplant may be due in part to the very slow reconstitution of memory B cells.
ACKNOWLEDGMENT
Supported by National Institutes of Health Grants No. CA 18221 and CA68496.