Abstract
Although monoclonal antibody (MoAb) therapy of the human malignant lymphomas has shown success in clinical trials, its full potential for the treatment of hematologic malignancies has yet to be realized. To expand the clinical potential of a promising human-mouse chimeric antihuman B-cell MoAb (chCLL-1) constructed using the variable domains cloned from the murine Lym-2 (muLym-2) hybridoma, fusion proteins containing granulocyte-macrophage colony-stimulating factor (GM-CSF) (chCLL-1/GM–CSF) or interleukin (IL)-2 (chCLL-1/IL–2) were generated and evaluated for in vitro cytotoxicity and in vivo tumor targeting. The glutamine synthetase gene amplification system was employed for high level expression of the recombinant fusion proteins. Antigenic specificity was confirmed by a competition radioimmunoassay against ARH-77 human myeloma cells. The activity of chCLL-1/GM–CSF was established by a colony formation assay, and the bioactivity of chCLL-1/IL–2 was confirmed by supporting the growth of an IL-2–dependent T-cell line. Antibody-dependent cellular cytotoxicity against ARH-77 target cells demonstrated that both fusion proteins mediate enhanced tumor cell lysis by human mononuclear cells. Finally, biodistribution and imaging studies in nude mice bearing ARH-77 xenografts indicated that the fusion proteins specifically target the tumors. These in vitro and in vivo data suggest that chCLL-1/GM–CSF and chCLL-1/IL–2 have potential as immunotherapeutic reagents for the treatment of B-cell malignancies.
WITH THE EXCEPTION of a chimeric anti-CD20 monoclonal antibody (MoAb), which has produced tumor regressions in patients with relapsed B-cell non-Hodgkin's lymphoma (NHL),1 unconjugated MoAbs have demonstrated limited therapeutic responses.2 Radioimmunotherapy, on the other hand, has shown considerable promise in clinical studies, particularly in the treatment of B-cell NHL.3 The efficacy of radioimmunotherapy is restricted, however, either by dose-limiting thrombocytopenia or more severely by the presence of bone marrow disease. In these settings, effective therapy with unconjugated MoAbs would be desirable for the induction of tumor remission. For this purpose, the combination of MoAbs and biologic response modifiers has been investigated as a means of increasing tumor lysis. Cytokines including interleukin-2 (IL-2) and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been shown to enhance both in vitro cytotoxicity mediated by MoAbs against tumor targets and in vivo killing of tumor xenografts in animal model systems.4-9 Because of the toxicity of systemically administered cytokines, however, methods are needed to target these biologically potent immunologic mediators to the tumor site. One approach, gene transfer, has demonstrated that tumor cells engineered to secrete cytokines stimulate antitumor immunity and rejection in animal models,10-17 illustrating the importance of localizing cytokines to tumors. However, at the present time, this approach is impractical in the clinical setting. An alternative method is the use of antibody-cytokine fusion proteins to direct such immunologically active molecules to tumor sites.18-20 In this way high local concentrations of cytokines within tumors can be achieved and systemic toxicity is minimized or avoided.
In this report, we describe the development of such molecules for the treatment of hematologic malignancies. Lym-2 is a murine IgG1 MoAb directed against a human major histocompatability complex (MHC) class II variant that is strongly reactive with a high percentage of human B-cell NHL, chronic lymphocytic leukemia, and multiple myeloma cell lines and biopsy specimens.21 Lym-2 has recently been shown to have a direct inhibitory effect on human lymphoma cell lines in vitro and to improve the survival of human lymphoma-bearing severe combined immunodeficiency (SCID) mice by the induction of apoptosis (Funakoshi et al, manuscript in preparation). In antibody-dependent cellular cytotoxicity (ADCC) assays, however, murine Lym-2 mediates low tumor lysis with human mononuclear effector cells. A human-mouse chimeric derivative designated chCLL-1 has therefore been constructed to increase its effector functions. To further enhance the immunotherapeutic potential of this chimeric antibody for the treatment of B-cell malignancies, antibody fusion proteins containing human GM-CSF and IL-2 have been generated. In this study, we describe the effector functions mediated by these recombinant molecules and demonstrate their tumor targeting abilities in a nude mouse xenograft model.
MATERIALS AND METHODS
Reagents
The plasmid pcD-hGM/Eo-CSF containing the human GM-CSF cDNA22 was obtained from the American Type Culture Collection (clone 57594; Rockville, MD). The plasmid pBC12/HIV/IL-2 containing the human IL-2 cDNA23 was obtained from the American Type Culture Collection (clone 67618). The plasmids pEE6hCMV-B and pEE12 were purchased with the Glutamine Synthetase Gene Amplification System from Celltech Biologics (Slough, UK). Restriction endonucleases, T4 DNA ligase, and other molecular biology reagents were purchased from New England Biolabs (Beverly, MA) or Boehringer Mannheim (Indianapolis, IN). RPMI-1640 medium, minimal essential medium (MEM) nonessential amino acids solution, penicillin-streptomycin solution, Dulbecco's phosphate-buffered saline (PBS), dialyzed fetal bovine serum, Sephadex, buffer salts, and other reagents such as chloramine T, sodium metabisulfite, hydrogen peroxide, and ABTS (2,2′-azino–bis(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt) were purchased from Sigma Chemical Co (St Louis, MO). Hybridoma-SFM medium with and without glutamine was purchased from Life Technologies (Gaithersburg, MD). Fetal bovine serum was obtained from HyClone Laboratories, Inc (Logan, UT). Iodine-125 and iodine-131 were obtained as sodium iodide in 0.1 N sodium hydroxide from DuPont/New England Nuclear (North Billerica, MA). Balb/C and athymic nude mice were purchased from Harlan Sprague Dawley (Indianapolis, IN).
Antibodies and Cell Lines
The murine MoAb Lym-2 (muLym-2, IgG1), directed against a B-cell surface antigen,21 was obtained from Techniclone International, Inc (Tustin, CA). The human-mouse chimeric MoAb Lym-1 (chLym-1, IgG1κ) was generated as previously described.24 The chimeric MoAb CLL-1 (chCLL-1, IgG1κ) was produced as previously described (Funakoshi et al, in preparation). The chimeric MoAb TNT-1 (chTNT-1, IgG1κ), the cDNAs for whose variable regions were cloned from the murine TNT-1 hybridoma,25 was constructed and expressed in the same manner as chCLL-1. The murine Lym-2 antiidiotype MoAb (7E2) was generated as previously described for the antiidiotype to Lym-1 (1A7).24 Iodine-125 and iodine-131-labeled MoAbs were prepared using a modified chloramine T method as previously described.24 The NS0 murine myeloma cell line, which was obtained from Celltech Biologics, was grown in nonselective medium consisting of Hybridoma-SFM supplemented with 10% fetal bovine serum, L-glutamine, MEM nonessential amino acids solution, penicillin G (100 U/mL), and streptomycin (100 μg/mL). Selective medium consists of Hybridoma-SFM without glutamine supplemented with 10% dialyzed fetal bovine serum, glutamic acid, asparagine, nucleosides, penicillin G, and streptomycin, according to the protocol provided with the Glutamine Synthetase Gene Amplification System (Celltech Biologics). The ARH-77 human myeloma cell line,26 obtained from the American Type Culture Collection, was grown in RPMI-1640 medium supplemented with 10% fetal bovine serum, L-glutamine, penicillin G, and streptomycin.
Construction of Expression Vectors
The expression vectors were constructed using standard techniques. The expression vector for chCLL-1, 12/chCLL-1/HL, was used as the parent vector. This plasmid contains the cDNA sequences for the human-mouse chimeric CLL-1 heavy and light chains, each under the control of the cytomegalovirus (CMV) major immediate early promoter, and the cDNA sequence for glutamine synthetase, under the control of the SV40 early promoter. Two oligonucleotide primers, 5′- GGTAAAGCGGCCGCAGGAGGTGGTAGCGCACCCGCCCGCTCGCCCAGC - 3′ and 5′ - TCAATGCGGCCGCTCACTCCTGGACTGGCTCCCAGCA - 3′, were used to amplify by polymerase chain reaction (PCR) the human GM-CSF cDNA from the pcD-hGM/Eo–CSF plasmid template. To amplify the human IL-2 cDNA from the pBC12/HIV/IL-2 plasmid template, two primers, 5′ - GGTAAAGCGGCCGCAGGAGGTGGTAGCGCACCTACTTCAAGTTCTACA - 3′ and 5′ - TCATGCGGCCGCTCAAGTTAGTGTTGAGATGATGCT - 3′, were used. The PCR fragments were each inserted into the Not I site of 12/chCLL-1/HL, resulting in the expression vectors 12/chCLL-1/HL/GM-CSF and 12/chCLL-1/HL/IL-2, encoding the chimeric light chain and a fusion protein consisting of the chimeric CLL-1 heavy chain with human GM-CSF or human IL-2 at its C-terminus.
Expression and Purification of Fusion Proteins
The fusion proteins were expressed from NS0 murine myeloma cells according to the protocol of the manufacturer (Celltech Biologics). Briefly, linearized plasmids were electroporated into NS0 cells, which were plated in nonselective Hybridoma-SFM medium. Selective glutamine-free medium was added 24 hours later. When transfectants appeared approximately 3 weeks later, supernatants were tested for the presence of chimeric fusion protein by indirect enzyme-linked immunosorbent assay (ELISA). The highest-producing clones were identified by 24-hour rate of production assays. To maximize the yield of chCLL-1/GM-CSF, amplification of vector copy number was achieved by expanding the clone and incubating the cells in increasing concentrations of methionine sulfoximine, a specific inhibitor of glutamine synthetase. Three to 4 weeks later, viable clones were again assayed for rate of chimeric fusion protein production. After subcloning by limiting dilution, the highest-producing clones were expanded, incubated in 10 L bioreactors, and chCLL-1/GM-CSF and chCLL-1/IL-2 were purified stepwise from cell culture medium by protein A affinity chromatography and ion-exchange chromatography, as described previously.24 The purity of each fusion protein was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a reducing gel according to the method of Laemmli27 and by high performance liquid chromatography (HPLC). The samples were filtered through a 0.22 μm Nalgene disposable filter unit before injection. The fusion proteins were analyzed with a Beckman HPLC Gold System (Beckman Instruments, Fullerton, CA) equipped with two 110B solvent pumps, a 210A valve injector, a 166 programmable UV detector, and a 406 analog interface module. Size exclusion chromatography was performed on a G4000SW column (TosoHaas; Montgomeryville, PA) with 0.1 mol/L PBS, pH 7.2 as the solvent system, eluting at a flow rate of 1 mL/min. The UV absorbance of the HPLC eluate was detected at 280 nm.
Immunoassays
ELISA.Chimeric fusion protein-containing supernatants were initially identified by indirect ELISA using murine Lym-2 antiidiotype 7E2 MoAb, as described previously.24 For production rate assays, 106 cells were plated in 1 mL of selective medium and allowed to incubate for 24 hours. ELISA was then performed as before. Supernatants were serially diluted and applied to wells of microtiter plates coated with goat antihuman IgG (H+L) (CalTag, South San Francisco, CA). Dilutions of a control chimeric antibody were used to generate a standard curve using 4-parameter fit by an automated ELISA reader (Bio-Tek Instruments, Inc, Winooski, VT), from which concentrations of unknowns were estimated. Rates of production expressed as μg/mL/106 cells/24 hours were compared to identify the highest producing clones.
ARH-77 cell competition radioimmunoassay.The antigen-binding activity of chCLL-1/GM-CSF and chCLL-1/IL-2 was determined by a competition radioimmunoassay for binding to fixed ARH-77 myeloma cells. For these studies, 2 × 106 ARH-77 cells previously fixed in 2% paraformaldehyde28 were incubated with 20 ng of 125I-labeled muLym-2 and serial dilutions of cold muLym-2, chCLL-1/GM-CSF, chCLL-1/IL-2, or an irrelevant MoAb (chLym-1). The cells and MoAbs or fusion proteins were incubated for 1 hour at room temperature with constant mixing. The cells were then washed twice, and the cell pellet-associated radioactivity was measured in a gamma counter. Maximal binding was determined from tubes containing no cold antibodies.
Determination of Avidity
To determine the avidity constants of chCLL-1/GM-CSF and chCLL-1/IL-2, a fixed cell radioimmunoassay was performed using the method of Frankel and Gerhard.29 Each experimental variable was run in duplicate. ARH-77 myeloma cell suspensions containing 106 cells/mL were incubated with 10 to 110 ng of 125I-labeled chCLL-1/GM-CSF or chCLL-1/IL-2 in 200 μL PBS for 1 hour at room temperature with constant mixing. The cells were then washed three times with PBS containing 1% bovine serum albumin to remove unbound antibody and counted in a gamma counter. The amount of fusion protein bound was then determined by the remaining cell-bound radioactivity (cpm) in each tube and the specific activity (cpm/ng) of the radiolabeled fusion protein. Scatchard plot analysis was used to obtain the slope. The equilibrium or avidity constant Ka was calculated by the equation K = −(slope/n), where n is the valence of the antibody (2 for IgG).
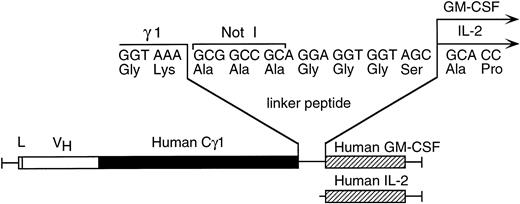
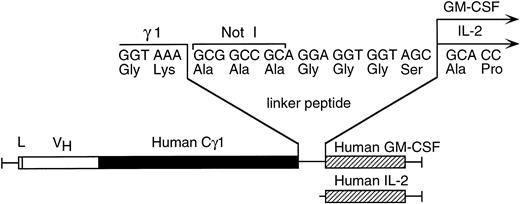
Schematic diagram depicting the linker containing the Not I cloning site between the human γ1 and human GM-CSF or human IL-2 cDNAs in the chimeric CLL-1 heavy chain/cytokine fusion genes.
Schematic diagram depicting the linker containing the Not I cloning site between the human γ1 and human GM-CSF or human IL-2 cDNAs in the chimeric CLL-1 heavy chain/cytokine fusion genes.
Isolation of Bone Marrow Cells
Bone marrow samples were obtained in preservative-free heparin from normal donors after receiving their informed consent (with the approval of UCLA Institutional Review Board). Cells were diluted with an equal volume of PBS containing 0.6% ACD-A (anticoagulant citrate dextrose solution, Formula A; Baxter-Fenwal Corp, Deerfield, IL) and mononuclear cells (MNC) were isolated by gradient centrifugation on Ficoll-Paque (Pharmacia LKB; Uppsala, Sweden) followed by two washes with PBS/ACD-A. CD34+ cells were purified from MNC using a CD34+ Progenitor Cell Isolation Kit (Miltenyi Biotec; Auburn, CA) without modification of the manufacturer's instructions.
Colony Assays
Bone marrow MNC (7.5 × 104 cells/well) or CD34+ cells (1 × 104 cells/well) were plated in triplicate in 24-well plates in semisolid medium containing 0.3% bacto agar in Iscove's Modified Dulbecco's Medium, 20% fetal bovine serum (Atlanta Biological, Norcross, GA), 50 μg/mL gentamicin, 0.4 mmol/L L-glutamine. Colony assays were supplemented with either hu-GM-CSF (generously provided by Amgen; Thousand Oaks, CA), chCLL-1/GM-CSF, or chCLL-1. Cultures were maintained humidified at 37°C in 5% CO2. Colonies containing more than 30 cells were enumerated after 14 to 16 days in culture.
IL-2 Bioassay
Biologic activity of chCLL-1/IL-2 was determined by a standard IL-2–dependent T-cell proliferation assay.30 Carrier-free recombinant IL-2 obtained from Hoffmann La Roche, Inc (Nutley, NJ) was used as a standard. Roche IL-2 stock (7.8 mg/mL, specific activity ≈12 × 106 IU/mg) was diluted to yield a stock solution containing 2 × 106 IU/mL. Growth of the IL-2–dependent murine T-cell line, CTLL-2, was used to determine the amount of IL-2 bioactivity in a sample. Briefly, serially diluted samples and standard were incubated with 2 × 104 CTLL-2 cells in triplicate for 20 hours at 37°C in 96-well flat bottom microtiter plates. The cells were then pulsed with 0.5 μCi of 3H-thymidine for 6 hours, and the samples were harvested and counted.
Cytotoxicity Assays
ADCC was performed using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI), which is a colorimetric assay that quantitatively measures lactate dehydrogenase release.31 Effector cells were peripheral blood MNC or neutrophilic polymorphonuclear leukocytes (PMN). MNC were isolated from healthy human donors by Ficoll-Paque gradient centrifugation, and PMN were purified by centrifugation through a discontinuous percoll gradient (70% and 62%) followed by hypotonic lysis to remove residual erythrocytes as described previously.32 ARH-77 myeloma cells were used as target cells. ARH-77 cells were suspended in Hybridoma-SFM medium supplemented with 2% fetal bovine serum and plated in 96-well V-bottom microtiter plates at 2 × 104 cells/well. Antibody or fusion protein preparations (chCLL-1/GM-CSF, chCLL-1/IL-2, chCLL-1, muLym-2, or chTNT-1 as an isotype-matched irrelevant control) were added in triplicate to individual wells at 1 μg/mL, and effector cells were added at various effector:target cell ratios (12.5:1 to 50:1). The plates were incubated for 4 hours at 37°C, after which the supernatants were harvested, lactate dehydrogenase release was determined, and % specific lysis was calculated according to the protocol of the manufacturer. Data are reported as mean ± standard deviation (SD). Differences between groups were analyzed by unpaired Student's t-test.
Pharmacokinetic and Biodistribution Studies
Six-week-old Balb/C mice were used to determine the pharmacokinetic clearance of chCLL-1, chCLL-1/GM-CSF and chCLL-1/IL-2. Groups of mice (n = 5) were administered intraperitoneal (IP) injections of 125I-labeled fusion proteins (30 to 40 μCi/mouse). The whole body activity at injection and at selected times thereafter was measured with a CRC-7 microdosimeter (Capintec, Inc, Pittsburgh, PA). The data were analyzed and half-lives were determined using the RSTRIP pharmacokinetic program (MicroMath, Inc, Salt Lake City, UT). To determine the tissue biodistribution of chCLL-1/GM-CSF and chCLL-1/IL-2, 6-week-old female athymic nude mice were irradiated with 400 rads from a cesium source, 3 days after which they were injected with a 0.2 mL inoculum containing 4 × 107 ARH-77 cells and 4 × 106 human fetal lung fibroblast feeder cells subcutaneously (SC) in the left thigh. The tumors were grown for 3 weeks until they reached approximately 1 cm in diameter. Within each group (n = 5), individual mice were injected intravenously (IV) with a 0.1 mL inoculum containing 100 μCi/10 μg of 125I-labeled fusion protein. Animals were killed by sodium pentobarbital overdose at 72 hours postinjection, and various organs, blood, and tumors were removed and weighed. The radioactivity in the samples was then measured in a gamma counter. For each mouse, data were expressed as percent injected dose/gram (% ID/g) and tumor:organ ratio (cpm per gram tumor/cpm per gram organ). From these data, the mean and SD were calculated for each group.
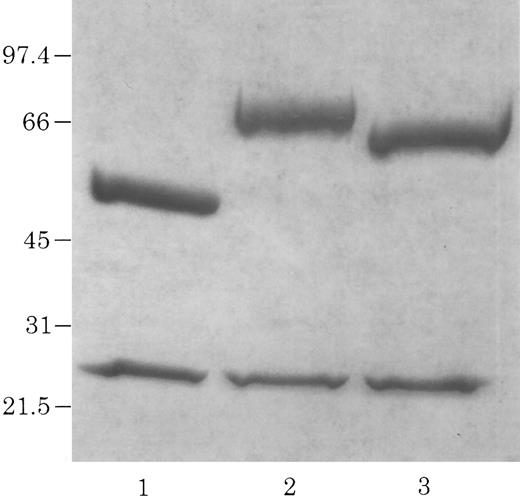
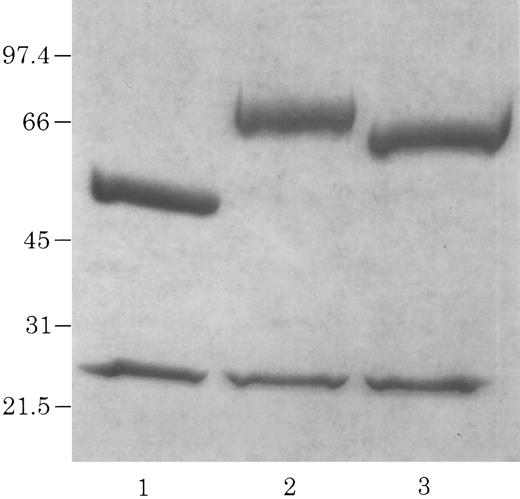
Electrophoretic identification of chCLL-1/cytokine fusion proteins. Coomassie Blue-stained 4% to 20% acrylamide gradient tris-glycine reduced gel of purified chCLL-1 (lane 1), chCLL-1/GM-CSF (lane 2), and chCLL-1/IL-2 (lane 3).
Electrophoretic identification of chCLL-1/cytokine fusion proteins. Coomassie Blue-stained 4% to 20% acrylamide gradient tris-glycine reduced gel of purified chCLL-1 (lane 1), chCLL-1/GM-CSF (lane 2), and chCLL-1/IL-2 (lane 3).
Imaging Studies
ARH-77 human myeloma tumors were grown in the left thighs of athymic nude mice as described above. When the tumors had reached approximately 1 cm in diameter, the mice were injected IV with a 0.1 mL inoculum containing 100 μCi/10 μg of 131I-labeled chCLL-1, chCLL-1/GM-CSF, or chCLL-1/IL-2. At 1, 3, and 5 days postinjection, the mice were anesthetized with a SC injection of 0.8 mg sodium pentobarbital. The immobilized mice were then imaged in a prone position with a Spectrum 91 camera equipped with a pinhole collimator (Raytheon Medical Systems, Melrose Park, IL) set to record 5,000 to 10,000 counts using the Nuclear MAX Plus image analysis software package (MEDX Inc, Wood Dale, IL).
RESULTS
Construction, Expression, and Purification of chCLL-1/GM-CSF and chCLL-1/IL-2
A Not I site was previously appended immediately downstream of the terminal codon of the human γ1 sequence by PCR. A PCR fragment containing either the human GM-CSF cDNA or the human IL-2 cDNA preceded by a seven amino acid linker peptide was then inserted into the Not I site, producing CLL-1 VH/human γ1/human GM-CSF or CLL-1 VH/human γ1/human IL-2 fusion genes (Fig 1). This resulted in the expression vectors 12/chCLL-1/HL/GM-CSF and 12/chCLL-1/HL/IL-2, encoding the chimeric light chain and a fusion protein consisting of the chimeric CLL-1 heavy chain with human GM-CSF or human IL-2 at its C-terminus. The fusion proteins were expressed from NS0 murine myeloma cells using the glutamine synthetase gene amplification system (Celltech Biologics). After subjection to vector amplification, the highest chCLL-1/GM-CSF–producing subclone secreted approximately 26 μg/mL/106 cells/24 hours in static culture. The highest chCLL-1/IL-2–producing subclone expressed approximately 16 μg/mL/106 cells/24 hours. Upon scale-up, greater than 100 μg/mL of chCLL-1/GM-CSF were obtained after purification. When the chCLL-1/IL-2–producing cell line was grown in a 10-L bioreactor, approximately 70 μg/mL of fusion protein were obtained. Both chimeric antibody fusion proteins were properly assembled as demonstrated by reducing SDS-PAGE; two well-defined bands were resolved for chCLL-1/GM-CSF at approximately 25 and 66 kD and for chCLL-1/IL-2 at approximately 25 and 65 kD, corresponding to the molecular weights of the immunoglobulin light chain and heavy chain plus cytokine (Fig 2). Both fusion proteins appeared as a single peak by HPLC analysis (data not shown).
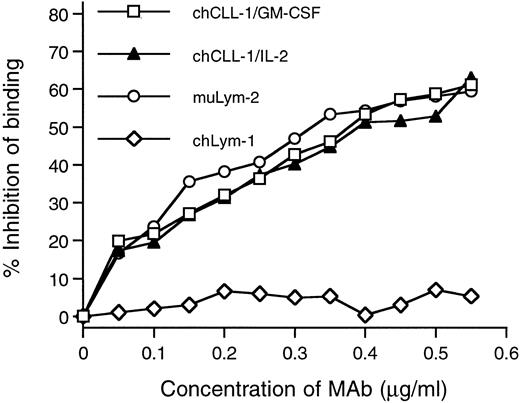
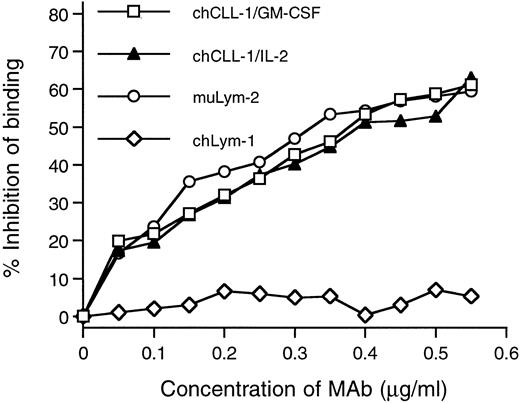
Competitive binding radioimmunoassay with chCLL-1/GM-CSF and chCLL-1/IL-2. Purified antibody fusion proteins were assayed for their ability to inhibit the binding of 125I-labeled muLym-2 to ARH-77 human myeloma cells. muLym-2 and chLym-1 served as positive and negative controls, respectively.
Competitive binding radioimmunoassay with chCLL-1/GM-CSF and chCLL-1/IL-2. Purified antibody fusion proteins were assayed for their ability to inhibit the binding of 125I-labeled muLym-2 to ARH-77 human myeloma cells. muLym-2 and chLym-1 served as positive and negative controls, respectively.
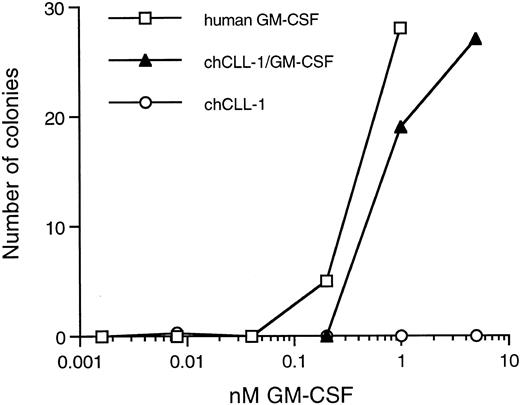
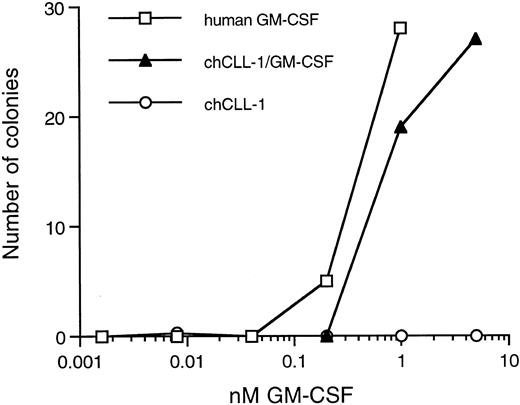
Colony-forming activity of chCLL-1/GM-CSF. Various concentrations of recombinant human GM-CSF, chCLL-1/GM-CSF, or chCLL-1 were cultured with 7.5 × 104 bone marrow MNC in triplicate in semisolid medium for 14 to 16 days at 37°C until colonies containing more than 30 cells formed.
Colony-forming activity of chCLL-1/GM-CSF. Various concentrations of recombinant human GM-CSF, chCLL-1/GM-CSF, or chCLL-1 were cultured with 7.5 × 104 bone marrow MNC in triplicate in semisolid medium for 14 to 16 days at 37°C until colonies containing more than 30 cells formed.
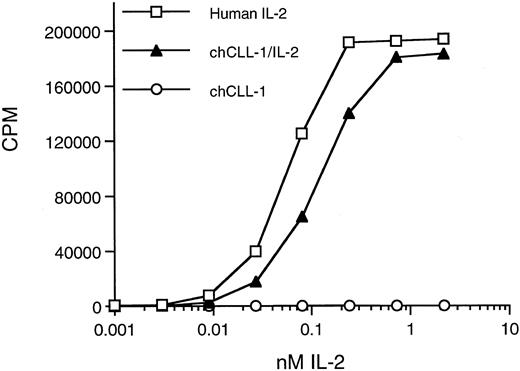
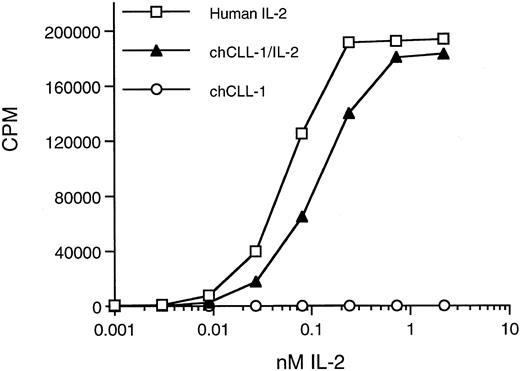
Biologic activity of chCLL-1/IL-2 as determined by the ability to support proliferation of CTLL-2 cells. Serial dilutions of chCLL-1/IL-2, chCLL-1, or recombinant IL-2 standard were incubated with 2 × 104 CTLL-2 cells in triplicate for 20 hours at 37°C. The cells were pulsed with 0.5 μCi of 3H-thymidine for 6 hours, and the samples were harvested and counted.
Biologic activity of chCLL-1/IL-2 as determined by the ability to support proliferation of CTLL-2 cells. Serial dilutions of chCLL-1/IL-2, chCLL-1, or recombinant IL-2 standard were incubated with 2 × 104 CTLL-2 cells in triplicate for 20 hours at 37°C. The cells were pulsed with 0.5 μCi of 3H-thymidine for 6 hours, and the samples were harvested and counted.
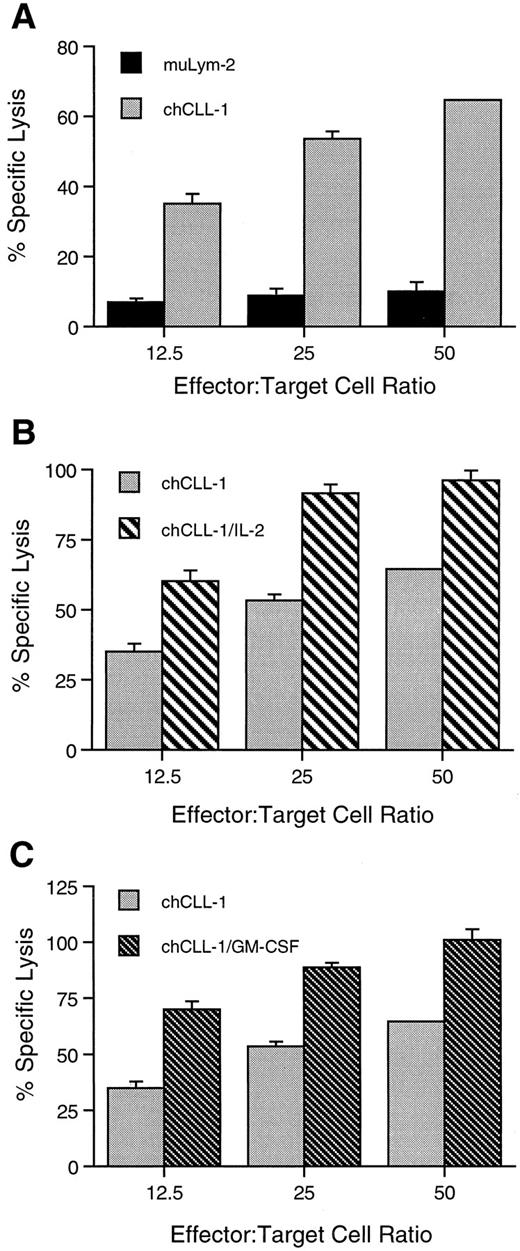
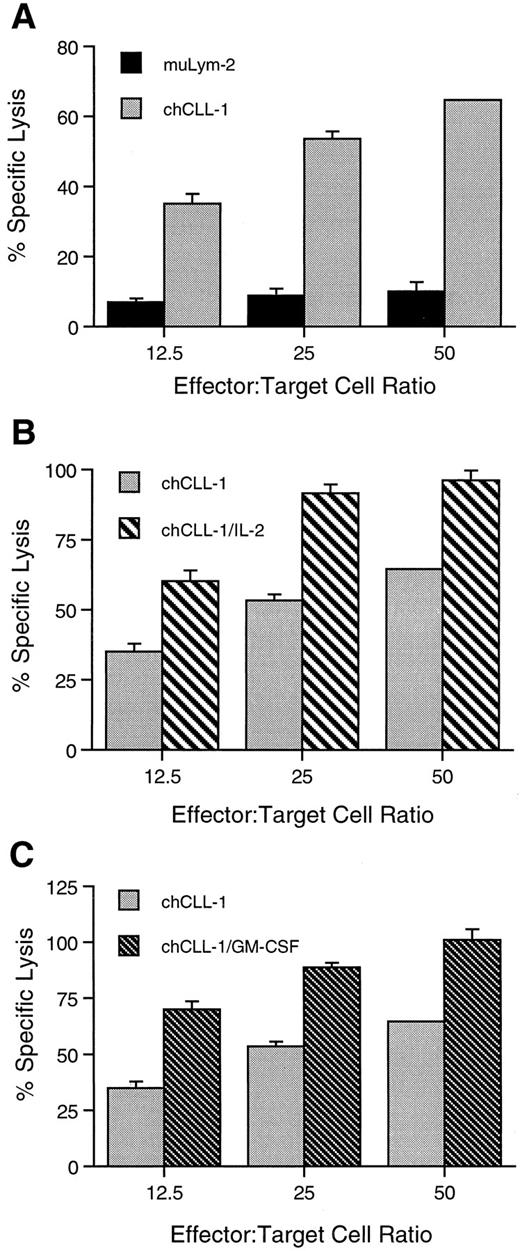
ADCC activity of chCLL-1 and fusion proteins. MoAb or fusion protein (1 μg/mL) was cultured with ARH-77 human myeloma target cells and human mononuclear effector cells at varying effector:target cell ratios as indicated. (A) Comparison between ADCC mediated by muLym-2 and chCLL-1. (B) Comparison between ADCC mediated by chCLL-1 and chCLL-1/IL-2. (C) Comparison between ADCC mediated by chCLL-1 and chCLL-1/GM-CSF. Specific lysis with the isotype-matched negative control (chTNT-1) was <5% (data not shown). Expressed as mean ± SD. At each effector:target cell ratio, the difference between pairs is significant (P < .001).
ADCC activity of chCLL-1 and fusion proteins. MoAb or fusion protein (1 μg/mL) was cultured with ARH-77 human myeloma target cells and human mononuclear effector cells at varying effector:target cell ratios as indicated. (A) Comparison between ADCC mediated by muLym-2 and chCLL-1. (B) Comparison between ADCC mediated by chCLL-1 and chCLL-1/IL-2. (C) Comparison between ADCC mediated by chCLL-1 and chCLL-1/GM-CSF. Specific lysis with the isotype-matched negative control (chTNT-1) was <5% (data not shown). Expressed as mean ± SD. At each effector:target cell ratio, the difference between pairs is significant (P < .001).
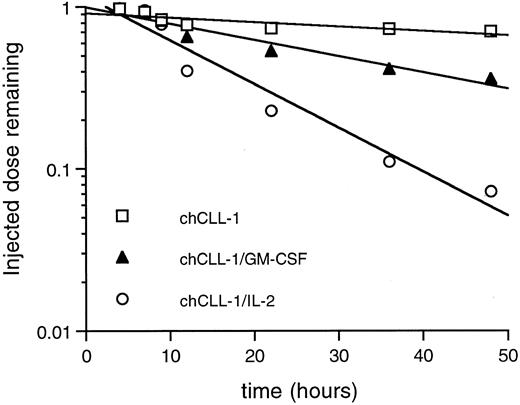
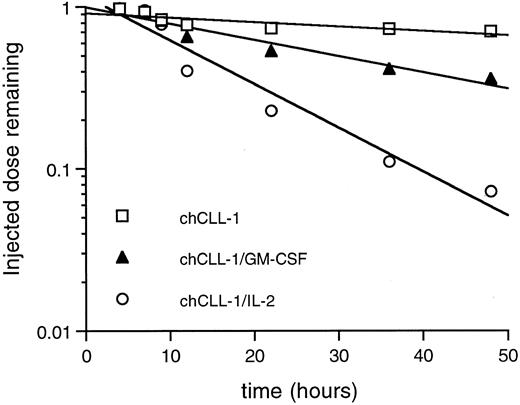
Whole body pharmacokinetic clearance of 125I-labeled chCLL-1, chCLL-1/GM-CSF, and chCLL-1/IL-2 in nontumor-bearing mice. Activity at injection and at selected times thereafter was measured with a microdosimeter.
Whole body pharmacokinetic clearance of 125I-labeled chCLL-1, chCLL-1/GM-CSF, and chCLL-1/IL-2 in nontumor-bearing mice. Activity at injection and at selected times thereafter was measured with a microdosimeter.
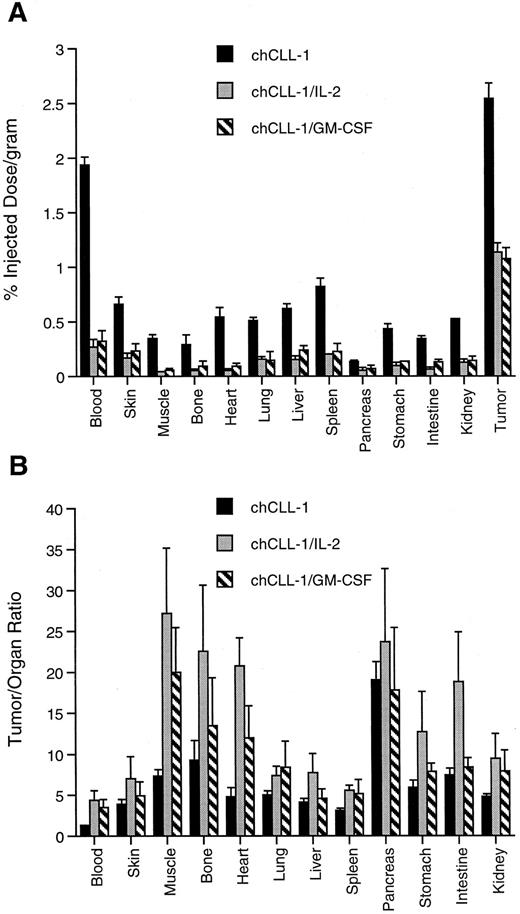
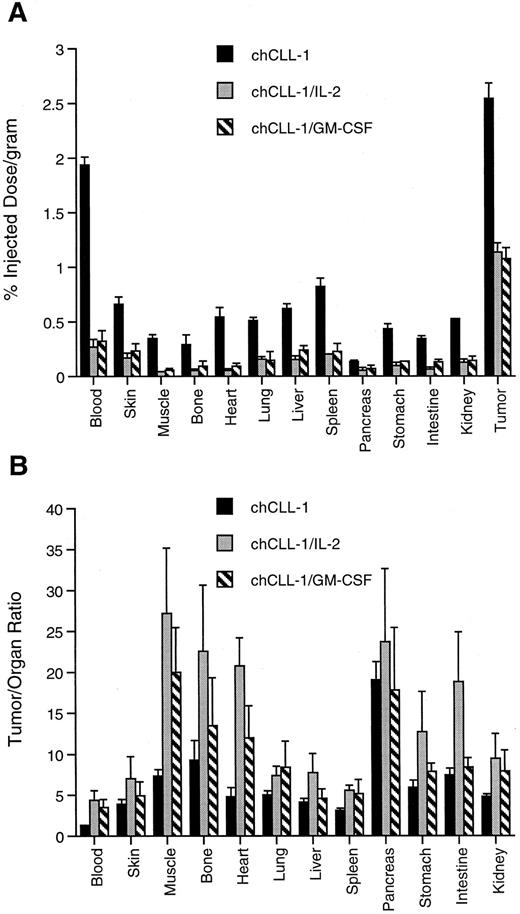
Tissue biodistribution and tumor uptake of chCLL-1, chCLL-1/GM-CSF, and chCLL-1/IL-2 at 72 hours postinjection in ARH-77 myeloma tumor-bearing nude mice. (A) Tumor uptake measured by percent injected dose/gram of 125I-labeled MoAb or fusion protein in the indicated tissues. (B) Tumor:organ ratios expressed as mean ± SD.
Tissue biodistribution and tumor uptake of chCLL-1, chCLL-1/GM-CSF, and chCLL-1/IL-2 at 72 hours postinjection in ARH-77 myeloma tumor-bearing nude mice. (A) Tumor uptake measured by percent injected dose/gram of 125I-labeled MoAb or fusion protein in the indicated tissues. (B) Tumor:organ ratios expressed as mean ± SD.
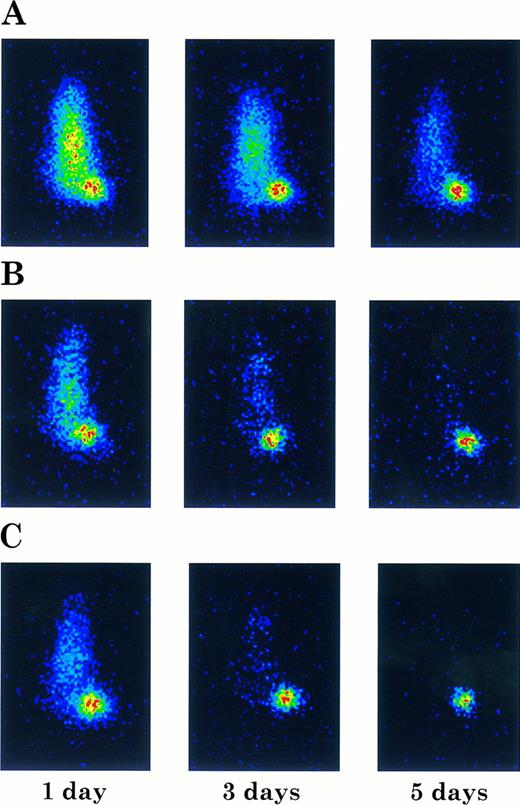
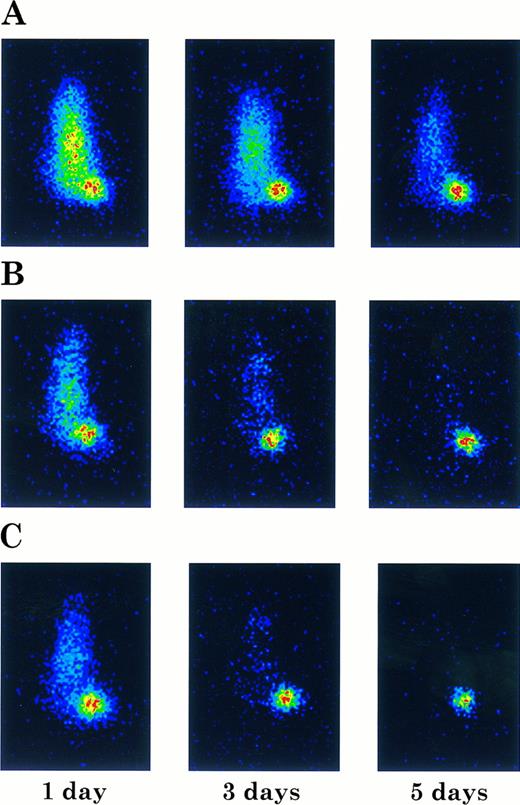
Imaging of ARH-77 myeloma tumor-bearing nude mice injected with 131I-labeled chCLL-1 (A), chCLL-1/GM-CSF (B), or chCLL-1/IL-2 (C). Mice were imaged in a prone position at the indicated times postinjection.
Imaging of ARH-77 myeloma tumor-bearing nude mice injected with 131I-labeled chCLL-1 (A), chCLL-1/GM-CSF (B), or chCLL-1/IL-2 (C). Mice were imaged in a prone position at the indicated times postinjection.
Immunobiochemical Analysis
The immunoreactivity of purified chCLL-1/GM-CSF and chCLL-1/IL-2 with the target antigen of muLym-2 was assessed by determining the binding to antigen-bearing ARH-77 myeloma cells. In a radioimmunoassay, increasing concentrations of chCLL-1/GM-CSF, chCLL-1/IL-2, muLym-2, or an irrelevant MoAb (chLym-1) were evaluated for their ability to inhibit the binding of 125I-labeled muLym-2 to ARH-77 cells (Fig 3). Because it binds to a nonoverlapping epitope, chLym-1 was unable to compete with 125I-labeled muLym-2, but chCLL-1/GM-CSF and chCLL-1/IL-2 inhibited 125I-labeled muLym-2 binding to ARH-77 cells. These studies confirm that chCLL-1/GM-CSF and chCLL-1/IL-2 maintain the immunoreactivity of muLym-2.
Avidity binding studies were then conducted in which 125I-labeled chCLL-1/GM-CSF or chCLL-1/IL-2 was incubated with ARH-77 cells and the bound radioactivity used to calculate the avidity constant Ka by Scatchard analysis as described in the Materials and Methods. chCLL-1/GM-CSF and chCLL-1/IL-2 had similar binding constants of 3.3 × 108 mol/L−1 and 3.0 × 108 mol/L−1, respectively. The binding constant of muLym-2 was determined to be 2.9 × 108 mol/L−1. These studies demonstrate that the presence of the cytokines on the C-terminus of the heavy chain does not affect binding to the antigenic target.
Colony-Forming Activity of chCLL-1/GM-CSF
Biologic activity of the GM-CSF moiety was determined by colony assays using both bone marrow MNC and CD34+ cells. As indicated in Fig 4, chCLL-1/GM-CSF compares favorably with recombinant human GM-CSF in its ability to stimulate colony formation from the MNC fraction of normal bone marrow. In addition, the fusion protein is capable of inducing the formation of colonies from isolated CD34+ progenitor cells (data not shown). No colonies formed in the presence of chCLL-1.
IL-2 Bioactivity of chCLL-1/IL-2
Biologic activity of the IL-2 moiety was determined by assaying the ability of chCLL-1/IL-2 to support IL-2-dependent T-cell proliferation. A bioassay with the IL-2–dependent CTLL-2 line was performed in which chCLL-1/IL-2 was assayed along with chCLL-1 and the IL-2 standard (Fig 5). On a molar basis, chCLL-1/IL-2 had approximately 50% of the activity required to produce 50% maximum proliferation of the IL-2-dependent cell line compared with the recombinant IL-2 standard. This corresponds to a specific activity of approximately 8 × 105 IU/mg of fusion protein. At higher concentrations (eg, >1 nmol/L), maximum proliferation was achieved as evidenced by the plateau of the incorporation of 3H-thymidine into DNA. As expected, chCLL-1 had no activity.
Cytotoxicity Studies
chCLL-1/GM-CSF, chCLL-1/IL-2, chCLL-1, and muLym2 were evaluated for their ability to mediate ADCC by colorimetric lactate dehydrogenase release assays against ARH-77 myeloma target cells. At a concentration of 1 μg/mL, chCLL-1 mediated 65% cytotoxicity, while muLym-2 mediated only 10% specific lysis of tumor cells by human MNC at an effector:target cell ratio of 50:1 (Fig 6A). At the same effector:target cell ratio, both fusion proteins mediated approximately 100% specific lysis of target cells (Fig 6B and C). Similar enhancement of specific lysis mediated by both fusion proteins over chCLL-1 and by chCLL-1 over muLym-2 can be seen at lower effector:target cell ratios. The isotype-matched irrelevant control (chTNT-1) mediated <5% specific lysis at all effector:target cell ratios (data not shown). Neither the fusion proteins nor antibodies mediated specific lysis of target cells by human PMN at an effector:target cell ratio of 50:1 (<5%, data not shown).
In Vivo Pharmacokinetic and Tumor Targeting Studies
Whole body clearance studies were performed to establish differences in pharmacokinetics among chCLL-1/GM-CSF, chCLL-1/IL-2, and chCLL-1. Mice were injected with 125I-labeled fusion proteins or chimeric antibody, and the whole body activity at injection and selected times thereafter was measured with a microdosimeter. chCLL-1/IL-2 cleared rapidly with a whole body half-life of 11 hours (Fig 7). chCLL-1/GM-CSF had a half-life of approximately 30 hours, while chCLL-1 cleared slowly, with a half-life of 100 hours.
The difference among clearance rates was evident when tumor and normal organ biodistribution was examined in ARH-77 myeloma-bearing nude mice. As indicated in Fig 8A, tumor uptake of chCLL-1 after 72 hours was 2.54% ± 0.14% injected dose/gram, while tumor uptake of chCLL-1/IL-2 and chCLL-1/GM-CSF was significantly lower (1.14 ± 0.08 and 1.07 ± 0.10, respectively; P < .001). However, uptake of the fusion proteins in normal tissues was considerably lower than chCLL-1, which can be attributed to the rapid clearance of the fusion proteins. This low normal tissue uptake produces higher tumor/organ ratios, as can be seen in Fig 8B.
Imaging studies were also performed to examine tumor targeting with the fusion proteins. Tumor-bearing nude mice were injected with 131I-labeled chimeric antibody or fusion protein and imaged at 1, 3, and 5 days postinjection. In Fig 9, the difference in clearance is manifested by the unambiguous localization of the fusion proteins to the tumor after 24 hours, while the mouse injected with chCLL-1 demonstrated high signal throughout the body at 1 and 3 days postinjection. Nevertheless, by day 5, localization of chCLL-1 to the tumor site is clear. In mice that received chCLL-1/GM-CSF or chCLL-1/IL-2, by day 5 no signal remained except in the tumor. These data demonstrate that chCLL-1/GM-CSF and chCLL-1/IL-2 effectively localize to the ARH-77 human myeloma xenografts.
DISCUSSION
In this study, recombinant fusion proteins containing the chimeric MoAb CLL-1 and human GM-CSF or IL-2 have been generated, which retain both tumor targeting and cytokine functions. The GS gene amplification system was used for high level expression of the fusion proteins from myeloma cells so that large-scale production can yield sufficient products to enable clinical studies to be undertaken. With this expression system, gram quantities of the fusion proteins can be produced in batch cultures. Biochemical analysis demonstrates the presence of two GM-CSF or IL-2 molecules per chimeric antibody molecule (Fig 2). GM-CSF or IL-2 is located at the C-terminus of the heavy chain following a short linker peptide to facilitate proper folding of the cytokine. The immunoreactivity of the fusion proteins was retained, as evidenced by competition with 125I-labeled muLym-2 for binding to antigen-bearing ARH-77 myeloma cells (Fig 3). Moreover, the binding affinity of the fusion proteins was unaffected by the presence of the cytokine molecules. In addition, the biological activity of the cytokines within the fusion proteins was confirmed by appropriate assays; chCLL-1/GM-CSF possesses colony-forming activity (Fig 4), while chCLL-1/IL-2 is able to support the proliferation of an IL-2–dependent T-cell line (Fig 5).
Cytotoxicity studies clearly demonstrate the improved effector functions of chCLL-1 over muLym-2 and of both fusion proteins over chCLL-1 (Fig 6). Human IgG1 constant regions were selected for construction of the chimeric MoAb based on earlier observations of the enhanced antitumor cytotoxic activity of chimeric IgG1 over chimeric MoAbs of other isotypes.33 At each effector:target cell ratio, chCLL-1/IL-2 mediates higher specific tumor lysis by human MNC than the chimeric MoAb alone (Fig 6B). This is in agreement with previous reports of augmented MNC ADCC either by free recombinant IL-24-7,9 or by a recombinant MoAb/IL-2 fusion protein.34,35 chCLL-1/GM-CSF also mediates higher specific tumor lysis by human MNC than chCLL-1 (Fig 6C). Ragnhammar et al36 have previously shown that short-term preincubation of MNC with GM-CSF enhances ADCC against colorectal carcinoma and lymphoma cell lines. Other investigators have observed no effect of GM-CSF on MNC ADCC against malignant B-cell lines.9 There is evidence that GM-CSF and IL-2 can act synergistically in vitro. In this regard, GM-CSF has been shown to augment the induction of lymphokine-activated killer (LAK) activity by IL-2 against a human Burkitt's lymphoma cell line through monocytes.37 In addition, GM-CSF and IL-2 enhance ADCC against a colorectal carcinoma cell line,38 leading the investigators to suggest combination therapy consisting of low dose IL-2, GM-CSF, and MoAb.
No specific lysis of target cells by PMN in ADCC mediated by chCLL-1 or chCLL-1/GM-CSF was observed in our studies. It has recently been demonstrated that antibodies recognizing HLA class II mediate lysis of malignant B-cell lines by PMN, while antibodies to other B-cell antigens fail to mediate such ADCC.32 In these studies, Lym-1 and 1D10, both of which recognize HLA class II related epitopes, did not mediate ADCC by PMN from healthy donors, although both MoAbs mediated ADCC with PMN from patients treated with granulocyte colony-stimulating factor. Similar results have been observed with Lym-1 in combination with GM-CSF.9 GM-CSF has also been shown to enhance PMN ADCC against solid tumor cell line targets, including neuroblastoma, melanoma, and colorectal carcinoma.36,39,40 It is as yet unclear why MoAbs directed against particular antigens on malignant B cells possess the ability to mediate PMN ADCC, while those with specificity for other B-cell antigens do not. Based on in vitro ADCC data and clinical experience with a murine MoAb, a clinical trial using the combination of the MoAb and GM-CSF for the treatment of metastatic colorectal carcinoma was initiated.41 In this study, complete remissions were achieved in some patients, providing clinical evidence for the benefit of combination therapy.
In the current study, pharmacokinetic analysis in Balb/C mice demonstrated the marked difference in whole body clearance among chCLL-1 and the fusion proteins (Fig 7). We have recently shown that a fusion protein consisting of chLym-1 and IL-2 has a half-life of 11 hours,35 which is identical to that observed for chCLL-1/IL-2. chCLL-1/GM-CSF has a whole body half-life intermediate between the chimeric MoAb and the IL-2–containing fusion protein. The relatively longer half-life of a GM-CSF–containing antibody fusion protein compared with those containing other cytokines has previously been described for the antiganglioside MoAb ch14.18.42 It has yet to be demonstrated whether similar differences in clearance between chimeric MoAbs and cytokine-containing antibody fusion proteins exist in patients. Biodistribution and imaging studies in human myeloma-bearing nude mice illustrate the tumor targeting abilities of chCLL-1/IL-2 and chCLL-1/GM-CSF (Figs 8 and 9). Despite their rapid clearance profiles, they retain the capacity to localize to tumor xenografts effectively. In fact, such rapid clearance might be beneficial in clinical applications, wherein potentially injurious cytokine exposure to normal tissues would be minimized. This is particularly true for IL-2, which induces a capillary leak syndrome when administered systemically in high doses.43-46
There is considerable evidence that high local concentrations of cytokines within tumors can stimulate antitumor immunity and rejection in animal models. The majority of such efforts have employed gene transfection to engineer tumor cell lines to secrete cytokines.10-17 Although these studies demonstrate the utility of delivering cytokines directly to tumors, they are presently impractical in the clinical setting. A more feasible approach to generating high local concentrations of cytokines within tumors is targeting cytokines via antibody fusion proteins.18,19 This approach combines the cytotoxicity that MoAbs can mediate against tumor targets with the host antitumor immune response, which is stimulated by high local concentrations of cytokines. Several groups have taken such an approach to delivering cytokines by engineering fusion proteins consisting of IL-2 and antibody fragments including F(ab′)19 and single-chain antibodies.47-49 Intact MoAbs may have greater effectiveness than fragments, however, because they can mediate ADCC. An alternative approach that also employs antibody-cytokine fusion proteins is engineering a cancer vaccine using idiotype-cytokine fusion proteins including IL-2 and GM-CSF.50,51 In a murine B-cell lymphoma model, such fusion proteins have been shown to induce antitumor responses. The efficacy of antibody-targeted IL-2 has been elegantly demonstrated in both a SCID mouse human neuroblastoma model20,52 and a syngeneic murine melanoma model.53,54 In these studies, the effector cell population responsible for antitumor responses was identified as CD8+ T cells. As the fusion protein retained a therapeutic effect in natural killer (NK) cell-deficient mice, the investigators concluded that tumor eradication was not dependent on NK cells.55 Whether such a mechanism of antitumor cytotoxicity holds for other antibody-cytokine fusion proteins in the treatment of other malignancies remains to be determined.
The chimeric antibody fusion proteins described in the current study have the potential for producing tumor killing by a number of mechanisms. The parent muLym-2 is reactive with a majority of human B-cell lymphomas, chronic lymphocytic leukemias, and multiple myeloma,21 suggesting that this MoAb and derivatives may be of use in treating a variety of B-cell malignancies. Both muLym-2 and chCLL-1 have a direct inhibitory effect on human lymphoma cell lines and can improve the survival of SCID mice injected with human lymphoma cells (Funakoshi et al, in preparation). Furthermore, both chCLL-1/GM-CSF and chCLL-1/IL-2 mediate enhanced ADCC against a human myeloma cell line. Finally, the combination of GM-CSF and IL-2 targeted to the tumor site may be sufficient to bring about the induction of effective cytotoxic T-cell responses. As the antigen recognized by chCLL-1 is not present in animal lymphomas and hence a syngeneic model is unavailable in which to evaluate immune responses induced by chCLL-1/GM-CSF and chCLL-1/IL-2, clinical trials will be undertaken to test the immunotherapeutic efficacy of these novel reagents against human B-cell malignancies.
ACKNOWLEDGMENT
The authors wish to thank Barbara H. Biela, Jahangir Sharifi, and Myra M. Mizokami for assistance with the animal studies.
Supported in part by Cancer Therapeutics, Inc (Los Angeles, CA), Techniclone Corp (Tustin, CA), Brilliance Pharmaceuticals (Shanghai, China), and a grant from Children's Cancer Research Fund, the Hedberg Foundation (Minneapolis, MN).
Address reprint requests to Alan L. Epstein, MD, PhD, Department of Pathology, University of Southern California School of Medicine, 2011 Zonal Ave, HMR 210, Los Angeles, CA 90033.