Abstract
Bcl-2 is an oncogene that confers deregulated growth potential to B lymphocytes through its ability to inhibit apoptotic cell death. A specific molecular activity for the Bcl-2 protein has not been identified, but several lines of evidence have supported a role in protection of cells from oxidative stress. We investigated whether there is a correlation between expression of high levels of Bcl-2 and susceptibility of human Burkitt's lymphoma cell lines to H2O2 -induced killing. The amount of H2O2 required to kill 50% of cells in 24 hours varied widely in the seven different lymphoma cell lines that were tested, ranging from 35 to 500 μmol/L H2O2 . However, expression of high levels of endogenous Bcl-2 did not protect the cells from H2O2 -induced killing, even though it was effective in protecting the cells from apoptosis induced by agents such as A23187. Thus, Bcl-2 was functional in preventing apoptosis but did not act in an antioxidant capacity. The results were confirmed using a Burkitt's lymphoma cell line overexpressing transfected bcl-2. The results may be explained by the observation that H2O2 was inefficient at inducing apoptosis in these mature B-cell lines. Nonapoptotic death induced by H2O2 was not prevented by Bcl-2.
THE bcl-2 ONCOGENE is thought to confer oncogenic potential to cells by inhibiting programmed cell death, or apoptosis.1 It was first discovered by virtue of its translocation (t[14; 18]) and elevated expression in follicular B-cell lymphomas.2 Subsequent studies have shown that it is also expressed during normal B- and T-cell development.3-5 Transfection and overexpression of the bcl-2 gene protects a variety of different cell types from induction of apoptotic cell death.6-9 Treatments that induce apoptosis and from which Bcl-2 protects include calcium ionophores, serum and growth factor depletion, glucocorticoids, and γ-irradiation.7,8,10,11 The mechanism by which the Bcl-2 protein extends cell survival in the face of such a wide variety of treatments is unclear. No biochemical activity has thus far been ascribed to the oncoprotein and it has little or no significant homology with other known cellular proteins.12,13 Early reports indicated that Bcl-2 is localized to the mitochondrial inner membrane,6 suggesting that Bcl-2 might act by modulating mitochondrial function. However, Bcl-2 is also found in the endoplasmic reticulum and nuclear envelope.14 15
One theory of how Bcl-2 functions suggests that it acts by protecting cells from oxidative stress. It may do this by reducing cellular generation of reactive oxygen compounds or by blocking the activity of these compounds after they are formed.16,17 Oxidants such as superoxide and hydrogen peroxide (H2O2 ) are generated by a variety of conditions,18 including activation of phagocytes during inflammation19,20 and from reperfusion of ischemic tissue.21,22 Intracellular oxidants are generated by electron leakage from the electron transport chain23 and by the action of mixed function oxidases within the cell.22,24 Hockenbery et al17 found that overexpression of the Bcl-2 protein in a murine pro-B–cell line protected those cells from killing induced by treatment with high levels of reagent H2O2 . Kane et al16 found that overexpression of Bcl-2 in a neural cell line protected the cells from killing induced by depletion of endogenous glutathione. Other studies have supported these initial findings (reviewed in Korsmeyer et al25 ). However, the theory that Bcl-2 acts through an antioxidant mechanism has recently been challenged by studies showing that oxygen depletion has no effect on the induction of apoptosis and that Bcl-2 protects against apoptosis without inhibiting the production or activity of reactive oxygen compounds.26 27
Almost all of the investigations showing that Bcl-2 protects cells from oxidant-induced killing were performed with cell lines transfected with an expression vector carrying an exogenous bcl-2 gene.16,17,28-31 In this report, we address the question of whether Burkitt's lymphoma (BL) cell lines that express different levels of endogenous Bcl-2 show a differential susceptibility to oxidant-induced killing. BL is a high-grade B-cell lymphoma that is distinguished by the presence of chromosomal translocations resulting in activation of the c-myc oncogene.32,33 This tumor is of germinal center origin,32 similar to follicular lymphoma, but is otherwise phenotypically and genotypically distinct.34,35 For example, unlike follicular lymphoma, elevated Bcl-2 expression is not generally found in primary BL tumors, but it may appear in some tumors upon relapse.36 Thus, activation of bcl-2 may lead to tumor progression in BL, but it is not a feature of primary BL development. The level of Bcl-2 expression varies among established BL cell lines in tissue culture depending on the stage at which it was introduced into culture and the Epstein-Barr virus (EBV) infection status37-40; infection of BL cell lines with EBV can lead to induction of the bcl-2 oncogene and to failure to undergo apoptosis.37,40 In contrast to bcl-2, mutations in the p53 gene are a common feature of primary BL tumors and cell lines.41 42
Using BL cell lines that were matched for their p53 and EBV status, we found no correlation between the level of expression of endogenous Bcl-2 and resistance to H2O2 -induced killing. In fact, H2O2 was the only agent against which a Bcl-2hi BL cell line was not resistant. The results with endogenous Bcl-2 were confirmed using cells overexpressing transfected Bcl-2. The data argue against the generalization that Bcl-2 protects B cells from oxidant-induced cell killing.
MATERIALS AND METHODS
Cells.The Burkitt's lymphoma cell lines used in this study were provided by Dr Kishor Bhatia (National Cancer Institute, National Institutes of Health, Bethesda, MD). Lymphoblastoid cell lines were derived in the laboratory of Dr Giovanna Tosato (Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, MD). The mutation status of the p53 gene of these cell lines is taken from previous reports.43-45 Cells were grown in RPMI 1640 containing 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, and 50 μmol/L β-mercaptoethanol at 37°C in 5% CO2 in air.
Cell treatments.Exponentially growing cells (7.5 to 12 × 105 cells/mL) were harvested and resuspended in fresh growth media (10% FCS media except for the serum depletion experiments) to achieve a culture density 5 × 105 cells/mL. H2O2 was added to the cell suspensions at the beginning of the experiments and the cells were incubated for 6 to 48 hours as indicated in the text. The calcium ionophore A23187 (Calbiochem, San Diego, CA) was dissolved in dimethyl sulfoxide to make a 10 mmol/L stock solution that was stored in the dark at 4°C. It was added to cell suspensions (5 × 105 cell/mL) to achieve final concentrations of 0.5 to 2.0 μmol/L. γ-Irradiation was performed using a 137Cs irradiator (Nordion 137Cs Gammacell Elite; Nordion International, Kanada, Ontario, Canada) at a dose rate of 22.2 rads/s. Cells in fresh growth media (5 × 105 cells/mL) were irradiated at room temperature for 27 seconds (600 rads) or 54 seconds (1,200 rads) and incubated for 12 to 72 hours before harvesting. For serum depletion experiments, cells were harvested, washed with phosphate-buffered saline (PBS), resuspended in RPMI 1640 containing 0.2% or 1% heat-inactivated FCS, and incubated for 24 to 72 hours as indicated in the text. Etoposide was prepared as a 10 mg/mL stock solution in PBS. All incubations were performed at 37°C in 5% CO2 in air.
Viability assay.Cell viability was determined by trypan blue exclusion. Cells that were treated with various agents and incubated for the times indicated were suspended in an equal volume of 0.4% trypan blue. Dead (blue) and live (clear) cells were counted using a hemacytometer. The percentage of viability is defined as the number of live cells divided by the number of live and dead cells.
H2O2 assay.Cells were suspended at a density of 5 × 105 cells/mL in PBS containing 1 mg/mL glucose and 0.2 mg/mL phenol red.46 At time 0, H2O2 was added to each cell suspension and the mixtures were incubated at 37°C for 0 to 3 hours. Aliquots were taken at timed intervals and the cells were removed immediately by centrifugation. Horseradish peroxidase (50 μg/mL final concentration) was added to the cell supernatants and the samples were incubated at room temperature for approximately 5 minutes. NaOH was then added to a final concentration of 20 mmol/L and the resulting color was allowed to stabilize for at least 5 minutes. The absorbance of reduced phenol red (A610 ) was measured on a Hewlett Packard 8452A diode array spectrophotometer (Hewlett Packard Inc, Palo Alto, CA). The concentration of H2O2 was calculated based on standard curves using reagent H2O2 .
Western blotting analysis.Cells were harvested by centrifugation; washed once with cold PBS; resuspended in lysis buffer containing 62.5 mmol/L Tris (pH 6.8), 2% sodium dodecyl sulfide (SDS), 10% glycerol, and 1 mmol/L diethylenetriaminepentaacetic acid; and heated for 12 minutes at 100°C. The total cell lysates (∼50,000 cells per lane; 5 to 20 μg protein) were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (4% to 12% gradient SDS-polyacrylamide gels; Novex, San Diego, CA).47 The protein bands were then transferred electrophoretically48 to Immobilon membranes (Millipore, Bedford, MA). After electrotransfer, the membrane was blocked for 1 hour in 3% bovine serum albumin at room temperature (for monoclonal antibody treatments) or overnight at 4°C in 5% skim milk (for polyclonal antibody treatments). Primary antibodies were incubated overnight at 4°C (for monoclonals) or for 1 hour at room temperature (for polyclonals). Anti–Bcl-2 (monoclonal mouse antibody to a synthetic peptide sequence comprising amino acids 41-54 of human Bcl-2; DAKO, Carpinteria, CA) was used at 1:10,000, anti-Bax (rabbit polyclonal antibody against amino acids 11-30 of human Bax; Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:4,000, and anti–Bcl-x (rabbit polyclonal antibody against amino acids 2-19 of human Bcl-x; Santa Cruz Biotechnology) was used at 1:400. Immunoreactivity was visualized using peroxidase detection systems and chemiluminescence (Renaissance kit; Dupont NEN, Boston, MA). X-ray films were scanned (Microtek Scanmaker, Microte K Inc, Redondo Beach, CA) and analyzed using the Macintosh desitometry program IMAGE (National Institutes of Health, Bethesda, MD).
Assessment of apoptosis by agarose gel electrophoresis.The fragmentation of total cellular DNA was analyzed by the procedure of Smith et al.49 The extracts from 106 cells were subjected to electrophoresis (30 V, 12 to 15 mA, 16 hours) on 2% (wt/vol) agarose gels containing 0.1 μg/mL ethidium bromide. DNA was visualized by fluorescence under UV light. Molecular weight standards (HaeIII-digested φ X174; New England Biolabs, Beverly, MA) were run in adjacent lanes.
Assessment of apoptosis by TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-X nick end labeling) and flow cytometry.The TUNEL assay used in this study was adapted from Gold et al,50 with little modification. Cell samples (2 × 106/mL) were fixed by mixing with PBS containing 2.5% formaldehyde and incubating for 30 minutes at room temperature. The fixed cells were washed with fluorescence-activated cell sorting (FACS) buffer (PBS containing 0.5% bovine serum albumin and 0.01% sodium azide) and permeabilized with 0.1% Triton X-100/0.1% sodium citrate for 2 minutes on ice. After washing with FACS buffer, the permeabilized cells were resuspended in apoptosis cell labeling mixture consisting of 1× terminal deoxynucleotidyl transferase (TdT) buffer, 25 U of TdT enzyme, 250 μmol/L CoCl2 , 0.6 μmol/L fluorescein-dUTP, and deoxynucleotide mixture (dATP, dGTP, and dCTP at 2 μmol/L and dTTP at 1.4 μmol/L) and then incubated at 37°C for 1 hour. All reagents were purchased from Boehringer Mannhein (Indianapolis, IN). Reactions were stopped by adding 1 mL FACS buffer. The cells were washed once more, resuspended in 1 mL FACS buffer, and analyzed (10,000 cells per sample) on a FACScan (Becton Dickinson, San Jose, CA) using CELLQUEST flow cytometric analysis software.51 The fluorescein-dUTP incorporated into the cells was detected using a 530/30-nm bandpass filter (FL1 channel). The percentage of apoptosis was calculated as the number of cells in the high fluorescence intensity population (reflecting fluorescein dUTP incorporation) divided by the total number of cells analyzed. In the absence of TdT, there was no shift in fluorescent staining upon treatment of the cells with any agent. Forward and side light scatter (FSC and SSC) were also measured for each sample and analyzed according to Dive et al.52
Morphologic assessment of apoptosis using Hoechst/propidium iodide nuclear staining and fluorescence microscopy.Cells (∼5 × 106 cells/mL) were incubated for 15 minutes at 37°C with Hoechst 33342 dye (5 μg/mL in PBS), centrifuged, washed once in PBS, and then resuspended at an approximate density of 2.5 × 107 cells/mL. Propidium iodide (50 μg/mL from a 1 mg/mL stock in PBS) was added just before microscopy. Cells were visualized using a Nikon Optiphot microscope equipped with a fluorescent light source and a UV-2A filter cube (Nikon, Japan); (excitation wavelength, 330 to 380 nm; barrier filter, 420 nm). Cell morphology was scored as follows. (1) Viable cells had blue-stained normal, smooth nuclei. (2) Viable, apoptotic cells had blue-stained nuclei with multiple bright specks of condensed chromatin. (3) Nonviable, necrotic cells had red-stained, smooth nuclei that were roughly the same size as normal (control) nuclei. (4) Nonviable, apoptotic cells had red-stained nuclei with either multiple bright specks of fragmented chromatin or one or more spheres of condensed chromatin (significantly more compact than normal nuclei). Samples were randomized and examined after blinding. At least 200 cells for each treatment were counted. Experiments were repeated at least three different times.
Transfection of BL cells with bcl-2. A human bcl-2 expression plasmid was prepared by subcloning a 910-bp EcoRI complementary DNA from the plasmid pB4 (American Type Culture Collection, Rockville, MD) into the pcDNA3.1 expression vector (Invitrogen, San Diego, CA). The resulting recombinant plasmid pcDNA3.1–bcl-2 and the parental vector were linearized with Sca I and transfected into JLP 119 cells (20 μg DNA/6 × 106 cells) by electroporation (200 V, 1,600 μF ). Stably transfected cells were selected with 1 mg/mL G418 (GIBCO BRL, Gaithersburg, MD), expanded, and screened for expression of the Bcl-2 protein by Western blotting.
RESULTS
Susceptibility of BL cell lines to killing by H2O2 .The goal of this research was to identify tumor cells with differing degrees of resistance to oxidative stress so that the cellular pathways responsible for conferring resistance could be examined. To this end, nine different cell lines were seeded at a density of 5 × 105 cells/mL and exposed to a bolus addition of different concentrations of H2O2 (50 to 500 μmol/L). The panel of cell lines examined included Burkitt's lymphoma cell lines that are infected (SHO) or not infected (BJAB, ST-486, JLP 119, EW-36, CA-46, and JD-38) with EBV and cells that contain only wild-type (wt) p53 (JLP 119, EW-36, and SHO) or either one (BJAB and ST-486) or two (CA-46 and JD-38) mutated (mut) alleles of p53. EBV-immortalized lymphoblastoid cell lines (LCLs; Tory and VDSO) were used as nontumor controls. Viabilities were assessed by trypan blue staining after 24 hours of incubation. As shown in Fig 1, a range of different susceptibilities to H2O2 -induced killing was observed. LD50 values (concentration of H2O2 required to kill 50% of the cells) ranged from approximately 35 to 500 μmol/L H2O2 . SHO (EBV-positive, p53 wt/wt) and BJAB (EBV-negative, p53 wt/mut) were the most resistant to H2O2 -induced killing (LD50 , ∼500 μmol/L), followed by the LCLs (Tory and VDSO; LD50s, ∼350 μmol/L). CA-46 (EBV-negative, p53 mut/mut) showed an intermediate level of resistance to H2O2 -induced killing (LD50 , ∼100 μmol/L). The remaining lymphoma cell lines were relatively sensitive to H2O2 treatment (LD50s, ∼35 to 75 μmol/L). In general, there was no correlation between p53 mutation status and susceptibility to H2O2 toxicity.
Relative susceptibility and resistance of BL cell lines and LCLs to killing by H2O2 . Cells were seeded at a density of 5 × 105/mL in growth medium. H2O2 was added as a bolus at the indicated concentrations. After 24 hours of incubation at 37°C, the number of live and dead cells was determined using trypan blue. The percentage of viability is defined as the number of live cells divided by the number of live plus dead cells. Also indicated are the p53 mutation (m indicates a mutated allele) and EBV infection status (+ indicates infected) for each cell line. Cell line (EBV, p53): (⊡) SHO (+, w/w); (▴) BJAB (−, w/m); (×) Tory (+, w/w); (▪) VDSO (+, w/w); (▵) CA-46 (−, m/m); (○) EW-36 (−, w/w); (⊞) JD-38 (−, m/m); (•) JLP 119 (−, w/w); (□) ST-486 (−, w/m).
Relative susceptibility and resistance of BL cell lines and LCLs to killing by H2O2 . Cells were seeded at a density of 5 × 105/mL in growth medium. H2O2 was added as a bolus at the indicated concentrations. After 24 hours of incubation at 37°C, the number of live and dead cells was determined using trypan blue. The percentage of viability is defined as the number of live cells divided by the number of live plus dead cells. Also indicated are the p53 mutation (m indicates a mutated allele) and EBV infection status (+ indicates infected) for each cell line. Cell line (EBV, p53): (⊡) SHO (+, w/w); (▴) BJAB (−, w/m); (×) Tory (+, w/w); (▪) VDSO (+, w/w); (▵) CA-46 (−, m/m); (○) EW-36 (−, w/w); (⊞) JD-38 (−, m/m); (•) JLP 119 (−, w/w); (□) ST-486 (−, w/m).
To determine whether different levels of expression of Bcl-2 might be responsible for the observed differences in the H2O2 -sensitivity of Burkitt's lymphoma cells, Bcl-2 protein was assayed in cell extracts by Western blot immunoassay using a monoclonal antibody to human Bcl-2 (Fig 2). The EBV-immortalized LCLs (Tory and VDSO) were used as positive controls because they are known to express elevated levels of Bcl-2.38 39 As shown in Fig 2, the Bcl-2 level varied among the BL cell lines: EW-36 expressed an unusually high level of Bcl-2 protein, whereas JD-38 and SHO expressed moderately elevated levels that were similar to those found in the LCLs. All of the other cell lines showed little or no Bcl-2 expression. There was no clear correlation between the level of expression of Bcl-2 and either the EBV infection status (eg, compare SHO with EW-36 and JD-38) or the p53 mutation status.
Western blot analysis of Bcl-2 levels in BL cell lines and EBV-transformed LCLs. Total cell extracts from equal numbers of cells (∼50,000) were loaded in each lane. The figure shows immunoblot results obtained using a monoclonal antibody to human Bcl-2 (see the Materials and Methods). Also indicated are the p53 mutation (m indicates presence of 1 or 2 mutated alleles) and EBV infection status (+ indicates infected) for each cell line.
Western blot analysis of Bcl-2 levels in BL cell lines and EBV-transformed LCLs. Total cell extracts from equal numbers of cells (∼50,000) were loaded in each lane. The figure shows immunoblot results obtained using a monoclonal antibody to human Bcl-2 (see the Materials and Methods). Also indicated are the p53 mutation (m indicates presence of 1 or 2 mutated alleles) and EBV infection status (+ indicates infected) for each cell line.
As shown in Fig 3, the relative susceptibilities of the different cell lines to H2O2 -induced killing (as defined by LD50 values) did not correlate with the respective levels of Bcl-2 expression. EW-36, which expresses Bcl-2 at unusually high levels, and JD-38, which expresses a moderately elevated level of Bcl-2, were among the most sensitive cell lines (LD50s, ∼75 μmol/L), whereas BJAB and CA-46, which do not show detectible Bcl-2, were relatively resistant to H2O2 treatment (LD50 , ∼500 and 150 μmol/L, respectively). Treatment with H2O2 did not alter the steady-state Bcl-2 protein levels in the cells (data not shown). There was no apparent correlation between the presence of a mutated p53 allele and resistance to H2O2 -induced killing.
Correlation between susceptibility to H2O2 toxicity and the level of expression of Bcl-2 protein. The data are compiled from Figs 1 and 2. LD50 is defined as the concentration of H2O2 that kills 50% of the cells after 24 hours of incubation. The level of Bcl-2 protein expression was unaffected by H2O2 treatment (not shown).
Correlation between susceptibility to H2O2 toxicity and the level of expression of Bcl-2 protein. The data are compiled from Figs 1 and 2. LD50 is defined as the concentration of H2O2 that kills 50% of the cells after 24 hours of incubation. The level of Bcl-2 protein expression was unaffected by H2O2 treatment (not shown).
The different BL cell lines were tested for their ability to degrade H2O2 to establish whether differences in their antioxidant defense systems (eg, peroxidase activities) might be affecting their susceptibility to H2O2 toxicity. The ability of the cells to deplete H2O2 from the surrounding medium was measured by the phenol red assay,46 as described in Materials and Methods. As shown in Fig 4, there was very little difference in the rate of removal of H2O2 by the BL cell lines. The cell line with the highest Bcl-2 level (EW-36) was somewhat (∼2-fold) slower in removing H2O2 than the other cell lines, but there was no consistent correlation between the level of expression of Bcl-2 and the rate of removal of H2O2 . JD-38 has an intermediate level of Bcl-2 expression, but removed H2O2 at the same rate as cell lines with little or no Bcl-2 protein expression. All six lines removed at least 80% of the H2O2 within 2 hours. The viability assays shown in Fig 1 were conducted 18 to 24 hours after the addition of H2O2 .
Depletion of H2O2 by BL cell lines. The ability of BL cell lines to degrade H2O2 (peroxidase-like activity) was assessed as described in the Materials and Methods. A concentration of 100 μmol/L H2O2 was added to each cell suspension (5 × 105 cells/mL). After various times of incubation at 37°C, aliquots were removed and the medium was assayed for the presence of residual H2O2 . The X's show the stability of H2O2 incubated in medium in the absence of cells. (▵) ST-486; (○) CA-46; (•) BJAB; (⋄) JD-38; (▪) EW-36; (□) JLP 119; (×) H2O2 only.
Depletion of H2O2 by BL cell lines. The ability of BL cell lines to degrade H2O2 (peroxidase-like activity) was assessed as described in the Materials and Methods. A concentration of 100 μmol/L H2O2 was added to each cell suspension (5 × 105 cells/mL). After various times of incubation at 37°C, aliquots were removed and the medium was assayed for the presence of residual H2O2 . The X's show the stability of H2O2 incubated in medium in the absence of cells. (▵) ST-486; (○) CA-46; (•) BJAB; (⋄) JD-38; (▪) EW-36; (□) JLP 119; (×) H2O2 only.
Correlation between Bcl-2 expression and protection from apoptosis induced by calcium ionophore, serum depletion, and γ-irradiation.One possible explanation for the lack of protection of BL cells from H2O2 -induced killing could be that the Bcl-2 expressed in these cells is not functional. Because activity of Bcl-2 is defined by its ability to inhibit apoptosis, we tested this possibility by subjecting cells to treatments that have been shown to induce apoptosis in different cells, including BL cell lines.10,11 53 Among these are the calcium ionophore A23187, serum depletion, and γ-irradiation.
To eliminate possible confounding effects caused by EBV infection and p53 mutations, two cell lines that express widely different levels of Bcl-2 but are both EBV-negative and p53 wild-type were compared. These are EW-36 (Bcl-2hi) and JLP 119 (Bcl-2lo). The LD50 values for H2O2 -induced killing in these two cell lines differed by less than twofold, whereas the Bcl-2 levels differed by more than 20-fold. There was no difference in the levels of expression of other Bcl-2 family members, Bax54 and Bcl-x55 (Fig 5).
Western blot analysis of Bax and Bcl-x levels in JLP 119 and EW-36. Total cell extracts from 50,000 cells were loaded in each lane. The figures show typical immunoblot results obtained using a polyclonal antibody to human Bax or human Bcl-x (see the Materials and Methods). The cell line BJAB was found to express high levels of bcl-xL and is shown as a positive control.
Western blot analysis of Bax and Bcl-x levels in JLP 119 and EW-36. Total cell extracts from 50,000 cells were loaded in each lane. The figures show typical immunoblot results obtained using a polyclonal antibody to human Bax or human Bcl-x (see the Materials and Methods). The cell line BJAB was found to express high levels of bcl-xL and is shown as a positive control.
Apoptosis was assayed by induction of DNA ladders using agarose gel electrophoresis49 and a TUNEL method coupled with FACS analysis.50 Cell viability was determined by trypan blue exclusion. This latter assay is not ideal, because cells at the early stages of apoptosis do not manifest membrane leakage and may still have the ability to exclude vital dye.56 57 Thus, trypan blue exclusion underestimates cell death when it occurs by an apoptotic mechanism and the values obtained can only be viewed as estimates. As shown in Fig 6A, A23187 (1 and 2 μmol/L), serum depletion (reduction from 10% to 1% or 0.2%), and γ-irradiation (600 and 1,200 rads) killed both cell lines in a concentration/dose-dependent manner. However, JLP 119 was significantly more sensitive to all treatments than EW-36. The biggest difference in viability between the two cell lines was seen with A23187, which is also the agent that induced the most profound degree of apoptosis. Agarose gel electrophoresis of total DNA (Fig 6B) shows that cell killing by these agents is mainly apoptotic in JLP 119. In contrast, EW-36 was resistant to induction of apoptosis.
Effect of A23187, serum depletion, and γ-irradiation on cell viability or induction of DNA fragmentation in EW-36 and JLP 119. Cells (5 × 105/mL) were treated with A23187 (1 or 2 μmol/L), serum depletion (reduced from 10% to either 1% or 0.2%), or γ-irradiation (600 or 1,200 rads). After 48 hours of incubation, cell viability was determined by trypan blue exclusion (A). Fragmentation of total cellular DNA (1 × 106 cells) was assessed by agarose gel electrophoresis (B). The viability results (▪, EW-36; , JLP 119) represent the mean ± SEM of quadruplicate (A23187) or duplicate (serum depletion and γ-irradiation) experiments. The DNA fragmentation data shown are from a representative study that was repeated two to four times.
Effect of A23187, serum depletion, and γ-irradiation on cell viability or induction of DNA fragmentation in EW-36 and JLP 119. Cells (5 × 105/mL) were treated with A23187 (1 or 2 μmol/L), serum depletion (reduced from 10% to either 1% or 0.2%), or γ-irradiation (600 or 1,200 rads). After 48 hours of incubation, cell viability was determined by trypan blue exclusion (A). Fragmentation of total cellular DNA (1 × 106 cells) was assessed by agarose gel electrophoresis (B). The viability results (▪, EW-36; , JLP 119) represent the mean ± SEM of quadruplicate (A23187) or duplicate (serum depletion and γ-irradiation) experiments. The DNA fragmentation data shown are from a representative study that was repeated two to four times.
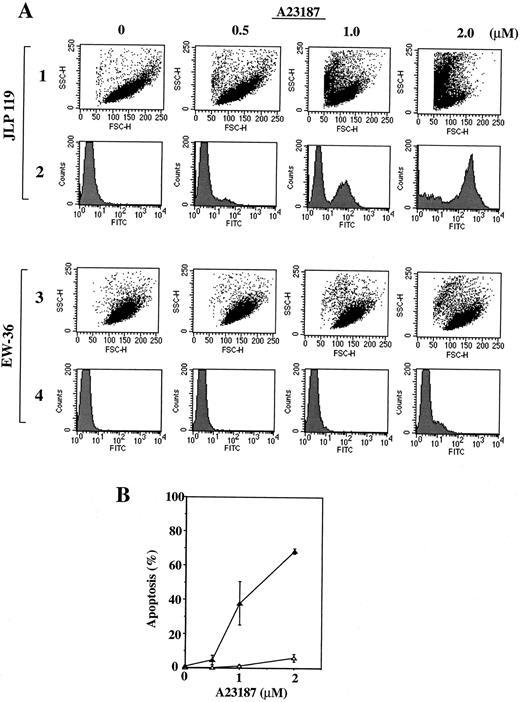
The results obtained by agarose gel electrophoresis were confirmed by FACS analysis of cells treated with A21387. Two types of measurements were performed; a TUNEL assay was used to quantify apoptotic cells,50 whereas forward and side light scatter were used to estimate the number of total live and dead cells.52 Cells incorporating high levels of fluorescein isothiocyanate (FITC)-labeled dUTP in the presence of terminal deoxynucleotidyl transferase (TUNEL assay) represent apoptotic cells containing fragmented DNA. Cells exhibiting high forward scatter (FSC) and low side scatter (SSC) are considered to be live cells, whereas those exhibiting low FSC and high SSC are considered to be dead.52 When these analyses were performed on JLP 119 and EW-36 treated with A23187, the results shown in Fig 7A were obtained. A23187 induced a significant, concentration-dependent incorporation of FITC-conjugated dUTP into the DNA of JLP 119 cells and caused a clear shift in the cell population from one of high FSC/low SSC (live cells) to one of low FSC/high SSC (dead cells). In contrast, identical treatment of EW-36 had no such effect; there was little incorporation of FITC-labeled dUTP and the cell population remained in the high FSC/low SSC region of the scattergram. The percentage of apoptotic cells induced by treatment of both cell lines with different concentrations of A23187 was quantified from the TUNEL assay and the results are shown in Fig 7B. Roughly 70% of JLP 119 cells underwent apoptosis in response to 2 μmol/L A23187 compared with only 5% of EW-36 cells.
Flow cytometric analysis of JLP 119 and EW-36 treated with A23187. (A) Flow cytometer plots. Cells (5 × 105/mL) were incubated in growth medium containing the indicated concentrations of A23187 (0, 0.5, 1.0, or 2.0 μmol/L) for 48 hours. FACS analysis of fixed cells was performed as described in the Materials and Methods. Rows 1 and 3, cytograms showing forward and side light scatter (FSC and SSC, respectively) for 10,000 cellular events. Rows 2 and 4, histograms of FITC-labeled DNA for the cells shown in the cytograms. Rows 1 and 2, results for JPL-119. Rows 3 and 4, results for EW-36. The population with high FITC intensity in each histogram (right-hand peak) represents apoptotic cells. The data shown are from a representative study that was repeated three times. (B) Proportion of JLP 119 (▴) and EW-36 (▵) cells that are undergoing apoptosis at each concentration of A23187. The percentage of apoptosis equals the high FITC-labeled population divided by the total cell population. Each point is derived from 10,000 cellular events and represents the mean ± SEM of triplicate cultures.
Flow cytometric analysis of JLP 119 and EW-36 treated with A23187. (A) Flow cytometer plots. Cells (5 × 105/mL) were incubated in growth medium containing the indicated concentrations of A23187 (0, 0.5, 1.0, or 2.0 μmol/L) for 48 hours. FACS analysis of fixed cells was performed as described in the Materials and Methods. Rows 1 and 3, cytograms showing forward and side light scatter (FSC and SSC, respectively) for 10,000 cellular events. Rows 2 and 4, histograms of FITC-labeled DNA for the cells shown in the cytograms. Rows 1 and 2, results for JPL-119. Rows 3 and 4, results for EW-36. The population with high FITC intensity in each histogram (right-hand peak) represents apoptotic cells. The data shown are from a representative study that was repeated three times. (B) Proportion of JLP 119 (▴) and EW-36 (▵) cells that are undergoing apoptosis at each concentration of A23187. The percentage of apoptosis equals the high FITC-labeled population divided by the total cell population. Each point is derived from 10,000 cellular events and represents the mean ± SEM of triplicate cultures.
The ability of the TUNEL apoptosis assay to reflect differences in cellular Bcl-2 levels was confirmed using two additional cell lines: JD-38, which expresses an intermediate level of Bcl-2, and ST-486, in which little or no Bcl-2 is detected. As shown in Fig 8, JD-38 was much more resistant to A23187-induced apoptosis than ST-486 but was more sensitive than EW-36, which expresses the highest bcl-2 levels.
Correlation between TUNEL activity and the level of Bcl-2 expressed in BL cells. The flow cytometric TUNEL assay was performed and analyzed on the cell lines indicated in the figure as described in the legend to Fig 7. The relative levels of Bcl-2 expression (see Fig 2) were EW-36 << JD-38 <<ST-486 ∼ JLP-119. (•) ST-486; (○) JLP 119; (▴) JD-38; (▵) EW-36.
Correlation between TUNEL activity and the level of Bcl-2 expressed in BL cells. The flow cytometric TUNEL assay was performed and analyzed on the cell lines indicated in the figure as described in the legend to Fig 7. The relative levels of Bcl-2 expression (see Fig 2) were EW-36 << JD-38 <<ST-486 ∼ JLP-119. (•) ST-486; (○) JLP 119; (▴) JD-38; (▵) EW-36.
Inefficient induction of apoptosis in BL cell lines by H2O2 .The comparisons mentioned above show that the Bcl-2hi expressor cell line EW-36 is more resistant to being killed by A23187, serum depletion, and γ-irradiation than JLP 119. However, EW-36 was highly sensitive to toxicity from H2O2 (see Fig 1). Because Bcl-2 is believed to protect cells primarily from apoptosis, we investigated whether the reason for the discrepancy might derive from the mechanisms of cell killing, ie, that H2O2 kills by inducing necrosis and not apoptosis. The following experiments show that this is partly correct. When total DNA was extracted from cells treated with H2O2 and checked for the presence of ladders by agarose gel electrophoresis, we found that H2O2 induced little if any of the DNA fragmentation that is characteristic of apoptosis in either the low or high Bcl-2 expressor. Because it is thought that apoptotic death occurs at low levels of cellular damage, whereas necrotic death is induced under more extreme conditions,58 we tested concentrations of H2O2 (25 to 100 μmol/L) that show a range of degrees of cell killing (see Fig 1): 25 μmol/L H2O2 , which kills less than 10% of the cells; 50 μmol/L H2O2 , which induces moderate killing (20% to 60%); and 75 and 100 μmol/L H2O2 , which kill the majority (∼80%) of the cells. As shown in Fig 9, there was no detectible DNA fragmentation in EW-36 at any concentration of H2O2 tested. Fragmentation of a relatively small portion of the DNA of JLP 119 cells treated with 50 or 75 μmol/L H2O2 was seen, but most of the DNA still remained in the high molecular weight fraction. At 100 μmol/L H2O2 , more than 80% of cells from both lines were dead, but the DNA still remained in the high molecular weight fraction, suggesting that most of the cell killing by H2O2 was nonapoptotic. This poor induction of DNA ladders by H2O2 was seen additionally with four other lymphoma cell lines (CA-46, BJAB, JD-38, and ST-486; data not shown). This phenomenon is in stark contrast to the effects of A23187 and serum depletion, which both led to complete loss of high molecular weight DNA in JLP 119 at the highest concentrations tested (see Fig 6B). The low level of induction of nucleosomal DNA fragmentation by H2O2 was confirmed by the TUNEL assay coupled with FACS analysis (Fig 10). Increasing the concentration of H2O2 induced significant shifts in the cell populations of both JLP 119 and EW-36 from high FSC/low SSC to low FSC/ high SSC populations, consistent with the results obtained by trypan blue counting. However, only a small number of cells (≤20%) from either cell line showed significantly increased incorporation of FITC-dUTP, indicating that most of the cells did not exhibit the DNA fragmentation characteristic of death by apoptosis. The Bcl-2hi cell line showed the same amount of TUNEL activity as the Bcl-2lo cell line, indicating that Bcl-2 had no effect on this activity.
Effect of H2O2 concentration on induction of DNA fragmentation in EW-36 and JLP 119. Cells (5 × 105/mL) were incubated for 24 hours in growth medium containing the indicated concentrations of H2O2 (0, 25, 50, 75, or 100 μmol/L). Fragmentation of total cellular DNA (extract of 1 × 106 cells/lane) was assessed by agarose gel electrophoresis. The data shown are from a representative study that was repeated three times.
Effect of H2O2 concentration on induction of DNA fragmentation in EW-36 and JLP 119. Cells (5 × 105/mL) were incubated for 24 hours in growth medium containing the indicated concentrations of H2O2 (0, 25, 50, 75, or 100 μmol/L). Fragmentation of total cellular DNA (extract of 1 × 106 cells/lane) was assessed by agarose gel electrophoresis. The data shown are from a representative study that was repeated three times.
Flow cytometric analysis of JLP 119 and EW-36 treated with different concentrations of H2O2 . (A) Flow cytometer data. Cells (5 × 105/mL) were incubated in growth medium for 24 hours after the addition of the indicated concentrations of H2O2 (0, 25, 50, 75, or 100 μmol/L). FACS analysis of fixed cells was performed as described in the Materials and Methods. Rows 1 and 3, cytograms showing forward and side light scatter (FSC and SSC, respectively) for 10,000 cellular events. Rows 2 and 4, histograms of FITC-labeled DNA for the cells shown in the cytograms. Rows 1 and 2, results for JPL-119. Rows 3 and 4, results for EW-36. Cells with a high FITC-labeling intensity represent apoptotic cells. The data shown are from a representative study that was repeated three times. (B) Proportion of JLP 119 (▴) and EW-36 (▵) cells that are undergoing apoptosis at each concentration of H2O2 . The percentage of apoptosis equals the high FITC-labeled population divided by the total cell population. Each point is derived from 10,000 cellular events and represents the mean ± SEM of triplicate cultures.
Flow cytometric analysis of JLP 119 and EW-36 treated with different concentrations of H2O2 . (A) Flow cytometer data. Cells (5 × 105/mL) were incubated in growth medium for 24 hours after the addition of the indicated concentrations of H2O2 (0, 25, 50, 75, or 100 μmol/L). FACS analysis of fixed cells was performed as described in the Materials and Methods. Rows 1 and 3, cytograms showing forward and side light scatter (FSC and SSC, respectively) for 10,000 cellular events. Rows 2 and 4, histograms of FITC-labeled DNA for the cells shown in the cytograms. Rows 1 and 2, results for JPL-119. Rows 3 and 4, results for EW-36. Cells with a high FITC-labeling intensity represent apoptotic cells. The data shown are from a representative study that was repeated three times. (B) Proportion of JLP 119 (▴) and EW-36 (▵) cells that are undergoing apoptosis at each concentration of H2O2 . The percentage of apoptosis equals the high FITC-labeled population divided by the total cell population. Each point is derived from 10,000 cellular events and represents the mean ± SEM of triplicate cultures.
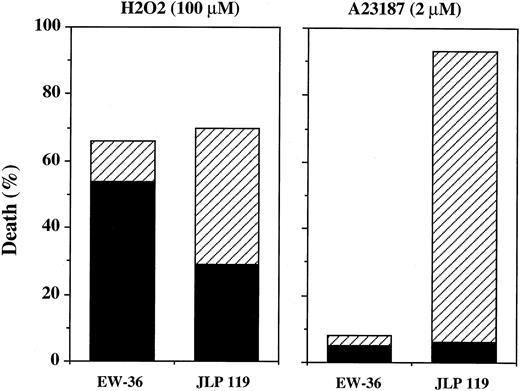
Morphologic assessment of apoptosis.Both of the assays described above measure apoptosis based on induction of nucleosomal DNA fragmentation. Because A23187 produced a positive response using both methods, we conclude that Burkitt's lymphoma cells can be induced to undergo nucleosomal DNA fragmentation but that H2O2 is inefficient at accomplishing this. However, these results do not rule out the possibility that H2O2 induces other features of apoptosis that may be independent of endonuclease activity.59-61 This possibility was examined using nuclear staining and fluorescence microscopy to look for the ability of H2O2 to induce the morphologic changes that define apoptotic death.62 Hoechst 33342 is a blue fluorescent dye that is permeable to both live and dead cells, whereas propidium iodide (red fluorescence) only enters cells in which the membrane integrity has been damaged (defined as dead). Based on this assay, we found that A23187 (2 μmol/L) induced substantial nuclear fragmentation in 87% of JLP 119 but only 3% of EW-36 cells. Very little necrotic cell death (∼5%) was observed in either cell line after treatment with A23187. In contrast, H2O2 induced morphologic changes characteristic of both apoptosis and necrosis in JLP 119, whereas the predominant form of death in EW-36 was necrotic (Fig 11). It should be pointed out that, in general, the morphologic changes induced by H2O2 were atypical. Nuclei classified as apoptotic were cleaved into 2 or 3 spheres that were significantly larger than the small fragments induced by A23187. Intact nuclei classified as necrotic were not necessarily larger than control (untreated) nuclei but were not adequately condensed to be defined as apoptotic .
Quantitation of extent and type of H2O2 - and A23187-induced cell death induced in BL cell lines by Hoechst/propidium iodide nuclear staining. EW-36 and JLP 119 cells were treated for 24 hours with 100 μmol/L H2O2 or for 48 hours with 2 μmol/L A23187. Cell death (the percentage of propidium iodide-stained cells) and morphology were quantified as described in the Materials and Methods. The data represent the results from four separate experiments. (▪) Necrotic death; (▨) apoptotic death.
Quantitation of extent and type of H2O2 - and A23187-induced cell death induced in BL cell lines by Hoechst/propidium iodide nuclear staining. EW-36 and JLP 119 cells were treated for 24 hours with 100 μmol/L H2O2 or for 48 hours with 2 μmol/L A23187. Cell death (the percentage of propidium iodide-stained cells) and morphology were quantified as described in the Materials and Methods. The data represent the results from four separate experiments. (▪) Necrotic death; (▨) apoptotic death.
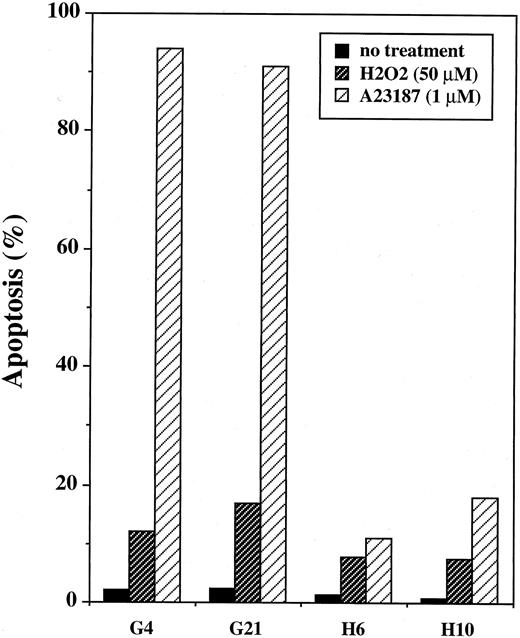
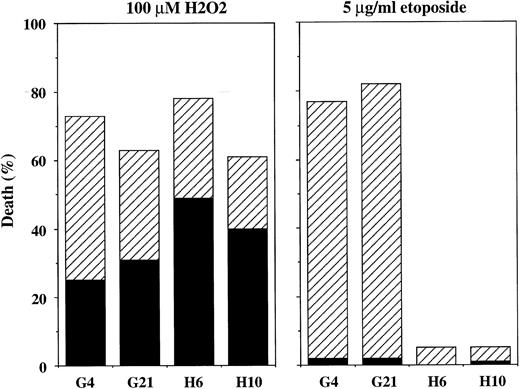
Effect of Bcl-2 overexpression induced by transfection.The results of the present study show that endogenous Bcl-2 is ineffective at protecting BL cells from H2O2 toxicity. However, interpretation of the results is complicated by the fact that the cell lines examined are not isogenic. Despite our efforts to use matched cell lines, the possibility exists that unknown pathways could be modulating the responses of these cells to the various agents tested. To test directly whether overexpression of Bcl-2 by itself can protect Burkitt's lymphoma cells from H2O2 -induced cytotoxicity, the human bcl-2 gene was transfected into JLP 119 cells. In Fig 12, the levels of expression of transfected Bcl-2 are shown for two positive clones (H6 and H10) and two control clones (G4 and G21). The results of TUNEL analyses performed with these cell lines are shown in Fig 13. Control cells carrying vector only were highly susceptible to A23187-induced DNA fragmentation, similar to the parental cell line, whereas both bcl-2–transfected cell lines exhibited marked resistance. However, H2O2 (50 μmol/L) induced little TUNEL activity in any of the four cell lines. Transfected Bcl-2 prevented some of this activity (positive TUNEL in ∼6% of cells compared with 12% to 14% of control cells). The overall viability of the cells was determined by trypan blue staining and it was found that transfected Bcl-2 was not protective (average of 61% and 66% viability in control and bcl-2–transfected cells, respectively). This result was confirmed using Hoechst/propidium iodide staining to quantify cell viability and mechanism of cell death. In this experiment, the topoisomerase II inhibitor etoposide was used as a positive control for induction of apoptosis because it acts more quickly than A23187 and can thus be assayed at the same time as H2O2 (ie, after 24 hours). As shown in Fig 14, treatment with 100 μmol/L H2O2 killed 60% to 75% of cells regardless of whether they expressed transfected Bcl-2. The mechanism of cell killing was both necrotic and apoptotic. In contrast, treatment with etoposide (5 μg/mL) killed ∼80% of control cells but only ∼5% of bcl-2–transfected cells. Almost all of the cell death induced by this agent was apoptotic.
Western blot immunoassay of JLP 119 cells transfected with bcl-2. JLP 119 cells with a stable transfection of either vector alone (G4 and G21) or plasmid expressing the human bcl-2 gene (H6 and H10) were tested for expression of the Bcl-2 protein as described in the legend to Fig 2. The level of expression of endogenous Bcl-2 in EW-36 cells is shown for comparison.
Western blot immunoassay of JLP 119 cells transfected with bcl-2. JLP 119 cells with a stable transfection of either vector alone (G4 and G21) or plasmid expressing the human bcl-2 gene (H6 and H10) were tested for expression of the Bcl-2 protein as described in the legend to Fig 2. The level of expression of endogenous Bcl-2 in EW-36 cells is shown for comparison.
Flow cytometric TUNEL assay of JLP 119–bcl-2 transfectants treated with H2O2 or A23187. JLP 119 cells with a stable transfection of either control plasmid (G4 and G21) or plasmid expressing the human bcl-2 gene (H6 and H10) were tested for induction of TUNEL activity as described in the legends to Figs 7 and 10. (▨) H2O2 was added at 50 μmol/L and cells were assayed 24 hours later. (▨) A23187 was added 1 μmol/L and the cells were assayed 48 hours later. The data are taken from one representative experiment. (▪) No treatment.
Flow cytometric TUNEL assay of JLP 119–bcl-2 transfectants treated with H2O2 or A23187. JLP 119 cells with a stable transfection of either control plasmid (G4 and G21) or plasmid expressing the human bcl-2 gene (H6 and H10) were tested for induction of TUNEL activity as described in the legends to Figs 7 and 10. (▨) H2O2 was added at 50 μmol/L and cells were assayed 24 hours later. (▨) A23187 was added 1 μmol/L and the cells were assayed 48 hours later. The data are taken from one representative experiment. (▪) No treatment.
Quantitation of extent and type of H2O2 -induced cell death induced in bcl-2–transfected JLP 119 cells by Hoechst/propidium iodide nuclear staining. JLP 119 cells transfected with either control plasmid (G4 and G21) or plasmid expressing the bcl-2 gene (H6 and H10) were treated with 100 μmol/L H2O2 or 5 μg/mL etoposide. In both cases, cells were collected after 24 hours and examined for cell death (propidium iodide staining) and morphologic features of apoptosis (chromatin condensation) as described in the Materials and Methods. The data represent the average of four independent experiments. (▪) Necrotic death; (▨) apoptotic death.
Quantitation of extent and type of H2O2 -induced cell death induced in bcl-2–transfected JLP 119 cells by Hoechst/propidium iodide nuclear staining. JLP 119 cells transfected with either control plasmid (G4 and G21) or plasmid expressing the bcl-2 gene (H6 and H10) were treated with 100 μmol/L H2O2 or 5 μg/mL etoposide. In both cases, cells were collected after 24 hours and examined for cell death (propidium iodide staining) and morphologic features of apoptosis (chromatin condensation) as described in the Materials and Methods. The data represent the average of four independent experiments. (▪) Necrotic death; (▨) apoptotic death.
DISCUSSION
There is a substantial body of literature that suggests that Bcl-2 protects cells from death by protecting them from oxidative stress.16,17,28-31,63 We intended to build on this phenomenon so that we could investigate specific oxidative processes from which Bcl-2 protects but found that Bcl-2 had no effect on H2O2 -induced killing in our experimental system. Two BL cell lines, EW-36 and JLP 119, that contained significantly different levels of Bcl-2 but were wild-type for p53 protein, were EBV-negative, and showed similar levels of expression of Bax and Bcl-x were compared. The cells showed markedly different susceptibilities to induction of apoptosis by A23187 that correlated with their respective levels of expression of Bcl-2, consistent with the conclusion that Bcl-2 prevents cell death by inhibiting apoptosis.5,10,11,26,27,39 64 But these cell lines showed little difference in susceptibility to H2O2 -induced killing. The cell line with low Bcl-2 (JLP 119) was slightly more susceptible (≤2-fold) to toxicity (loss of viability) from 50 μmol/L H2O2 compared with the high Bcl-2 expressor (EW-36; see Fig 1), but the differences were small relative to the substantial (>20-fold) differences in both the levels of expression of Bcl-2 (see Fig 2) and the amount of protection afforded from A23187-induced apoptosis (see Figs 6 and 7).
One experimental difference that might have explained the disparity between our findings and those reported previously17,29-31 is that most previous investigations were performed using murine or rat cell lines that were transfected with the human bcl-2 gene,16,26,27,29,31,63,65 whereas we examined BL cells expressing different levels of endogenous Bcl-2 protein. Transfection experiments offer the advantage that the protein is expressed in an otherwise isogenic background. However, transfected genes are under different regulatory control from endogenous genes and functional differences between transfected and endogenous Bcl-2 proteins can influence the experimental results.30 53 We tested this possibility directly by transfecting human bcl-2 into JLP 119 BL cells and obtained results that were very similar to those achieved with the endogenous protein, ie, that transfected Bcl-2 did not prevent H2O2 -induced killing in these cells.
The absence of a protective effect of Bcl-2 on H2O2 -induced cell death in BL cells may be explained in part by the fact that H2O2 was relatively inefficient at inducing apoptosis. Using nucleosomal DNA fragmentation to assay for apoptosis (agarose gel electrophoresis and TUNEL assays), none of the cell lines that we examined (a total of 6) showed a high degree of apoptotic death in response to H2O2 treatment even though a range of concentrations was tested. In contrast, A23187 and serum depletion killed the cells primarily by apoptosis and cells expressing high levels of Bcl-2 were protected from these treatments. Using a morphologic assay for apoptosis, a somewhat different result was seen; by this measure, roughly half of the bcl-2lo (JLP 119) cells died by apoptosis and half by necrosis. However, it should be noted that the morphologic apoptosis induced by H2O2 was different from that seen with A23187. H2O2 induced nuclear pyknosis and cleavage into 2 to 3 spheres, but the fragmentation into the smaller particles that was characteristic of A23187 treatment was uncommon. At the same time, the necrosis induced by H2O2 was also somewhat atypical in that the nuclei were intact but were not necessarily larger than control nuclei. Additional experiments will be required to characterize the mechanism of cell death induced by H2O2 . The poor induction of chromatin fragmentation at the microscopic level correlated with the low levels of molecular DNA fragmentation assessed through biochemical techniques. Taken together, the results obtained to date suggest that H2O2 induces only partial apoptosis, as has been observed in some other experimental systems.59-61
Our studies suggest that Bcl-2 prevents cell death only when the mechanism of cell death is primarily apoptotic. The apoptotic death induced by H2O2 was inhibited by overexpression of Bcl-2. However, the cells still died (as determined by trypan blue and propidium iodide staining), apparently by a necrotic mechanism. This finding is inconsistent with previous reports suggesting that Bcl-2 protects cells from necrotic as well as apoptotic death. Kane et al16,28 concluded that, in neural cell lines, diminution of antioxidant defenses (glutathione depletion) kills cells by a necrotic mechanism, consistent with our results with H2O2 . But they found that transfection of bcl-2 into the cells provided protection and concluded that the oncogene protects from both necrotic and apoptotic modes of cell death.28 At present, it is unclear whether their finding was unique to neural cell lines.
The theory that Bcl-2 prevents apoptosis by acting through an antioxidant pathway is supported by the finding that bcl-2 transfected cells show a greater resistance to various pro-oxidant treatments than mock-transfected cells29,31,63,65 and that antioxidants protect some cells from apoptosis induced by nonoxidative agents.17,66-69 However, several recent findings have cast doubt on the interpretation of these results. Shimizu et al26 found that Bcl-2 protects rat hepatoma and pheochromocytoma cells from hypoxia-induced death even though there was no apparent oxidative damage to the cells. Jacobson and Raff27 reported that agents that induce apoptosis do so under anaerobic condition and that Bcl-2 protects cells from death even in the absence of molecular oxygen. Miyashita and Reed10 reported that transfected bcl-2 protects T-lymphoma cells from a variety of agents but not from H2O2 , similar to what we observed with both endogenous and transfected Bcl-2. Taken together with the present investigation, these findings challenge the theory that apoptosis is mediated by oxidative processes and that Bcl-2 protects against apoptosis by decreasing the activity of reactive oxygen species.
ACKNOWLEDGMENT
The authors thank Kishor Bhatia, Ian McGrath, and Barry Cherney for supplying the cell lines and their p53 mutation and EBV infection statuses. We are grateful to Apurva Sarin for invaluable instructions on performing and interpreting the Hoechst/propidium iodide morphology assays. Finally, we thank Giovanna Tosato, Wendy Shores, Melanie Vacchio, and Joy Williams for careful reading of the manuscript and for many useful suggestions.
Address reprint requests to Emily Shacter, PhD, FDA/CBER, HFM-538, Bldg 29A, Room 2A-11, Bethesda, MD 20892-4555.