Abstract
Multinucleated giant cells (MGC) are a common feature of granulomas that develop during various inflammatory reactions. MGC originate from fusion of monocytes or macrophages, but the exact mechanism of their generation is still unclear. In the present study, we investigated the influence of monocyte to macrophage maturation on the ability of human monocytes/macrophages to fuse with each other. MGC were generated in vitro by stimulation of human peripheral blood monocytes with cytokine containing supernatants. With freshly isolated monocytes, fusion rates of up to 90% were obtained. When monocyte to macrophage maturation was induced by culturing the cells in human serum, fusion rates gradually decreased with advancing time of the preceding culture (corresponding to the stage of differentiation) and almost no MGC formation could be obtained with 8-day-old macrophages. In contrast, fusion rates did not decrease when monocytes had been cultured under serum free conditions before stimulation. When freshly isolated monocytes were added to 1-week cultured macrophages, which had been membrane-labeled with a fluorochrome, fusion between the two populations could be induced. Because the ability for intracellular killing of certain pathogens is reduced in macrophages, fusion with monocytes (newly arriving at the site of inflammation) may represent an attempt to restore this capacity.
MULTINUCLEATED giant cells (MGC) are a common feature of granulomas that develop during certain infections, the most prominent example being tuberculosis, or as a consequence of foreign body reactions.1,2 Although the occurrence of these cells has been recognized and described in the middle of the last century,3 their role during the development and/or progression and termination of these inflammatory reactions is still poorly understood.
MGC originate from fusion of monocytes or macrophages, but little is known about the mechanisms of the fusion process itself. Furthermore, it is not clear how monocyte fusion is induced in vivo and whether different mechanisms are involved in different pathological states. A better understanding of the mechanism(s) of formation should also be helpful to define the role of MGC.
A number of possibilities have been reported on how the formation of MGC can be induced in vitro. These include the use of conditioned medium4-6 or several different cytokines,7-14 the addition of lectins alone or in combination with interferon (IFN)-γ,2,15 the addition of antibodies or phorbol myristate acetate (PMA) or a combination of both.2,16-20 Furthermore, MGC occur to a varying degree during long-term culture.21
For several of these approaches, the results are quite conflicting. Interleukin-4 (IL-4), eg, has been reported to promote,11-13,22 but also to inhibit9 15 MGC formation.
Among the cytokines that have been found to induce or enhance monocyte fusion, IFN-γ has a prominent role. Apart from being the cytokine that has been most often used for the in vitro generation of MGC,7-10 there is strong evidence that IFN-γ is the essential factor in conditioned medium promoting monocyte fusion.4,6 Moreover, in two in vivo models of granulomatous inflammation, antibodies to IFN-γ inhibit or reduce the formation of MGC.23,24 But even for IFN-γ the opposite effect, ie, prevention of fusion, has been described.25,26 Probably the most simple explanation for these differences would be that different culture conditions might have influenced MGC formation.6 In an attempt to clarify this problem, we started to investigate the influence of varying culture conditions and observed that the presence of human serum seemed to have a negative effect on the generation of MGC.
Human serum is known to induce the maturation of human monocytes into macrophages in vitro (reviewed in Dougherty and McBride21 ). Since there are conflicting results whether MGC are derived from monocytes or macrophages or both, we decided to investigate in more detail whether monocyte to macrophage maturation has an influence on the ability of monocytes/macrophages to fuse with each other.
In the following study, we present evidence that the ability of monocytes to form MGC when stimulated with cytokine containing supernatants is gradually lost during maturation into macrophages in vitro. However, macrophages are still able to fuse with freshly isolated monocytes. Since there is evidence that the monocytes' antimicrobial activity declines during maturation,21 27-29 fusion with monocytes could be of benefit for macrophages that have taken up certain bacteria or parasites.
MATERIALS AND METHODS
Isolation and culture of monocytes.Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood of healthy adult volunteers (members of laboratory staff ) by density gradient centrifugation with Ficoll-Paque (Pharmacia, Uppsala, Sweden). The cells were washed twice in phosphate-buffered saline (PBS) and were resuspended in medium RPMI 1640 (HyClone, Cramlington, UK) supplemented with 2 mmol/L glutamine (PAA Laboratories, Linz, Austria) and 10% fetal calf serum (FCS; PAA Laboratories). Finally, cells were added to 96-well microtiter plates (Costar, Cambridge, MA) at a density of 2 × 105 cells per well. After incubation for 1 hour at 37°C, the nonadherent cells were removed by repeated vigorous washings. For the evaluation of fusion between monocytes and macrophages (see below), monocytes were isolated on hydrophilic petri dishes (Petriperm; Bachofer, Reutlingen, Germany). The resulting cultures contained at least 90% monocytes as shown by morphology and phenotype fluorescence-activated cell sorting (FACS) analysis.6 30 Monocytes were cultured in the following media: Ultradoma (Bio-Whittaker, Verviers, Belgium), Ultradoma-PF (protein-free; Bio-Whittaker), RPMI 1640, RG (HyClone), CG (Vitromex, Vilshofen, Germany), and SF3 (Sebak, Suben, Austria). All these cultures were done without antibiotics and without FCS.
Viability was determined by trypan blue dye exclusion (trypan blue was purchased from Sigma, Munich, Germany).
Monocyte to macrophage maturation.Maturation of monocytes was induced by the addition of 10% human (autologous) serum. During the next 8 days differentiation into macrophages was followed with an inverted microscope (IMT-2; Olympus, Tokyo, Japan).
Maturation-associated changes in the expression of several antigens were evaluated with the aid of a cell–enzyme-linked immunosorbent assay31 (with minor modifications). Briefly, PBMC were added to a 96-well plate at a density of 1 × 106 cells per well. After incubation for 1 hour, the nonadherent cells were removed as described above. For detection of antigen expression, the following monoclonal antibodies (MoAbs) were used: INN-hFSH-8 (negative control, IgG1), INN-hFSH-9 (negative control, IgG2a; both MoAbs are directed against human follicle stimulating hormone and were kindly provided by Dr Peter Berger, Institut für experimentelle Pathologie, Universität Innsbruck, Innsbruck, Austria),32 DAKO-HLA-ABC (anti-HLA-class I antigen, IgG2a; DAKO, Glostrup, Denmark), DAKO-CD16 (anti-FcγRIII, IgG1; DAKO), MAX.1 and MAX.3 (directed against carboxypeptidase M and against another, as yet undefined maturation associated antigen, respectively; both MoAbs are IgG1 and were kindly provided by Dr Reinhard Andreesen, Klinik für Innere Medizin, Universität Regensburg, Regensburg, Germany).33,34 Detection of bound MoAbs was performed as described and the levels of antigen expression were quantified in relation to the expression of class I antigens.31
Generation of MGC.For most experiments, the formation of MGC was induced with conditioned medium.6 PBMC were cultured in RPMI 1640 supplemented with 10% FCS and 2 mmol/L glutamine at a density of 2 × 106 cells/mL and stimulated for 72 hours by addition of 16 μg/mL Concanavalin A (Con A; Sigma). The cell-free supernatant (conditioned medium) was stored at −20°C and added to monocyte/macrophage cultures at a final concentration of 50%. Alternatively, the supernatant of Herpesvirus saimiri-transformed T-cell clones35 was applied. The clones do not produce viral particles and were kindly provided by Dr Helmut Fickenscher (Institut für Klinische und Molekulare Virologie, Universität Erlangen, Erlangen, Germany). Two different controls were included in these experiments: supernatant from unstimulated PBMC and, second, medium containing 10% FCS, 2 mmol/L glutamine, and 16 μg/mL Con A. Both “control media” were obtained after incubation for 72 hours.
For a few experiments, a combination of PMA (Sigma; 5 nmol/L) and an anti-HLA-DR MoAb (IgG2a, DAKO; 1 μg/mL) was used to generate MGC.18
To disclose a possible influence of the stage of monocyte to macrophage maturation on MGC formation, monocytes were cultured with or without human serum and different preparations were stimulated on the day of isolation (day 0), on day 1, day 3, day 5, and day 8. Three days after each stimulation, the medium was removed and cells were stained with Giemsa (Sigma) in the microtiter plate. The plates were evaluated microscopically and the fusion rate of monocytes/macrophages was determined as described6 by counting the number of nuclei within MGC (more than 2 nuclei per cell) in a given area per total number of nuclei in that same area: fusion rate (%) = (number of nuclei within MGC/total number of nuclei counted) × 100. Between 300 and 400 nuclei from selected representative fields were counted for each single preparation.
Fusion between monocytes and macrophages.To determine whether macrophages could fuse with monocytes and thus could become part of MGC, we had to develop a method that allowed discrimination between MGC composed of monocytes and macrophages, and MGC consisting of monocytes alone. Several possibilities (ie, different staining protocols) were tested before the following approach was selected for further experiments. Macrophages, but not monocytes (which were known to be able to fuse), were labeled with the fluorescent dye PKH-2 (Sigma). After excitation at 490 nm PKH-2 is fluorescing green (emission maximum at 504 nm). The dye is stably incorporated into the lipid bilayer of cytoplasmic membranes and because of its inherent insolubility in aqueous environments, the probe is trapped once incorporated into the membrane.36 In a previous study, we could show that staining with this dye does not affect the fusion capacity of monocytes.37 Labeling of the cells was performed as described.37 Briefly, PBMC were isolated as described above and 1 × 107 cells/mL were mixed with an equal volume of PKH-2 at a concentration of 4 × 10−6 mol/L. The labeled PBMC were added to a 96-well microtiter plate at a density of 1 × 105 cells per well. The nonadherent cells were removed and the remaining monocytes were cultured with 10% human serum to induce maturation into macrophages. On day 8, PBMC were isolated either from the same or from a second donor.37 Monocytes were purified by adherence on hydrophilic petri dishes as described above. After 30 minutes of incubation on ice, monocytes were removed from the dishes by vigorous pipetting and added to the cultured macrophages at a concentration of 1 × 104 cells per well. These cocultures, as well as control cultures consisting of monocytes or macrophages alone (in twofold density), were stimulated with conditioned medium. After 3 days, nuclei were stained with bisbenzimide H 33258 fluorochrome (the so-called HOECHST dye; Calbiochem, San Diego, CA) and evaluation and documentation was performed with the inverted microscope supplemented with the appropriate equipment for dual wavelength fluorescence (filters for blue and UV light excitation).
Statistical analysis.Data are expressed as mean ± standard deviation (SD) of the indicated number of experiments. Differences between groups were analyzed for significance by Student's t-test.
RESULTS
Monocyte to macrophage maturation.During culture in medium Ultradoma-PF supplemented with 10% human serum, monocytes acquired the characteristic morphological features of maturating macrophages, whereas not much change in morphology was observed during culture with medium alone (Fig 1). Viability of freshly isolated monocytes was 98% or more and decreased during 1 week of culture to 85% to 90% (without serum) and 94% to 98% (with serum), respectively. Similar to observations made by others,38 a relatively wide variability was found between different experiments regarding loss of cells in the serum free preparations. Since this did not significantly influence the extent of MGC formation, we made no further attempts to quantify this loss.
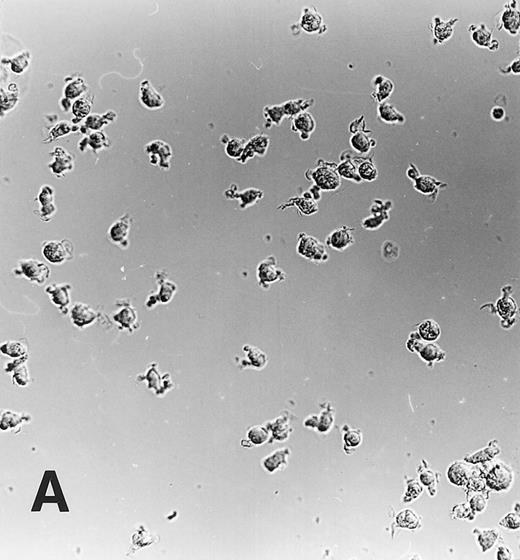
Maturation of monocytes into macrophages. Monocytes were cultured in the protein-free medium Ultradoma-PF without (A) or with (B) 10% human serum. In the presence of serum, the cells acquire the typical morphologic features of cultured macrophages. Photographs were taken on day 6 of culture. Giemsa staining, original magnification × 50.
Maturation of monocytes into macrophages. Monocytes were cultured in the protein-free medium Ultradoma-PF without (A) or with (B) 10% human serum. In the presence of serum, the cells acquire the typical morphologic features of cultured macrophages. Photographs were taken on day 6 of culture. Giemsa staining, original magnification × 50.
Maturation of monocytes could also be induced without the addition of human serum when cells were cultured in the serum free media CG and RG, but not (Ultradoma, RPMI 1640) or only partially (SF3) with the three others (not shown).
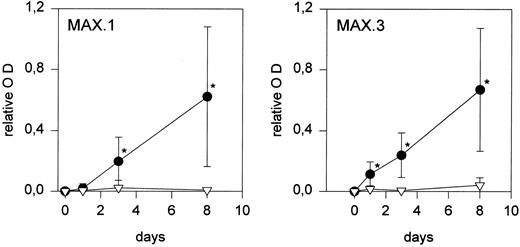
To further confirm that culture in human serum induced maturation into macrophages, the expression of differentiation associated antigens during an 8-day culture was determined. As expected, a markedly increased expression of the antigens recognized by MoAbs MAX.1 and MAX.3 was observed in cells cultured in serum (macrophages), whereas expression on serum free cultured cells (monocytes) remained low (Fig 2). A similar increase in the expression of FcγRIII was detected on macrophages (not shown).
Changes in antigen expression during monocyte to macrophage maturation. The expression of maturation-associated antigens MAX.1 and MAX.3 increases during culture in human serum (•), but not when monocytes are cultured under serum free conditions (▿). Each point represents the mean of five experiments performed in duplicate. Relative OD is the optical density of MAX.1 and MAX.3 divided by the optical density of the HLA class I MoAb (values of isotype specific controls were subtracted before). Statistically significant differences (P < .05) are indicated by an asterisk.
Changes in antigen expression during monocyte to macrophage maturation. The expression of maturation-associated antigens MAX.1 and MAX.3 increases during culture in human serum (•), but not when monocytes are cultured under serum free conditions (▿). Each point represents the mean of five experiments performed in duplicate. Relative OD is the optical density of MAX.1 and MAX.3 divided by the optical density of the HLA class I MoAb (values of isotype specific controls were subtracted before). Statistically significant differences (P < .05) are indicated by an asterisk.
Induction of monocyte fusion and influence of monocyte to macrophage maturation on the formation of MGC.For most experiments, the formation of MGC was induced by stimulation with conditioned medium for 3 days (Fig 3A). With freshly isolated monocytes, fusion rates of up to 90% were obtained. As already noticed previously,37 a relatively wide variation of fusion rates between different donors was observed. In control cultures to which supernatant from unstimulated PBMC had been added for the same time, no MGC formation occurred. When monocytes were stimulated with medium containing FCS and Con A in the same concentrations as in the conditioned medium, isolated MGC were seen in a few experiments, fusion rates being always less than 5%.
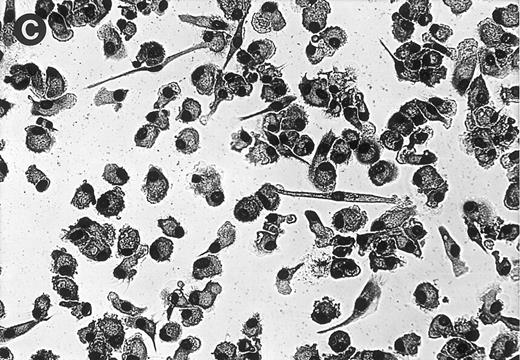
(A) MGC derived from freshly isolated monocytes (day 0) stimulated with conditioned medium for 3 days. Giemsa, original magnification × 25. (B) Generation of MGC with PMA and HLA class II MoAb. Monocytes were cultured for 6 days in the presence of 10% human serum before stimulation. Giemsa, original magnification × 25.
(A) MGC derived from freshly isolated monocytes (day 0) stimulated with conditioned medium for 3 days. Giemsa, original magnification × 25. (B) Generation of MGC with PMA and HLA class II MoAb. Monocytes were cultured for 6 days in the presence of 10% human serum before stimulation. Giemsa, original magnification × 25.
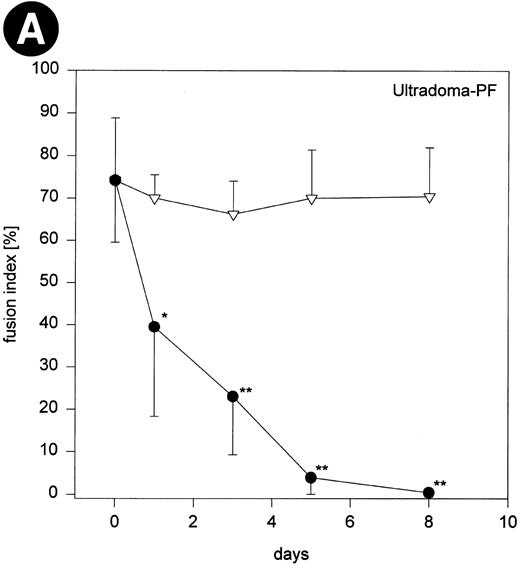
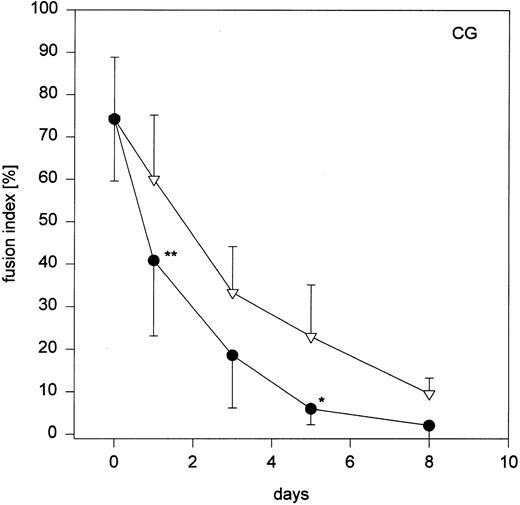
When monocytes that had been cultured in Ultradoma-PF with human serum were stimulated with conditioned medium, fusion rates gradually declined with advancing time of the preceding culture (corresponding to the stage of differentiation) and almost no MGC formation could be obtained with 8-day-old macrophages (Fig 4). In contrast, fusion rates did not decrease when monocytes had been cultured for the same time in Ultradoma-PF under serum free conditions. Very similar results were obtained when monocytes were cultured in Ultradoma, RPMI 1640 or SF3 (Fig 4). However, a comparable decrease of fusion rates was observed following culture in the two media that are able to induce maturation without the addition of serum (CG, RG), regardless of whether serum was present or not (Fig 4). To determine whether the loss of readiness for fusion during maturation is a more general phenomenon for cytokine-induced MGC formation, we used supernatants from Herpesvirus saimiri-transformed T-cell lines to induce fusion of monocytes/macrophages cultured in Ultradoma-PF. Although the fusion rates that could be obtained with this kind of stimulation were generally lower (mean 38% ± 12.5%), a trend similar to that observed with the conditioned medium was seen. Corresponding to the stage of maturation, fusion rates decreased to almost zero after a week of culture in human serum (not shown).
Fig 4 (cont'd). (A) Influence of monocyte to macrophage maturation on the formation of MGC. Monocytes were cultured with (•) and without (▿) human serum to induce/prevent macrophage maturation and stimulated with conditioned medium. Six different media were compared (Ultradoma-PF, Ultradoma, RPMI 1640, SF3, CG, and RG, the latter two inducing monocyte maturation by themselves). Each point represents the mean of six experiments performed with cells obtained from different donors. Deviations from the mean are caused mainly by the donor variability found on day 0 (fusion rates ranging from 55% to 90%). The capacity to fuse is gradually lost during maturation. Asterisks indicate statistically significant differences between cultures with and without human serum (*P < .05, **P < .01). Two typical examples are shown in the photographs. Following culture in protein-free medium (Ultradoma-PF ) without (B) or in the presence of (C) human serum, formation of MGC is still possible (B) or markedly reduced (C). Stimulation was performed on day 5. Giemsa, original magnification × 50.
Fig 4 (cont'd). (A) Influence of monocyte to macrophage maturation on the formation of MGC. Monocytes were cultured with (•) and without (▿) human serum to induce/prevent macrophage maturation and stimulated with conditioned medium. Six different media were compared (Ultradoma-PF, Ultradoma, RPMI 1640, SF3, CG, and RG, the latter two inducing monocyte maturation by themselves). Each point represents the mean of six experiments performed with cells obtained from different donors. Deviations from the mean are caused mainly by the donor variability found on day 0 (fusion rates ranging from 55% to 90%). The capacity to fuse is gradually lost during maturation. Asterisks indicate statistically significant differences between cultures with and without human serum (*P < .05, **P < .01). Two typical examples are shown in the photographs. Following culture in protein-free medium (Ultradoma-PF ) without (B) or in the presence of (C) human serum, formation of MGC is still possible (B) or markedly reduced (C). Stimulation was performed on day 5. Giemsa, original magnification × 50.
When PMA and class II MoAb, a combination that has been reported to induce the formation of MGC,18 were added to freshly isolated monocytes, most cells died during the next 3 days. However, when added to one or more days cultured monocytes/macrophages, this kind of stimulation readily induced the formation of MGC. Interestingly, in the presence of human serum, a very high fusion rate could be obtained (consistently ranging between 80% and 90% from day 1 to day 8), whereas under serum free conditions, fusion rates decreased from about 50% on day 1 to 10% to 20% on day 8 (not shown). Furthermore, a quite different appearance of MGC with very large syncytia was seen in the presence of serum (Fig 3B).
Fusion between monocytes and macrophages.To find out whether the macrophages' ability to fuse following stimulation with conditioned medium is lost completely or not, we wanted to test whether macrophages could still be fused to freshly isolated monocytes. For this purpose, a possibility to differentiate between the two cell populations was thought to be crucial. This discrimination was achieved by labeling the cell membranes of the macrophages with the fluorescent dye PKH-2 as described above. The combination of membrane labeling and nuclear staining allowed a clear-cut identification of MGC together with the assessment of the composition of a particular MGC. An unexpected finding was that the nuclei became visible (green) also in the blue excitation filter mode after excitation with UV light used to evaluate staining with the HOECHST dye.
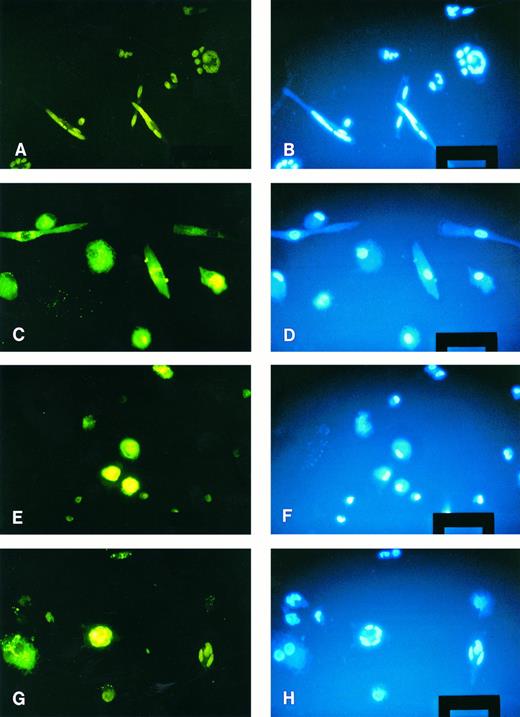
When freshly isolated monocytes were added to 1-week cultured stained macrophages, stimulation with conditioned medium induced the generation of MGC. About one half of these MGC displayed green fluorescence, indicating that macrophages had become part of these MGC and that fusion between the two populations had occurred (Fig 5G and H). Thus, the diminished fusion capacity of macrophages when stimulated with conditioned medium is relative rather than absolute. As can also be seen in Fig 5, labeling of the cells with PKH-2 or the HOECHST dye had no influence on the fusion capacity of monocytes/macrophages. High fusion rates were obtained with freshly isolated monocytes (Fig 5A and B) and no MGC formation was seen when 1-week cultured macrophages were stimulated (Fig 5C and D).
Fusion between monocytes and macrophages. Macrophages were membrane labeled with the fluorescent dye PKH-2 (green). Monocyte membranes were not labeled, nuclei of both cell types were stained with the HOECHST dye (blue). (A) and (B) Freshly isolated monocytes stimulated for 3 days; several MGC have formed. (C) and (D) 1-week cultured macrophages stimulated with conditioned medium; no fusions have occurred. (E) and (F ) Freshly isolated monocytes added to 1-week cultured macrophages without stimulation; no spontaneous MGC formation. (G) and (H) Stimulation of monocytes and macrophages; two MGC can be seen, one derived from monocytes only, the other containing at least one macrophage. Original magnification × 100 (bar = 50 μm).
Fusion between monocytes and macrophages. Macrophages were membrane labeled with the fluorescent dye PKH-2 (green). Monocyte membranes were not labeled, nuclei of both cell types were stained with the HOECHST dye (blue). (A) and (B) Freshly isolated monocytes stimulated for 3 days; several MGC have formed. (C) and (D) 1-week cultured macrophages stimulated with conditioned medium; no fusions have occurred. (E) and (F ) Freshly isolated monocytes added to 1-week cultured macrophages without stimulation; no spontaneous MGC formation. (G) and (H) Stimulation of monocytes and macrophages; two MGC can be seen, one derived from monocytes only, the other containing at least one macrophage. Original magnification × 100 (bar = 50 μm).
DISCUSSION
During our efforts to define the influence of varying culture conditions, we observed a negative effect on the generation of MGC when human serum was present. The addition of human serum to monocytes isolated from peripheral blood induces a process of maturation into macrophages comparable to that occurring in vivo during/following the migration of monocytes to the different tissues.21 We, therefore, investigated whether monocyte to macrophage maturation has an influence on the readiness of monocytes/macrophages to form MGC and could demonstrate that the ability of monocytes to fuse with each other after stimulation with cytokine-containing conditioned medium is gradually lost during in vitro maturation into macrophages. This difference is clearly due to maturation and not caused by the presence of a factor(s) in human serum, which could prevent or interfere with fusion. Human serum was most likely not present during the final 3 days of stimulation, since cells were thoroughly washed before the supernatant was added. If trace amounts of human serum were responsible for inhibition of fusion, the time dependence of the diminished fusion capacity could not be explained. Furthermore, a comparable effect was observed when maturation was induced with serum free media from different sources developed to promote the maturation of macrophages (though it is not clear which components of human serum are responsible for the maturation process, several factors have been identified which can, at least in part, produce this effect21 ).
The observation that the capacity to fuse following stimulation with conditioned medium is lost in mature macrophages was unexpected. First, during long-term culture of monocytes (and in most cases under conditions that promote maturation, ie, in the presence of human serum) “spontaneous” formation of MGC occurs to a varying degree.21 Second, many of the studies investigating MGC formation have been performed with macrophages from different species2,8,11,16,17,19,26,39 or in the presence of human serum,4,7,9,10,13 and only a few studies were done under conditions that do not promote monocyte maturation.5,6,15,20,40 Moreover, in some instances, MGC formation was either linked to maturation or maturation was considered as a prerequisite for the generation of MGC. Induction of MGC formation with PMA, eg, was only possible when macrophages had been cultured for 3 weeks before stimulation,19 and IL-3 and granulocyte macrophage colony-stimulating factor (GM-CSF ), in addition to their accelerating effect on maturation, enhanced fusion induced by IL-4 and IFN-γ.13 Finally, the phagocytic potential of macrophages (representing a different membrane dependent cellular function) is increased rather than diminished in comparison to monocytes,21 and macrophages appear to respond generally better than monocytes when stimulated with IFN-γ.41
To our knowledge, this study is the first systematic investigation regarding the time dependence of monocyte/macrophage fusion. However, changes in the readiness to fuse have been reported previously. With one exception,16 higher fusion rates were observed when cells were cultured for a few days5,13,42 or even weeks19 before stimulation.
Thus, at least in vitro different mechanisms for the induction of MGC formation appear to exist. This is supported by our finding that the observed time dependence of fusion capacity was not seen using a completely different kind of stimulation, ie, the addition of PMA and MHC class II MoAb. Nevertheless, the results obtained with supernatants from immortalized T-cell clones indicate that maturation dependence is a general feature of cytokine-induced MGC formation. At least in part the reported divergences may be caused by different responses of monocytes/macrophages in different stages of maturation, as has been suggested by McNally et al13 for the varying effects of IL-4 and by Nagasawa et al8 and Vignery et al26 for IFN-γ.
Despite the numerous possibilities to induce formation of MGC in vitro, the presence of MGC in inflammatory reactions in vivo is restricted to relatively few diseases. With this in mind, probably the most interesting question in this context is, which in vitro model most closely corresponds to the in vivo situation.
Only a few studies have addressed the question of whether in vivo MGC originate from fusion of monocytes or macrophages or both.43-46 It appears, however, that young or freshly infiltrating monocytes play the key role for the formation of MGC in these in vivo experiments. A second interesting observation in these studies was that the generation of MGC required only a few days (quite prolonged times were necessary for the generation of MGC in various in vitro systems4,8-10,13 25 ). Thus fusion occurred before infiltrating monocytes could have developed into mature macrophages (which does, of course, not exclude that resident macrophages have contributed to the formation of MGC). These observations fit quite well with the conclusions drawn from our data and suggest that at least some of our findings can be conferred to the in vivo situation.
In a third point, there is agreement between our in vitro system and the generation of MGC in vivo: the elimination of IFN-γ both in vivo23,24 and in vitro6 prevents or at least reduces the generation of MGC.
As far as the role of IFN-γ for fusion is concerned, it is interesting that this cytokine was reported to suppress the maturation of monocytes.47
During the preparation of this manuscript, Fais and Pallone48 reported that intestinal macrophages, in contrast to peripheral blood monocytes, were not able to fuse if stimulated with IFN-γ for 10 days. Despite the somewhat different kind of stimulation used, these findings closely resemble our results with in vitro differentiated macrophages, indicating that in vivo differentiated macrophages are also characterized by a reduced readiness for fusion. Taken together, we are convinced that there is considerable evidence that monocytes infiltrating the site of inflammation are the primary source of inflammatory MGC.
The molecular mechanisms responsible for the reduced fusion capacity of macrophages remain to be determined. Our observations open the possibility to study two clearly defined conditions with a marked difference in the ability to form MGC using the same kind of stimulation and the same cells, albeit in different stages of maturation.
Regarding the functional role of MGC, several quite different speculations have been put forward. MGC have been proposed to function as a disposal mechanism for effete macrophages,45 to exert specific functions in the phagocytosis and/or intracellular killing of microorganisms,5,49,50 or to be beneficial by supporting the sequestration of the pathogen during cryptococcal infection.51 But again, results concerning the functional capacity of MGC, in particular of phagocytosis and/or production of various factors have been controversial.5,45,49 50
Enelow et al9,50 have speculated that the ability to continually recruit fresh monocytes for fusion might confer to the MGC an enhanced ability to sequester organisms resistant to macrophage killing and to inhibit their growth. Based on our findings, we would like to emphasize this hypothesis by providing an additional explanation why the formation of MGC could be beneficial during the response of the organism against certain pathogens. There is evidence that monocytes lose their capacity for killing or inhibition of the intracellular replication of certain bacteria and parasites during maturation into macrophages.21 27-29 Fusion between macrophages having ingested such pathogens and monocytes newly arriving at the site of infection/inflammation could represent the attempt to restore the macrophages' antimicrobial activity, particularly when several monocytes fuse with one macrophage. If this assumption is correct, fusion between two macrophages would be of no benefit, moreover, it would be quite unfavorable. Thus, the observed inability of macrophages to fuse with other macrophages (in contrast to fusion with monocytes) could be most reasonable.
ACKNOWLEDGMENT
We thank Drs Reinhard Andreesen and Peter Berger for providing MoAbs, Dr Helmut Fickenscher for the T-cell clones, Drs Marina Kreutz and R. Andreesen for helpful discussions about monocyte maturation, and Reinhard Würzner for critical reading of the manuscript.
Supported by the Austrian Fonds zur Förderung der wissenschaftlichen Forschung (project P 09581).
Address reprint requests to Johannes Möst, MD, Institut für Hygiene, Fritz-Pregl-Str. 3, A-6020 Innsbruck, Austria.