Abstract
The susceptibility of Th1 and Th2 cell clones to apoptosis following HIV-gp120/CD4 cross-linking and TCR activation was investigated. We show that only Th1 clones are susceptible to HIV-gp120-sensitized apoptosis, although both types of clones express similar levels of CD4 and bind similar amounts of recombinant gp120. Both types of clones, however, undergo apoptosis induced by CD95 cross-linking with agonistic monoclonal antibody (MoAb). Apoptosis induced by gp120 in the Th1 clones is inhibited by either an antiCD95 neutralizing MoAb or an anti-CD95L neutralizing MoAb as well as by a specific interleukin-1β converting enzyme (ICE) inhibitor. When triggered to apoptosis by gp120, Th1 but not Th2 clones express both cell-associated and soluble CD95L. The CD95L produced by Th1 clones induces cell death, inhibitable by anti-CD95 neutralizing MoAb, of CD95 positive Jurkat cells. These data suggest that, like activation-induced apoptosis, HIV-gp120 sensitized apoptosis in Th1 clones occurs via CD95/CD95L interaction and that lack or insufficient production of CD95L is responsible, at least in part, for the resistance of Th2 clones to such apoptosis.
WE HAVE PREVIOUSLY reported that a human Th1 clone (clone 103)1 undergoes apoptosis (thereafter indicated as “gp120-apoptosis”), following gp120/CD4 cross-linking and CD3/TCR activation; in this system, gp120/CD4 cross-linking or CD3/TCR stimulation alone did not induce relevant levels of apoptosis. These observations were consistent with results obtained by others using polyclonal CD4+ populations2-5 and suggested a possible mechanism for CD4+ lymphocyte depletion in acquired immunodeficiency syndrome (AIDS) patients, a phenomenon that cannot be explained solely by the cytopathic effect of HIV.6-11 We also observed that such apoptosis could be inhibited by cytokines such as interleukin-12 (IL-12) and IL-2, especially when used in combination.1
It has been suggested that gp120-apoptosis, induced as in our experimental model, could represent an exacerbation of activation-induced cell death,12 a physiologic phenomenon responsible for T-cell peripheral selection and for controlling the expansion of activated clones to terminate an immune response.13,14 Involvement of the CD95(Apo-1/Fas)/CD95L pathway in activation-induced cell death has been shown, and activation through TCR/CD3 was reported to induce CD95L15-20 and to increase CD95 expression.19,20 Whether the CD95/CD95L interaction is involved in gp120-apoptosis has been suggested but not directly proven.12
The role of different Th populations in determining resistance or susceptibility to AIDS progression is debated. In vivo evidence suggests that CD4+ Th1 cells are the first to be impaired after human immunodeficiency virus (HIV) infection, because CD4+ cells with Th221,22 or Th023 phenotype are predominant during AIDS progression even if these cells are best recipients for HIV replication.24,25 The study of gp120-apoptosis in normal CD4+ T cells is relevant because apoptosis seems to occur mainly in bystander cells rather than in productively HIV-infected cells.26
In this study we analyze the ability of CD4+ clones with different Th phenotypes to bind gp120 and to undergo gp120-apoptosis. Our data show that Th1 clones are susceptible and Th2 clones resistant to gp120-apoptosis. Differential induction of CD95L and the consequent activation of the CD95/CD95L pathway plays a role to determine the differential sensitivity of Th1 and Th2 clones to gp120-apoptosis in vitro. Anti-CD95 neutralizing monoclonal antibody (MoAb) and anti-CD95L neutralizing MoAb, as well as an interleukin-1β converting enzyme (ICE) inhibitor, block gp120-sensitized apoptosis. Functional CD95L was detected on cells and in supernatants from Th1 but not Th2 clones treated for gp120-apoptosis. Our data provide the first evidence for a differential susceptibility of Th1 and Th2 clones to HIV-gp120-apoptosis depending on different regulation of CD95L, upregulated in Th1 but not in Th2 clones.
MATERIALS AND METHODS
Cells.Clone 103, a human CD3+ CD4+ Th1 clone, has been previously described.1 All the other T-cell clones used in the present study were CD3+ CD4+ and they were obtained from PBMC of four healthy seronegative donors.
CD4+ cells were positively isolated from PBMC of donors 1 to 3 by immunomagnetic beads (Dynabeads M-450 CD4; Dynal A S, Oslo, Norway), then cloned by limiting dilution (0.3 cell/well in 96 U bottomed well plates) in the presence of γ-irradiated (5,000 R) pooled allogeneic peripheral blood leukocytes (PBL) (5 × 104/well), 1 μg/mL phytohemaglutinin (PHA; Murex, Temple Hile Dartford, UK) and 180 IU/mL recombinant human IL-2 (Proleukin; Eurocetus, Amsterdam, The Netherlands). Clones from donor 4 were independently obtained as described.27
Cells were cultured at 37°C in a 5% CO2 atmosphere, in complete medium (CM) composed of RPMI 1640 (MA Bioproducts, Walkersville, MD) supplemented with 2 mmol/L glutamine (MA Bioproducts), 20 mmol/L HEPES buffer (MA Bioproducts), 100 U/mL penicillin, and 100 μg/mL streptomycin (Farmitalia Carlo Erba, Milan, Italy) plus 10% pooled human heat-inactivated serum. Cytokines were added to CM as follows: 180 IU/mL IL-2 (Th1 clones) or 180 IU/mL IL-2 plus 100 U/mL recombinant human IL-4 (Genzyme Corp, Cambridge, MA) (Th2 clones). All the clones (2 × 105 cells/well) were cultured in 24-well plates (Costar, Cambridge, MA) and weekly restimulated with irradiated allogeneic PBL (4 × 105 cells/well) and PHA (1 μg/mL). Six days after the last restimulation, cells were washed twice with CM, then used in experiments.
MoAbs.The following MoAbs were used: BMA031 (anti-TCR)28; OKT4A (Ortho, Raritan, NJ); CH-11 (anti-CD95 IgM, apoptosis inducing),29 fluorescein-conjugated UB2 (anti-CD95), ZB4 (anti-CD95 IgG, neutralizing)29 (all from MBL Co, Nagoya, Japan), 4H9 and biotinylated 4A5 (anti-CD95L neutralizing MoAb)30; biotinylated Leu-3a (anti-CD4) (Becton Dickinson, Sunnyvale, CA), biotinylated UC8-4B3 (anti-TNP), Streptavidin-PE (both from Pharmingen, San Diego, CA); anti-gp120 (HIV-1) (Intracel, London, UK); isotype-matched controls, fluorescein conjugated or not (Cymbus Bioscience LTD, Southampton Hants, UK).
Cytokine profile.CD4+ clones were characterized for the pattern of cytokine production following TCR triggering. Briefly, cells were collected 6 days after the last restimulation, washed twice, and seeded at 106 cells/mL in 96 U bottomed well plates precoated with anti-TCR MoAb (BMA031, 5 μg/mL in PBS, for 1 hour at 37°C). Supernatants were collected after 24 hours and the following cytokines were detected by commercial ELISA: IL-4 (Mabtech AB, Stockholm, Sweden), interferonγ (IFNγ, Mabtech AB), IL-10 (Pharmingen).
Immunofluorescence analysis.Cells (105 in 0.1 mL) were incubated in CM only or with suitable dilution of each MoAb for 30 minutes at 4°C, washed in CM, then treated with fluorescein-conjugated goat antimouse Ig F(ab′)2 (Technogenetics, S. Mauro Torinese, Italy) (1:50 dilution, 30 minutes at 4°C). After washing in CM, cells were resuspended in 0.2 mL of 1% paraformaldehyde (Sigma Chemical Co, St Louis, MO) in RPMI 1640 and analyzed with FACScan (Becton Dickinson).
For determination of gp120 binding, 107 cells/mL were treated with 10 to 100 μg/mL of recombinant HIV-1 gp120 (ABT Europe, London, UK) for 30 minutes at 37°C, then with 10 to 100 μg/mL anti-gp120 (HIV-1) for 30 minutes at 4°C, and finally with fluorescein-conjugated goat antimouse Ig F(ab′)2 (1:50 dilution, 30 minutes at 4°C); they were analyzed as described.
Quantitative analysis of indirect immunofluorescence staining was performed with QIFIKIT (Biocytex, Marseille, France): the mean number of antigenic sites/cell was determined on the basis of a standard curve obtained with beads with known numbers of MoAb sites.31 Analysis of surface CD95L was performed according to a published protocol30 with minor modifications. Briefly, cells (105 in 0.1 mL) were preincubated for 30 minutes on ice with 10% AB pooled human serum, washed and incubated for 1 hour with 0.5 μg/mL biotinylated anti-CD95L MoAb (4A5) in PBS. After extensive washing, cells were incubated for 30 minutes at 4°C with Streptavidin-PE 0.5 μg/mL, washed and analyzed with FACScan. In order to minimize interaction between CD95 and CD95L on adjacent cells, and possible masking of CD95L, before induction of CD95L, cells were pretreated with anti-CD95 neutralizing MoAb ZB4 (1 μg/mL, 1 hour incubation on ice, then extensive washing). Negative and positive controls were represented by cells incubated with biotinylated anti-TNP MoAb (unrelated isotype matched MoAb) UC8-4B3 and by cells incubated with biotinylated anti-CD4 MoAb Leu-3a, respectively.
Induction of apoptosis.The gp120 protocol was as follows: 107 cells/mL were treated with recombinant HIV-1 gp120 (Intracel) (10 μg/mL, 30 minutes at 37°C), washed once, then treated with mouse anti-gp120 (HIV-1) MoAb (10 μg/mL, 30 minutes at 4°C), washed once, and seeded (106 cells/well) in 48-well plates precoated with anti-TCR MoAb (BMA031, 5 μg/mL in PBS, 1 hour at 37°C). Apoptosis was detected at different times by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) and propidium iodide (PI) staining. The percentage of gp120-apoptosis was calculated as: % apoptotic cells after induction (gp120/anti-gp120/immobilized anti-TCR) − % apoptotic cells after activation (immobilized anti-TCR only). Positive and negative controls were γ irradiated cells (10,000 R) and cells maintained in IL-2 (180 IU/mL), respectively.
The anti-CD95 protocol was as follows: 106 cells/well were seeded in 48-well plates and cultured in the presence of different doses of agonistic anti-CD95 MoAb CH-11. Apoptosis was detected after 4 hours by TUNEL. The percentage of anti-CD95 apoptosis was calculated as: % apoptotic cells in the presence of CH-11 − % apoptotic cells without CH-11.
Inhibition of gp120-apoptosis.For inhibition experiments, cells (107/mL) were pretreated with the indicated doses of neutralizing anti-CD95 MoAb (ZB4),29 or anti-CD95L neutralizing MoAb (4H9)30 or specific ICE inhibitor (a modified peptide: Z-Val-Ala-Asp-CH2F ) (Kamiya Biochemical Co, Thousands Oaks, CA) for 30 minutes at 37°C, then induced to apoptosis following gp120 or anti-CD95 protocol. Inhibitors were maintained (at the same doses used for pretreatment) throughout all passages of apoptosis induction. The percentage of inhibition was calculated as follows: [1 − (% gp120-apoptosis with inhibitor)/(% gp120-apoptosis without inhibitor)] × 100.
Analysis of DNA fragmentation by TUNEL.TUNEL was performed as previously described.1 Briefly, 106 cells/sample were fixed with 2% paraformaldehyde in PBS (10 minutes on ice), washed three times with TBS (50 mmol/L Tris-HCl in saline solution, pH 7.5), then permeabilized with ice cold Acetone (Sigma) (1 minute on ice), washed twice in TBS and once in distilled water. Staining was performed by incubating cells for 1 hour at 37°C, in 20 μL final volume, with 1.5 μmol/L fluorescein-12-dUTP (Boehringer Mannheim GmbH, Mannheim, Germany), 0.5 U/μL terminal deoxynucleotidyl transferase (GIBCO-BRL, Gaithersburg, MD), in appropriate buffer (0.5 mol/L potassium cacodylate pH 7.2, 10 mmol/L CoCl2 , 1 mmol/L DTT; GIBCO). Samples were then analyzed by FACScan. Cells with fragmented DNA appeared positive.
Analysis of DNA content by PI staining.PI staining was performed as described.32 Briefly, 105 cells were incubated overnight at 4°C in 0.2 mL hypotonic fluorochrome solution, containing 50 μg/mL PI (Sigma), 0.1% sodium citrate (Sigma), and 0.1% Triton X-100 (Sigma). Analysis was performed with FACScan. Cells with subdiploid DNA content were considered apoptotic cells.
Enzyme-linked immunosorbent assay (ELISA) for determination of soluble CD95L in culture supernatants.Culture supernatants from Th clones were collected 30 minutes after induction of gp120-apoptosis or after stimulation with PMA/ionophore, and tested by ELISA for soluble CD95L, according to a published protocol.30 Purified anti-CD95L MoAb 4H9 and biotinylated anti-CD95L MoAb 4A5 were used for capture and detection, respectively. Recombinant human CD95L30 was used as a standard.
Western blot analysis of CD95L expression.T cell clones were treated for gp120-apoptosis induction or incubated with PMA/ionophore (as a positive control for CD95L production). After 1 hour, samples were collected and washed twice in PBS. Cell pellets were then lysed in Laemmli buffer (0.125 mol/L Tris-HCl, pH 6.8, 5% sodium dodecyl sulfate [SDS]) containing, as inhibitors, 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF ) 10 μg/mL leupeptin (all from Boehringer-Mannheim) and 1 mmol/L sodium orthovanadate (Sigma). The lysates were boiled for 2 minutes, sonicated for 20 seconds, and quantitated for protein content by the micro-BCA method (Pierce, Rockford, IL). Aliquots containing 15 μg/mL of protein plus 5% β-mercaptoethanol were loaded on 12% SDS polyacrylamide gel electrophoresis (PAGE), size-fractionated and electroblotted onto polyvinylidene fluoride (PVDF ) membranes (Millipore, Bedford, MA). After blotting, the membranes were blocked with 5% non-fat dried milk in PBS plus 0.1% Tween (Sigma) and incubated overnight with a dilution of 1:500 rabbit anti-CD95L antibody (N-20; Santa Cruz Biotechnology, Santa Cruz, CA). After extensive washing, the blots were incubated for 1 hour with 1:2000 dilution of peroxidase-labeled secondary antibody, washed again in PBS-T and revealed by enhanced chemiluminescence using a kit from Amersham. To normalize for protein content, the blots were probed with a rabbit antibody to β-actin (Sigma) diluted 1:2000.
Biological activity of soluble or cell-bound CD95L.In order to detect biological activity of soluble or cell-bound CD95L, Th clones were pretreated with 1 μg/mL anti-CD95 neutralizing MoAb ZB429 (1 hour incubation on ice, followed by extensive washing) to prevent possible soluble CD95L sequestration by CD95 receptors on the surface of the producing cells as well as CD95/CD95L interactions between adjacent cells. To detect soluble CD95L, supernatants were collected from Th clones cultured for 30 minutes following the gp120 protocol of apoptosis induction or for 1 hour in the presence of 10 ng/mL PMA (Sigma) and 500 ng/mL A23187 ionophore (Sigma). The presence of soluble CD95L was tested by 51Cr release assay following a previously described procedure.33 Briefly, CD95+ Jurkat cells were labeled with 51Cr (100 μCi Na512 CrO4 ) for 1 hour at 37°C, then washed and seeded (104 target cells/well in 0.1 mL) in 96 U bottomed well plates, in the presence of an equal volume of the various supernatants.
To test the biological activity of cell-bound CD95L, Th clones cultured for 1 hour after induction of gp120-apoptosis or stimulated with PMA/ionophore were extensively washed and seeded as effector cells in 96 U bottomed well plates with CD95+ Jurkat cells labeled with 51Cr, at an effector to target ratio of 20:1. For determination of spontaneous and total release, target cells were incubated in CM only or in 1% NP40, respectively. The cytotoxic assays were performed in triplicate, in the presence or absence of 50 ng/mL ZB429 (anti-CD95 neutralizing MoAb) or isotype-matched control MoAb. After 20 hours of incubation at 37°C, 51Cr release was determined by a microplate scintillation counter (Topcount; Packard Instrument Co, Meriden, CT).
RESULTS
Characterization of CD4+ Th clones.CD4+ clones were generated by limiting dilution from PBL of four different healthy donors. Clones were defined as Th1 or Th2 on the basis of their ability to produce IFN-γ or IL-4 respectively, upon 24 hour TCR stimulation. The levels of cytokines produced by the clones used throughout the study, including the previously described Th1 clone 103,1 were determined by ELISA. Although the amount of cytokines produced was variable, the phenotype of each clone was stable in 3 to 7 independent determinations on lymphocytes maintained in culture for different times (Table 1 and data not shown). Significant cytokine levels were detectable only in supernatants from cells stimulated with anti-TCR MoAb; in most cases, cytokine production, detectable after 4 hours stimulation, was significantly increased after 24 hours (data not shown). All cells from each clone analyzed expressed TCR, CD4, and CD95 antigens; differences in the mean number of antigenic sites per cell did not correlate with the pattern of cytokine production and therefore with the Th phenotype.
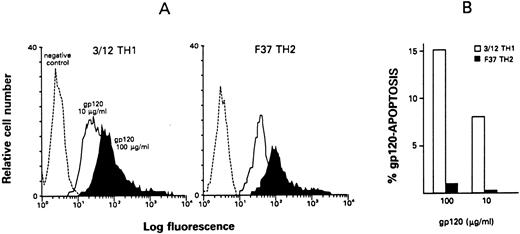
Differential susceptibility of Th clones to gp120-apoptosis.As shown in Fig 1A, no significant difference was detected in the ability of Th1 and Th2 representative clones to bind gp120 at the dose of 10 μg/mL, a concentration commonly used to induce apoptosis, and an equivalent increased binding was observed using a dose of 100 μg/mL. The susceptibility to gp120-apoptosis, in a preliminary experiment, was analyzed in Th1 clone 3/12 and Th2 clone F37 (Fig 1B). Although both clones bound gp120 to the same extent, gp120-sensitized apoptosis was seen, in a dose-dependent fashion, only in the Th1 clone after 4 hours stimulation, whereas the Th2 clone was resistant.
gp120 binding and susceptibility to gp120-apoptosis in Th1 and Th2 clones. (A) gp120 binding to Th1 and Th2 clones. gp120 binding was analyzed by indirect immunofluorescence on cells (clone 3/12, Th1 and F37, Th2) sequentially incubated with the indicated doses of gp120, equal amounts of anti-gp120 MoAb, and fluorescein-conjugated goat antimouse (for fluorescence staining see Materials and Methods). Negative controls were cells incubated with fluorescein-conjugated goat antimouse only. (B) Differential susceptibility of Th1 and Th2 clones to gp120-apoptosis. Th1 clone 3/12 and Th2 clone F37 were treated with the indicated doses of gp120, and equal concentration of anti-gp120 MoAb; after 4 hours incubation of the cells in the presence of immobilized anti-TCR MoAb, the percentage of apoptotic cells was determined by TUNEL. Data are expressed as net % gp120-apoptosis (see Materials and Methods).
gp120 binding and susceptibility to gp120-apoptosis in Th1 and Th2 clones. (A) gp120 binding to Th1 and Th2 clones. gp120 binding was analyzed by indirect immunofluorescence on cells (clone 3/12, Th1 and F37, Th2) sequentially incubated with the indicated doses of gp120, equal amounts of anti-gp120 MoAb, and fluorescein-conjugated goat antimouse (for fluorescence staining see Materials and Methods). Negative controls were cells incubated with fluorescein-conjugated goat antimouse only. (B) Differential susceptibility of Th1 and Th2 clones to gp120-apoptosis. Th1 clone 3/12 and Th2 clone F37 were treated with the indicated doses of gp120, and equal concentration of anti-gp120 MoAb; after 4 hours incubation of the cells in the presence of immobilized anti-TCR MoAb, the percentage of apoptotic cells was determined by TUNEL. Data are expressed as net % gp120-apoptosis (see Materials and Methods).
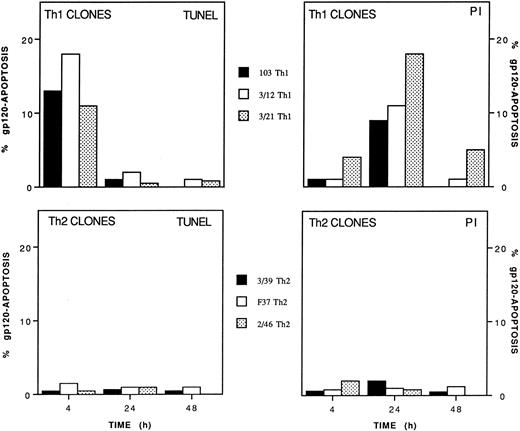
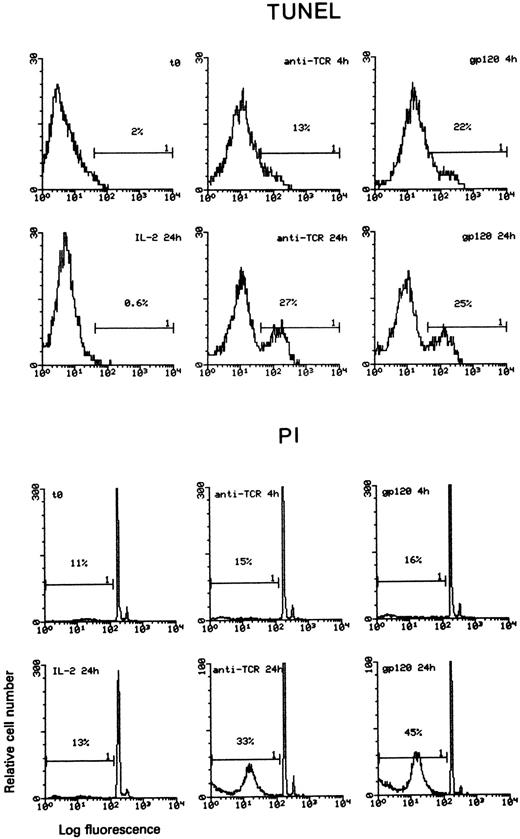
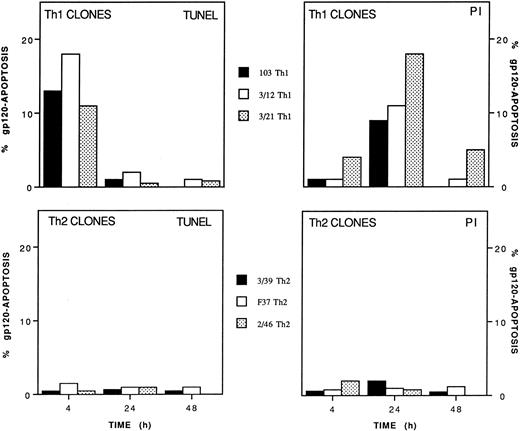
A total of 4 Th1 and 3 Th2 clones, independently obtained from four different donors, were further analyzed for susceptibility to gp120-apoptosis. Figure 2 shows the results obtained with three Th1 and three Th2 clones. All Th1 clones underwent apoptosis (range 8% to 18%) with rapid kinetics (4 to 24 hours). On the contrary, gp120-apoptosis could not be detected in Th2 clones, even 48 hours after induction. Apoptosis was detected better by TUNEL and by PI staining at 4 hours and at 24 hours, respectively, as expected based on the different sensitivity of the two assays.1 As an example, actual cytofluorimetric peaks obtained with TUNEL and PI methods from a representative experiment performed on Th1 clone 103 are shown in Fig 3. For calculation of the net gp120-apoptosis, the value of 13% obtained with anti-TCR MoAb only, should be subtracted to 22% which is the value obtained 4 hours after induction of gp120-apoptosis. Cells cultured in medium alone or treated with gp120-anti-gp120 without TCR triggering had a value of apoptosis ranging from 12% to 15% (not shown) like that of sample treated with anti-TCR (13%). Therefore, the net value of gp120-apoptosis revealed by TUNEL in this experiment was 9% at 4 hours. The same type of analysis performed after 24 hours revealed values of 25% and 27% in samples treated for gp120-apoptosis and with anti-TCR only, respectively; thus indicating that TUNEL does not allow calculation of net gp120-apoptosis. (For simplicity the figure shows only the value of anti-TCR–treated cells that does not exceed those of cells kept in medium alone or treated with gp120-anti-gp120 without TCR triggering, 30% and 28%, respectively, data not shown).
Differential susceptibility of Th1 and Th2 clones to gp120-apoptosis. Th1 clones 103, 3/12, and 3/21 and Th2 clones 3/39, F37, and 2/46 were treated with 10 μg/mL gp120, an equal dose of anti-gp120 MoAb, and seeded in the presence of immobilized anti-TCR MoAb. The percentage of apoptotic cells was determined at the indicated times with TUNEL and PI staining. Data are expressed as net % gp120-apoptosis (see Materials and Methods).
Differential susceptibility of Th1 and Th2 clones to gp120-apoptosis. Th1 clones 103, 3/12, and 3/21 and Th2 clones 3/39, F37, and 2/46 were treated with 10 μg/mL gp120, an equal dose of anti-gp120 MoAb, and seeded in the presence of immobilized anti-TCR MoAb. The percentage of apoptotic cells was determined at the indicated times with TUNEL and PI staining. Data are expressed as net % gp120-apoptosis (see Materials and Methods).
Comparison of TUNEL and PI staining for apoptosis detection. Th1 clone 103 was cultured with immobilized anti-TCR MoAb (sample named anti-TCR), treated with gp120-anti-gp120 and seeded with immobilized anti-TCR MoAb (here simply named gp120) or cultured with 180 IU/mL IL-2 (sample named IL-2). Cells were collected at the indicated times and stained with TUNEL or PI methods. Cytofluorimetric histograms are presented. Markers indicating the position of apoptotic cells are defined on the basis of a negative control represented by cells cultured 4 hours in IL-2 (not shown). Positive control were 10,000 Rad γ-irradiated cells (not shown). Untreated cells (here indicated as t0) were also analyzed to evaluate the background level of apoptosis.
Comparison of TUNEL and PI staining for apoptosis detection. Th1 clone 103 was cultured with immobilized anti-TCR MoAb (sample named anti-TCR), treated with gp120-anti-gp120 and seeded with immobilized anti-TCR MoAb (here simply named gp120) or cultured with 180 IU/mL IL-2 (sample named IL-2). Cells were collected at the indicated times and stained with TUNEL or PI methods. Cytofluorimetric histograms are presented. Markers indicating the position of apoptotic cells are defined on the basis of a negative control represented by cells cultured 4 hours in IL-2 (not shown). Positive control were 10,000 Rad γ-irradiated cells (not shown). Untreated cells (here indicated as t0) were also analyzed to evaluate the background level of apoptosis.
On the contrary, the PI staining applied to the same cells revealed a net gp120-apoptosis of 1% and 12% when measured 4 and 24 hours after induction, respectively. Therefore, in our system, the sensitivity of the two methods was quite different at the two time points of analysis; for this reason, the data obtained with both methods are shown. Cells kept with 180 IU/mL IL-2 were the negative control (TUNEL detected 1% and 0.6% apoptosis at 4 and 24 hours, respectively, whereas PI detected 11% and 13% apoptosis at 4 and 24 hours, respectively). γ-Irradiated cells (10,000 R), cultured for the same times, were the positive controls of apoptosis (TUNEL detected 24% and 30%, PI detected 16% and 72% at 4 and 24 hours, respectively) (not shown). Independent of the method used to measure apoptosis, the results concordantly indicate that Th1 but not Th2 clones undergo gp120-apoptosis.
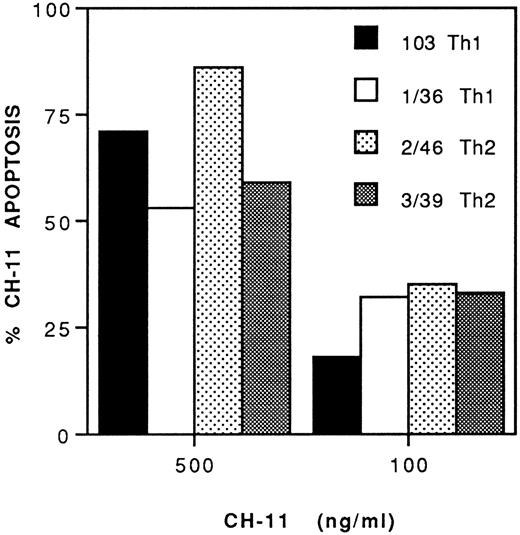
Apoptosis induced by anti-CD95 MoAb.To assess whether the different susceptibility of various Th clones to gp120-apoptosis depends on different sensitivity to CD95-dependent lysis, we analyzed the ability of the anti-CD95 agonistic MoAb CH-11 to cause apoptotic cell death. This MoAb induced apoptosis in all the Th clones, irrespective of their phenotype, in a dose-dependent fashion. Figure 4 shows two Th1 and two Th2 clones. Cells were analyzed after 4 hours culture in the presence of different doses of CH-11.
Both Th1 and Th2 clones are susceptible to anti-CD95 MoAb-induced apoptosis. Two Th1 (103 and 1/36) and two Th2 clones (2/46 and 3/39) were cultured in medium only or with the indicated doses of anti-CD95 agonistic MoAb CH-11. Cells were collected after 4 hours and stained by TUNEL. The percentage of CH-11 apoptosis was calculated by subtracting background apoptosis in medium alone (15%, 21%, 5%, and 10% in clones 103, 1/36, 2/46, and 3/39, respectively), to that obtained in the presence of the indicated doses of MoAb.
Both Th1 and Th2 clones are susceptible to anti-CD95 MoAb-induced apoptosis. Two Th1 (103 and 1/36) and two Th2 clones (2/46 and 3/39) were cultured in medium only or with the indicated doses of anti-CD95 agonistic MoAb CH-11. Cells were collected after 4 hours and stained by TUNEL. The percentage of CH-11 apoptosis was calculated by subtracting background apoptosis in medium alone (15%, 21%, 5%, and 10% in clones 103, 1/36, 2/46, and 3/39, respectively), to that obtained in the presence of the indicated doses of MoAb.
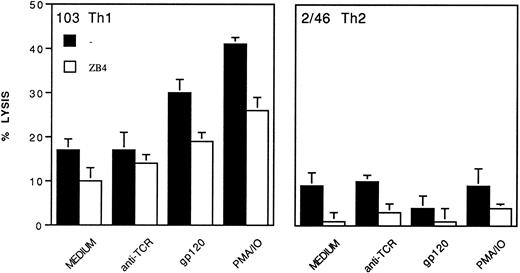
Involvement of the CD95/CD95L pathway in gp120-apoptosis.To test whether gp120-apoptosis involves the CD95/CD95L interaction, the gp120-apoptosis of Th1 clone 103 (Fig 5A and B) and of Th1 clone 3/12 (not shown) was assessed in the presence of the anti-CD95 MoAb ZB4 or of the anti-CD95L MoAb 4H9. Both MoAbs inhibited apoptosis in a dose-dependent fashion. The specific ICE inhibitor34 35 also prevented gp120-apoptosis of clone 103 (Fig 5C). Such inhibitors did not affect the background apoptosis of control samples treated with anti-TCR or with gp120/anti-gp120 without TCR triggering or kept in medium alone (not shown). Together the data indicate that gp120-apoptosis of Th1 clones involves the CD95/CD95L pathway.
Inhibition of gp120-apoptosis by anti-CD95 neutralizing MoAb (A), by anti-CD95L neutralizing MoAb (B), and by ICE inhibitor (C). Clone 103 (Th1) was triggered to undergo gp120-apoptosis, after preincubation (30 minutes at 37°C) with the indicated doses of anti-CD95 neutralizing MoAb ZB4 (A), anti-CD95L neutralizing MoAb 4H9 (B), or ICE inhibitor (C). ZB4, 4H9, and ICE inhibitor were then maintained throughout treatment with gp120 and anti-gp120 and culture on immobilized anti-TCR MoAb. After 4 hours, apoptosis was determined by TUNEL. gp120-Apoptosis in the absence of inhibitors was 15% to 18%. % inhibition was calculated as indicated in Materials and Methods.
Inhibition of gp120-apoptosis by anti-CD95 neutralizing MoAb (A), by anti-CD95L neutralizing MoAb (B), and by ICE inhibitor (C). Clone 103 (Th1) was triggered to undergo gp120-apoptosis, after preincubation (30 minutes at 37°C) with the indicated doses of anti-CD95 neutralizing MoAb ZB4 (A), anti-CD95L neutralizing MoAb 4H9 (B), or ICE inhibitor (C). ZB4, 4H9, and ICE inhibitor were then maintained throughout treatment with gp120 and anti-gp120 and culture on immobilized anti-TCR MoAb. After 4 hours, apoptosis was determined by TUNEL. gp120-Apoptosis in the absence of inhibitors was 15% to 18%. % inhibition was calculated as indicated in Materials and Methods.
gp120-apoptosis induces production of CD95L in Th1 clones.Because Th1 and Th2 clones have similar levels of CD95 expression and are both susceptible to CD95-mediated lysis, the above observations suggested the possibility that differential induction of CD95L may be responsible for the differential sensitivity of Th1 and Th2 cells to gp120-apoptosis.
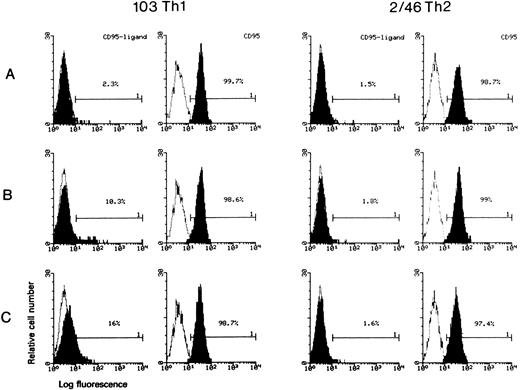
To test this hypothesis, we analyzed CD95L expression by immunofluorescence, Western blot, ELISA and bioassays. With the representative Th1 clone 103, membrane CD95L expression was observed, at 1 hour, only in the sample sensitized to gp120-apoptosis (Fig 6B) but not in control sample stimulated with anti-TCR MoAb only (Fig 6A). CD95L was undetectable also in samples treated with gp120/anti-gp120 without TCR triggering or with medium only (not shown). Approximately 10% of cells treated for gp120-apoptosis were CD95L+, roughly the same percentage of gp120-apoptosis detected by TUNEL at 4 hours. As a positive control for CD95L induction, cells were stimulated with PMA/ionophore (Fig 6C). Similar results were obtained with the Th1 clone 3/12 (not shown). On the contrary, the Th2 clone 2/46 analyzed in the same experiment did not show any induction of CD95L expression (Fig 6). Both Th1 and Th2 clones did not show variation in CD95 (Fig 6) or CD4 (not shown) expression.
Cytofluorimetric analysis of CD95L induction during gp120-apoptosis. Clone 103 (Th1) and clone 2/46 (Th2) were analyzed for CD95 and CD95L expression (shaded histograms) with an indirect immunofluorescence 1 hour after treatment for induction of gp120-apoptosis. Cells were incubated with fluorescein-conjugated anti-CD95 MoAb UB2 or biotinylated anti-CD95L MoAb 4A5 followed by Streptavidin-PE; the percentage of positive cells is indicated. Negative controls (empty histograms) were cells stained with fluorescein-conjugated or biotinylated isotype-matched control MoAb, respectively. (A) Cells treated with anti-TCR MoAb only; (B) cells sensitized to gp120 apoptosis (gp120/anti-gp120/anti-TCR); (C) cells stimulated with PMA/ionophore.
Cytofluorimetric analysis of CD95L induction during gp120-apoptosis. Clone 103 (Th1) and clone 2/46 (Th2) were analyzed for CD95 and CD95L expression (shaded histograms) with an indirect immunofluorescence 1 hour after treatment for induction of gp120-apoptosis. Cells were incubated with fluorescein-conjugated anti-CD95 MoAb UB2 or biotinylated anti-CD95L MoAb 4A5 followed by Streptavidin-PE; the percentage of positive cells is indicated. Negative controls (empty histograms) were cells stained with fluorescein-conjugated or biotinylated isotype-matched control MoAb, respectively. (A) Cells treated with anti-TCR MoAb only; (B) cells sensitized to gp120 apoptosis (gp120/anti-gp120/anti-TCR); (C) cells stimulated with PMA/ionophore.
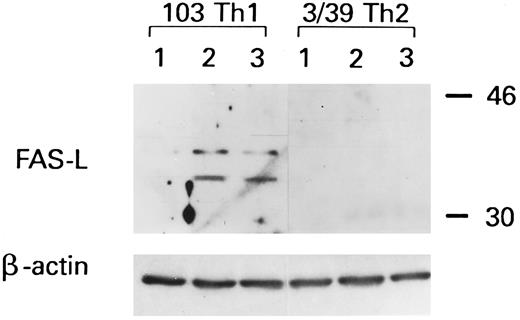
To confirm these data, a Western blot analysis was performed. As shown in Fig 7, two forms of approximately 32 and 42 kD, likely corresponding to the nonglycosylated and to the mature form of CD95L36 were detected in Th1 clone 103 but not in Th2 clone 3/39, 1 hour after stimulation for gp120-apoptosis or with PMA/ionophore. Moreover, ELISA was used to test whether soluble CD95L was released by gp120-apoptosis treated cells. As indicated in Table 2, soluble CD95L was detected only in the supernatants from Th1 clones 103 and 3/12 but not in those from Th2 clone 2/46.
Western blot analysis of CD95L expression in a Th1 and in a Th2 clone. Clones 103 (Th1) and 3/39 (Th2) were sensitized to gp120-apoptosis or stimulated with PMA/ionophore; after 1 hour, samples were collected and processed for Western blot analysis. Lane 1, untreated; lane 2, gp120/anti-gp120/antiTCR; lane 3, PMA/ionophore.
Western blot analysis of CD95L expression in a Th1 and in a Th2 clone. Clones 103 (Th1) and 3/39 (Th2) were sensitized to gp120-apoptosis or stimulated with PMA/ionophore; after 1 hour, samples were collected and processed for Western blot analysis. Lane 1, untreated; lane 2, gp120/anti-gp120/antiTCR; lane 3, PMA/ionophore.
Functional activity of CD95L produced by Th1 clones during gp120-apoptosis.We also tested whether supernatants from Th1 and Th2 clones treated for gp120-apoptosis could mediate CD95-dependent death of bystander cells, as a measure of their ability to produce functional soluble CD95L. As described in detail in Materials and Methods, surface CD95 was blocked with neutralizing anti-CD95 MoAb ZB4 to prevent possible sequestration of the produced soluble CD95L, then Th clones were treated for induction of gp120-apoptosis (ZB4 MoAb remains stuck to cell surface for at least 4 hours after treatment, not shown). Supernatants collected after 1 hour were tested for cytotoxicity on CD95+ Jurkat cells, an assay already described for determination of soluble CD95L.33 As shown in Fig 8, supernatants from the Th1 clone 103 (two different experiments are shown) or from the Th1 clone 3/12 were treated for gp120-apoptosis or stimulated with PMA/ionophore lysed Jurkat cells. The lysis was significantly higher (P < .05, Student's t-test) than that measured with control supernatants collected from cells untreated, treated with anti-TCR MoAb only (Fig 8) or with gp120/anti-gp120 MoAb only (not shown). Such lysis was CD95-dependent, as it was significantly inhibited (P < .05, Student's t-test) by anti-CD95 MoAb ZB4 (Fig 8) but not by isotype matched-control MoAb (normal mouse IgG1, not shown). Thus, the ability to lyse Jurkat cells is consistent with the presence of functional soluble CD95L. Moreover the positive supernatants were also able to lyse, in a CD95-dependent manner, CD95+ Th2 clone 3/39 (data not shown). Supernatants from similarly treated Th2 clones (3/39, F37 and 2/46) were unable to kill Jurkat cells (Fig 8) or the Th2 clone 3/39 used as target (not shown), suggesting an impaired production of soluble CD95L.
CD95-dependent lytic activity of supernatants from Th1 and Th2 clones. Th1 clones 103 (two independent experiments are shown) and 3/12 as well as Th2 clones 3/39, F37 and 2/46, were cultured in medium alone, or in the presence of immobilized anti-TCR MoAb, or treated with gp120/anti-gp120 and then cultured with immobilized anti-TCR (simply named gp120 in the figure), or stimulated with PMA/ionophore. After 30 minutes or 1 hour (just for the PMA/ionophore sample) supernatants were collected and tested on 51Cr labeled Jurkat cells, with or without the anti-CD95 neutralizing MoAb ZB4 (50 ng/mL). Mean ± SD of three replicates is shown.
CD95-dependent lytic activity of supernatants from Th1 and Th2 clones. Th1 clones 103 (two independent experiments are shown) and 3/12 as well as Th2 clones 3/39, F37 and 2/46, were cultured in medium alone, or in the presence of immobilized anti-TCR MoAb, or treated with gp120/anti-gp120 and then cultured with immobilized anti-TCR (simply named gp120 in the figure), or stimulated with PMA/ionophore. After 30 minutes or 1 hour (just for the PMA/ionophore sample) supernatants were collected and tested on 51Cr labeled Jurkat cells, with or without the anti-CD95 neutralizing MoAb ZB4 (50 ng/mL). Mean ± SD of three replicates is shown.
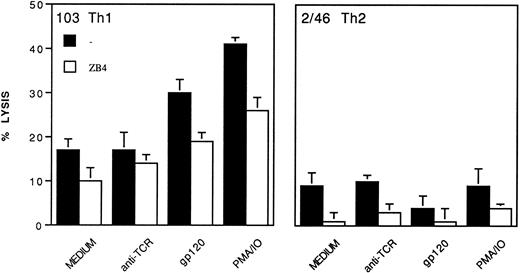
The ability of Th clones to kill bystander cells through membrane CD95L was also tested and shown in Fig 9. Also in this case, surface CD95 was preventively blocked with ZB4, then cells were treated for gp120-apoptosis or stimulated with PMA/ionophore. After 1 hour, the clones were tested as effector cells against 51Cr labeled Jurkat cells (ratio 20:1). Cells of Th1 clone 103 sensitized to gp120-apoptosis or stimulated with PMA/ionophore showed a significantly higher lytic activity (P < .05, Student's t-test) than control cells, which included untreated cells or cells treated with immobilized anti-TCR MoAb only (Fig 9) or with gp120/anti-gp120 without TCR triggering (not shown). The cytotoxic activity of Th1 cells sensitized to gp120-apoptosis or stimulated with PMA/ionophore was CD95-dependent, as it was significantly inhibited (P < .05, Student's t-test) by anti-CD95 neutralizing MoAb ZB4 (Fig 9) and not by isotype-matched control mouse MoAb (not shown).
CD95-dependent cell-mediated cytotoxicity of Th1 and Th2 clones on Jurkat cells. Th1 clone 103 and Th2 clone 2/46 were cultured in medium alone, or in the presence of immobilized anti-TCR MoAb, or treated with gp120/anti-gp120 and then cultured with immobilized anti-TCR (simply named gp120 in the figure), or stimulated with PMA/ionophore. After 1 hour, cells were collected, washed, and used as effectors in a 20-hour cytotoxicity test toward 51Cr labeled Jurkat cells, with or without the anti-CD95 neutralizing MoAb ZB4 (50 ng/mL). Effector to target ratio was 20:1. Mean ± SD of three replicates is shown.
CD95-dependent cell-mediated cytotoxicity of Th1 and Th2 clones on Jurkat cells. Th1 clone 103 and Th2 clone 2/46 were cultured in medium alone, or in the presence of immobilized anti-TCR MoAb, or treated with gp120/anti-gp120 and then cultured with immobilized anti-TCR (simply named gp120 in the figure), or stimulated with PMA/ionophore. After 1 hour, cells were collected, washed, and used as effectors in a 20-hour cytotoxicity test toward 51Cr labeled Jurkat cells, with or without the anti-CD95 neutralizing MoAb ZB4 (50 ng/mL). Effector to target ratio was 20:1. Mean ± SD of three replicates is shown.
DISCUSSION
Depletion of CD4+ T lymphocytes during AIDS progression cannot be explained only with a direct cytopathic effect of HIV.6-11 The number of CD4+ infected cells is well below that of CD4+ cells that die during progression11 and it has been proposed that uninfected CD4+ T lymphocytes are eliminated by apoptosis.6-11 Apoptosis of uninfected CD4+ cells was detected in vivo in lymph nodes from HIV-infected individuals26 and increased spontaneous apoptosis has been reported in PBMC from HIV+ patients compared with healthy controls.4,37,38 In vitro, gp120-apoptosis (following treatment with gp120/anti-gp120 MoAb and immobilized anti-TCR MoAb) could be induced on isolated CD4+ lymphocytes2-4 as well as on a CD4+ Th1 clone1 from seronegative donors. These in vitro models could mimic the in vivo situation where circulating gp120 as well as membrane associated gp120 expressed on the surface of HIV-infected cells or on viral particles could bind CD4 molecules of uninfected lymphocytes.3
Here we analyze the susceptibility to gp120-apoptosis of CD4+ T-cell clones with different Th phenotypes obtained from various seronegative donors. We show that Th1 clones are susceptible, whereas Th2 clones are resistant to gp120-apoptosis, despite the fact that both cell types bind gp120 to the same extent. Although the percentage of apoptotic cells never exceeded 25%, the strength of these data rely on the reproducibility of the results among 18 different experiments. However, it is a general finding that a low percentage of cells undergoes apoptosis after CD4/TCR triggering since similar results were reported by others using bulk PBL or purified CD4+ cells.2-5 When apparently higher numbers of apoptotic cells are reported, the data are not given as a crude number of apoptotic cells, but rather they are referred to a positive control to which a value of 100% is assigned.2-5 For example, using the calculation method published by Banda et al,3 the net value of 15% (clone 3/12 treated with 100 μg/mL gp120) in Fig 1B, became 51.7% if calculated against a value of 29% from γ-irradiated cells used as positive control in the same experiment.
Moreover, gp120-sensitized apoptosis is commonly tested 24 to 72 hours after induction,2-5 whereas in T-cell clones we were able to detect such apoptosis at as early as 4 hours by TUNEL. Several methods could detect apoptosis, but some are better than others when applied to different stages of apoptotic death.39 In our experiments we reproducibly observed that the early stages of apoptosis (4 hours) are better revealed by TUNEL, while the later stages (24 hours) by PI staining, possibly because TUNEL detects early DNA breaks while PI staining detects DNA loss following fragmentation.40 In addition, in our system, it seems that the cells undergoing gp120-apoptosis proceed through the apoptotic steps altogether. This may imply that most, if not all, apoptotic cells are in the early step of DNA fragmentation at 4 hours and then they enter a later stage of apoptosis in which DNA loss is the hallmark. Thus, in our system the differences between TUNEL and PI method for apoptosis determination are particularly evident. TUNEL poorly detects a late stage (24 hours) of gp120-apoptosis, whereas it is detectable by PI staining, possibly because reduction in DNA content or cell contraction and nuclear condensation could impair the fixing of dUTP-FITC by TdT. A similar phenomenon was described as occurring with other DNA dyes.40 However, the important point is that independently of the method used for detection of apoptosis, Th1 but not Th2 clones undergo gp120-apoptosis.
These data on differential susceptibility to gp120 apoptosis in Th1 versus Th2 clones are in agreement with clinical observations suggesting a progressive Th1/Th2 unbalance during the course of AIDS.21,22,25,41-44 Th1 cells are more susceptible than Th2 cells to gp120-apoptosis, and it is possible that early disappearance of Th1 clones accounts for the observed shift in cytokine production. The relative resistance of Th2 cells to gp120-apoptosis might also reflect a virus strategy for its survival. It has been hypothesized that HIV may not kill its host cells (representing a viral factory) but preferentially induces killing of bystander cells10: the former may well be represented by Th2 cells, which are better recipients for HIV replication,25 and the latter by Th1 cells. Although the in vivo situation is likely more complex because of cell-cell, cell-tissue interactions and other factors (including defects in Th cell renewal)45 that can contribute to CD4+ T-cell depletion, preferential elimination of uninfected Th1 cells could also favor HIV escape from host immune control.46-49 In several models, TCR stimulation can induce both T-cell proliferation and apoptotic cell death. This physiologic phenomenon, known as activation-induced apoptosis, regulates peripheral cell selection as well as duration of the immune responses.13,14 50
Previous studies have demonstrated that activation-induced apoptosis depends on CD95/CD95L interaction16,18-20: stimulation via TCR has been reported to increase CD95 expression in T-cell hybridomas19,20 and CD95L expression in CTL clones,15 in T-cell hybridomas17,19,20 and in the Jurkat cell line.18 gp120-Apoptosis was not analyzed in that contest. It has also been reported that CD4 cross-linking by anti-CD4 MoAb or by gp120-anti-gp120 MoAb could induce CD95 expression in PBL,51 and the hypothesis that gp120-apoptosis may represent an exacerbation of the activation-induced apoptosis has been proposed.12 CD4+ T-cell clones or hybridomas that do not undergo apoptosis after treatment with suboptimal doses of immobilized anti-TCR MoAb do so if pretreated with gp120 plus anti-gp120 MoAb. These investigators show that HIV-tat increased sensitivity to TCR and gp120-sensitized apoptosis by upregulating CD95L. However, the distinct role of tat and gp120 in inducing CD95L as well as the role of CD95L in the gp120-apoptosis, as we describe for Th subtypes, was not clarified. The data reported here provide, to our knowledge, the first direct demonstration that, like activation-induced apoptosis, the gp120-apoptosis occurs in Th1 clones via CD95/CD95L interaction. Several lines of evidence support this conclusion: (1) the anti-CD95 neutralizing MoAb ZB4 and the anti-CD95L neutralizing MoAb 4H9 inhibit gp120-apoptosis; (2) supernatants from gp120 treated Th1 clones contain CD95L, detected by specific ELISA; (3) Th1 clones triggered to gp120-apoptosis express surface CD95L, detected by immunofluorescence; (4) induction of CD95L in a Th1 but not in a Th2 clone, after triggering of gp120-apoptosis, was detected by Western blot analysis. This last assay revealed at least two forms of CD95L, which likely correspond to the mature and to the nonglycosylated forms already described by others36; (5) CD95L produced by Th1 clones treated for gp120-apoptosis is functional, as both cells and their supernatants efficiently lyse CD95+ Jurkat cells according to a published protocol.33 The lysis is inhibited by the anti-CD95 neutralizing MoAb ZB4. Jurkat cell killing measured by 51Cr release assay can also be followed by morphologic analysis and PI staining, both showing the apoptotic death of Jurkat cells (not shown).
Altogether, the results consistently indicate that Th1 clones triggered to gp120-apoptosis express CD95L in both cell-associated and soluble forms.52 However, bystander cell killing attributed to the cell-associated CD95L may be over-estimated, since the incubation of Th1 clone and Jurkat cells is protracted for 20 hours, and soluble CD95L released during this time may contribute to the Jurkat killing.
Inhibition of gp120-apoptosis by ICE inhibitor indicates that a member of the ICE family of proteases, commonly activated by many apoptotic triggers,53 is involved in the gp120-apoptosis, probably as a downstream signal that follows CD95/CD95L interaction. To be able to measure apoptosis sensitized by gp120 triggering of CD4 followed by TCR activation, it is necessary to calibrate the amount of anti-TCR MoAb below the threshold that alone induces T-cell apoptosis. In our system, anti-TCR MoAb was always used at 5 μg/mL and it never triggered the so-called activation induced apoptosis13,14: the value of apoptosis that followed anti-TCR treatment never exceeded that measured in cells cultured in medium only. Accordingly, when used alone, this dose of anti-TCR MoAb was unable to trigger CD95L production by Th1 clones, as shown by immunofluorescence, Western blot, ELISA, and bioassay on Jurkat cells. Higher doses (exceeding 20 μg/mL) of anti-TCR MoAb were, per se, capable of inducing CD95L and cytotoxicity of Jurkat cells (not shown). Accordingly, Westendorp et al12 showed that stimulation of Jurkat cells with 10 μg/mL of anti-CD3 MoAb for 1 hour (a suboptimal concentration chosen to give only minor apoptosis in the absence of HIV tat or gp120) did not induce CD95L mRNA; on the contrary, the same dose of anti-CD3 MoAb induced CD95L mRNA when combined with HIV-tat.
CD95L is reported to be preferentially inducible in Th1 rather than in Th2 clones54,55 and Th1 clones are more susceptible to activation-induced cell death than Th2 clones.54 Here we confirm these findings in the gp120-apoptosis model, in which functional CD95L is produced by Th1 but not Th2 cells. Based on these data, we propose that HIV-gp120 primes uninfected CD4+ T cells for activation-induced apoptosis, that then occurs in Th1 cells, through CD95/CD95L interaction, and that the resistance of Th2 clones to gp120-apoptosis depends, at least in part, on impaired production of CD95L.
Our data do not exclude that CD95+ Th2 cells may undergo apoptosis in vivo following interaction with the CD95L induced in Th1 clones in either membrane-anchored or soluble form. The results in vitro that Th2 clones do not undergo apoptosis when cultured alone in conditions capable of inducing gp120-apoptosis but die when exposed to supernatants containing CD95L from activated Th1 clones may correlate with the in vivo situation, in which CD95-dependent killing of Th2-Th0 clones by Th1 cells (expressing CD95L) has been hypothesized as a possible mechanism of regulation of immune responses.56 This model is in agreement with clinical studies showing both a high level of CD95 expression in T lymphocytes from HIV-infected patients,57-59 and a high susceptibility of HIV-transformed T-cell lines to anti-CD95–induced apoptosis.60
ACKNOWLEDGMENT
We are indebted to S. Nagata for kindly providing recombinant FasL and anti-FasL MoAbs and to B. Perussia for critically reviewing the manuscript. We are grateful to E. Fontanella for technical assistance.
Supported by the Italian Ministry of Health (VI, VII and VIII projects on AIDS). Paola Accornero and Marina Radrizzani equally contributed to this work.
Address reprint requests to Mario P. Colombo, PhD, Division of Experimental Oncology D, Istituto Nazionale Tumori, via Venezian 1, 20133 Milan, Italy.