Abstract
Interferon-α (IFN-α) is an established treatment for chronic myelogenous leukemia (CML) in chronic phase, but the mechanism of its antileukemic activity is not clear. One possible mechanism of action might include the induction of apoptosis, and especially Fas-mediated cell killing may play an important role in the elimination of malignant cells. We investigated Fas receptor (Fas-R) expression and the consequences of Fas-R triggering in CML patients. Using two-color flow cytometry, we found a significantly higher number of Fas-R–expressing CD34+ cells in the bone marrow (BM) of CML patients compared with normal subjects. We have previously shown that IFN-γ induces Fas-R expression on CD34+ cells; in this study, we investigated whether IFN-α induces Fas-R expression on CML progenitor cells. Dose-dependent induction of Fas-R expression was observed after IFN-α stimulation of CD34+ cells from CML BM. In methylcellulose culture, IFN-α alone at a therapeutic concentration showed only marginal antiproliferative effects on both normal and CML BM progenitors. In contrast, a Fas-R agonist, the anti-CD95 monoclonal antibody CH11, inhibited colony formation from normal progenitors, and the inhibition was even stronger on CML progenitors. When CML BM cells were cultured in the presence of IFN-α, Fas-R–mediated inhibition of colony growth was potentiated in a dose-dependent fashion, consistent with IFN-α induction of Fas-R expression. This functional effect did not require the presence of accessory cells, since similar results were obtained with purified CD34+ cells. In suspension cultures, we demonstrated that suppression of CML hematopoiesis by IFN-α and Fas-R agonist was exerted through Fas-R–mediated induction of apoptosis. Our findings suggest that the Fas-R/Fas-ligand system might be involved in the immunologic regulation of CML progenitor growth and that its effect can be amplified by IFN-α.
CHRONIC MYELOGENOUS leukemia (CML) arises from the clonal expansion of an altered stem cell capable of differentiation into mature myeloid cells.1-3 The Philadelphia chromosome (Ph) is the cytogenetic hallmark of the disease.4,5 The reciprocal translocation t(9,22) results in the creation of a chimeric bcr/abl gene6 believed to play a central role in leukemogenesis; mice transgenic for the fusion gene develop leukemia.7 Induction of clonal expansion by bcr/abl may be due to increased tyrosine kinase activity.8 Although the bcr/abl gene product may play a role in the inhibition of the apoptotic machinery,9-12 in some experimental systems a normal reaction of CML cells to death-inducing stimuli has been observed.13 CML bone marrow (BM) progenitor cells respond to colony-stimulating factors, but their adhesion to stroma is impaired, resulting in a loss of sensitivity to stromal inhibitory signals.14-17
Several clinical trials have demonstrated that administration of interferon-α (IFN-α) is an effective treatment for CML in chronic phase: cytogenetic remission can be induced in 20% of patients treated with IFN-α, and long-term survival is improved compared with regimens including hydroxyurea and busulfan.18-21 A number of in vitro effects of IFN-α on CML cells has been described, such as direct inhibition of CML BM progenitor growth22,23 and restoration of the adherence to stroma.24-26 It has also been reported that IFN-α regulates the paracrine release of growth factors by human BM stroma by inhibiting the production of stimulatory cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-1β (IL-1β), and granulocyte colony-stimulating factor (G-CSF), as well as by increasing the production of inhibitory cytokines such as IL-1 receptor antagonist, transforming growth factor-β, and macrophage inflammatory protein-1α.26-28 However, the exact mechanism and especially the selectivity of IFN-α effects on CML cells remains a subject of controversy.29
Inhibitory cytokines and cellular immune surveillance may play an important role in the control of expansion of the leukemic clone. In normal hematopoiesis, both direct T-cell–mediated hematopoietic suppression30 and inhibitory cytokine pathways have been described.31,32 IFN-γ and tumor necrosis factors (TNFs), alone or in combination with Fas receptor (Fas-R) agonists, inhibit the proliferation of hematopoietic progenitor cells, at least partially via induction of apoptosis.33-35 The inhibitory effects of IFN-γ seem to be mediated through induction of IFN regulatory factor-1 (IRF-1).36 Fas-R is expressed on activated lymphoid cells,37-41 in several hematologic malignancies,42-48 and also on normal CD34+ cells.35,36 Since the Fas-R/Fas ligand (Fas-L) system may be important in the elimination of virus-infected49,50 or malignant cells42-48 and since the Fas-R has been reported to be upregulated by IFN-γ and TNF-α on CD34+ cells34-36 and by IFN-α on Daudi cells,51 we hypothesized that the Fas-R/Fas-L system might be involved in the mechanisms responsible for the antileukemic effects of IFN-α in CML. Therefore, we investigated the expression and induction pathway of Fas and the consequences of Fas triggering in BM progenitor cells derived from CML patients.
MATERIALS AND METHODS
Specimen collection.BM samples were obtained from 41 normal subjects, 45 patients with CML, and five patients with acute myeloid leukemia. Informed consent was obtained according to the protocol approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (Bethesda, MD) and the Hematology Division, Federico II University (Naples, Italy). The diagnosis of CML was confirmed by the cytogenetic finding of the Ph chromosome. Of the patients with CML, 40 were in chronic phase and five in blastic crisis (and untreated at the time of specimen collection). None of the patients were receiving IFN-α at the time of BM sampling or at least within 2 months before BM sampling.
BM cell separation.BM was aspirated from the posterior iliac crest into syringes containing Iscove's modified Dulbecco's medium (IMDM) supplemented 1:10 with heparin (O'Neil & Feldman, St Louis, MO). Mononuclear cells (BM-MNC) were isolated by density-gradient centrifugation using lymphocyte separation medium (Organon, Durham, NC). After washing with Hanks' balanced salt solution (HBSS), cells were resuspended in IMDM supplemented with 5% fetal calf serum (FCS). HBSS, IMDM, and FCS were purchased from Life Technologies (Gaithersburg, MD).
Flow cytometric analysis.A fluorescein isothiocyanate (FITC)-conjugated (Fab′) fragment of a murine anti-human CD95 (clone UB2; Amac, Westbrook, ME) was used to determine the expression of Fas-R on BM cells. For two-color analysis, phycoerythrin (PE)-conjugated monoclonal antibody (MoAb) to CD34 (Becton Dickinson, Mountain View, CA) was used in combination with FITC-conjugated CD95 MoAb. Proper isotypic controls were used in all experiments. BM-MNC were resuspended in 100 μL phosphate-buffered saline ([PBS] Life Technologies) containing 2% FCS, incubated with 20 μL MoAb, washed three times with PBS, and analyzed by flow cytometry (Epics; Coulter, Hialeah, FL). Fas expression on CD34+ cells was analyzed in a uniformly set blast gate.
Separation of CD34+ cells.In some experiments, purified CD34+ cells were used for analysis of Fas-R expression or for colony assay. CD34+ cells were separated using affinity columns (Cellpro, Bothel, WA). Briefly, nonadherent BM cells were incubated at room temperature with streptavidin-conjugated murine anti-human CD34 IgM, washed with PBS, and applied to an affinity column containing biotin-coated beads; the CD34+ fraction was eluted with PBS. An aliquot of eluted cells was stained with PE-conjugated anti-CD34 HPCA-2 MoAb (Becton Dickinson) for purity assessment. Usually, 70% to 90% of separated cells were CD34+.
Macrophage and T-cell depletion.Macrophages and monocytes were depleted from BM samples using adherence to plastic. For T-cell depletion, BM-MNC were incubated on ice with CD3 MoAb (50 μL for 2 × 106 cells) for 30 minutes, washed with PBS, and then incubated with anti-mouse IgG–conjugated magnetic beads (Dynal, Oslo, Finland) on ice for 10 minutes before separation using a magnetic separator. This method yielded a virtually macrophage- and T-cell–free mononuclear BM cell population. The degree of residual contamination was evaluated using flow cytometry after staining cell aliquots with CD14 and CD3 MoAb. BM cell fractions used for further experiments usually contained less than 2% CD3+ cells and 1% CD14+ cells.
Hematopoietic colony assay and suspension cultures.Proliferation of hematopoietic progenitors was measured in methylcellulose cultures. Fresh total BM or isolated CD34+ cells were plated in methylcellulose (Stem Cell Technologies, Vancouver, CA) at a concentration of 1 × 105 or 1 × 103/mL medium (35-mm dishes, 1 mL medium per dish), respectively. The growth factor cocktail consisted of 10 ng/mL IL-3, 50 ng/mL G-CSF, 50 ng/mL GM-CSF, 20 ng/mL stem cell factor, and 2 U/mL erythropoietin (all factors kindly donated by Amgen, Thousand Oaks, CA). Recombinant IFN-α (Hoffmann La Roche, Basel, Switzerland) and anti-Fas MoAb (clone CH11; Amac) were used at a concentration range of 20 to 1,000 U/mL and 0.5 to 1.0 μg/mL, respectively. For control experiments, nonrelevant isotypic MoAbs (Dako, Carpinteria, CA) were used. All cultures were performed in duplicate. Suspension cultures were performed in 24-well plates in IMDM containing 20% FCS and growth factors at the concentrations already described. All experimental procedures were performed in endotoxin-free plasticware.
Apoptosis assay.DNA fragmentation was measured after extraction of low–molecular-weight DNA from a constant number of cells. Cells (2 × 106) were resuspended in 900 μL 1× Tris-EDTA buffer and lysed with 25 μL 20% sodium dodecyl sulfate. The high–molecular-weight DNA fraction was precipitated for 6 hours in the presence of 5 mol/L NaCl and pelleted by high-speed centrifugation. The fragmented DNA was then extracted from the aqueous phase with phenol and chloroform and then precipitated with ethanol. After resuspension in water, DNA was electrophoresed using 1.5% agarose gel and visualized by ethidium bromide staining.
Reverse transcriptase–polymerase chain reaction (RT-PCR) for human Fas RNA.Total RNA was extracted from constant numbers of purified CD34+ cells using RNAsol (Cina/Biotecx, Friendswood, TX). Contaminating DNA was digested using RNAse-free DNAse I (Boehringer, Indianapolis, IN). RNA was re-extracted with phenol/chloroform, precipitated with ethanol, and diluted in RNAse-free water. After reverse transcription using an oligo d(T)16 primer, Fas-R cDNA was amplified using the primer pairs 5′-GGACATGGCTTAGAAGTGGA-3′ and 5′-CTGCTGTG TCTTGGACATTG-3′ specific for human Fas-R simultaneously with primers specific for human β-actin (5′-CAATTGTGATGGACTCCGGAGACGG-3′ as an upstream primer and 5′-CATCTGCTG CTCGAAGTCTAGAGC-3′ as a downstream primer). For the amplification reaction, reagents supplied in the Amplimer kit (Perkin Elmer, Foster City, CA) were used. For reverse transcription and amplification, the following conditions were used: 45 minutes at 37°C and 5 minutes at 96°C for the RT reaction, and 30 cycles times 2 minutes at 96°C, 1.5 minutes at 55°C, and 2 minutes at 72°C for amplification. PCR products were electrophoresed in 1.2% agarose gels. The bands were visualized after staining with ethidium bromide and UV light exposure.
RESULTS
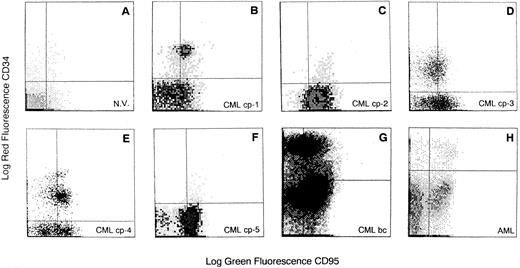
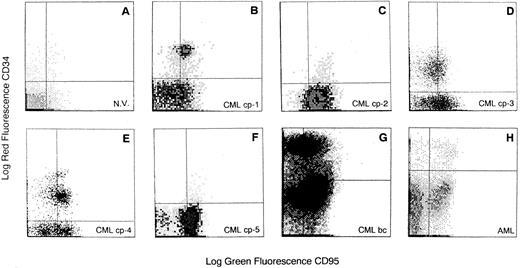
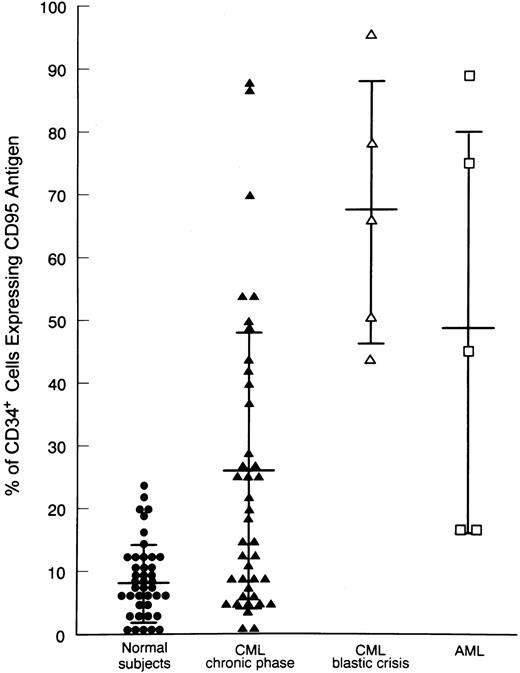
Expression of Fas antigen on BM cells from CML patients.We analyzed the expression of Fas-R (CD95) on normal BM cells derived from 41 donors and from 45 patients with CML. A significantly higher percentage of cells expressing Fas-R was found in CML patients (31.3 ± 24 v 10.7 ± 9, P < .05; Table 1). Using two-color flow cytometry with FITC-conjugated anti-CD95 and PE-conjugated anti-CD34 (Fig 1), we found in normal BM a mean of 8.4% ± 6% CD34+ cells expressing Fas-R, and in CML BM, 25.8% ± 22% (P < .05; Table 1). When five patients in blastic crisis were compared with those in chronic phase, a significantly increased Fas-R expression was found (Fig 2).
Flow cytometric analysis of Fas-R expression on CD34+ cells derived from BM of CML patients. Total BM cells were stained with PE-conjugated CD34 and FITC-conjugated CD95 MoAb. Quadrants were set on the basis of cells treated with PE- and FITC-labeled isotypic controls. N.V., BM sample from a healthy volunteer; CML cp-1 to cp-5, BM samples from 5 patients with CML in chronic phase; CML bc, BM sample from a patient with CML in blastic crisis; AML, BM sample from a patient with acute myeloid leukemia. BM CD34+ cells were analyzed in the blast gate for CD95 expression.
Flow cytometric analysis of Fas-R expression on CD34+ cells derived from BM of CML patients. Total BM cells were stained with PE-conjugated CD34 and FITC-conjugated CD95 MoAb. Quadrants were set on the basis of cells treated with PE- and FITC-labeled isotypic controls. N.V., BM sample from a healthy volunteer; CML cp-1 to cp-5, BM samples from 5 patients with CML in chronic phase; CML bc, BM sample from a patient with CML in blastic crisis; AML, BM sample from a patient with acute myeloid leukemia. BM CD34+ cells were analyzed in the blast gate for CD95 expression.
Expression of Fas-R on BM CD34+ cells from healthy subjects and CML patients. Values correspond to the percentage of CD95+CD34+ within total CD34+ cells (CD34+CD95+ + CD34+CD95−). Statistical analysis (nonparametric Kruskal-Wallis test): control v CML, P < .05. Horizontal bars are the mean values, and vertical bars are the SD.
Expression of Fas-R on BM CD34+ cells from healthy subjects and CML patients. Values correspond to the percentage of CD95+CD34+ within total CD34+ cells (CD34+CD95+ + CD34+CD95−). Statistical analysis (nonparametric Kruskal-Wallis test): control v CML, P < .05. Horizontal bars are the mean values, and vertical bars are the SD.
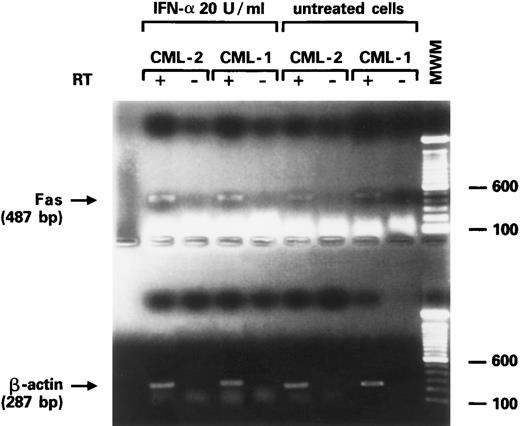
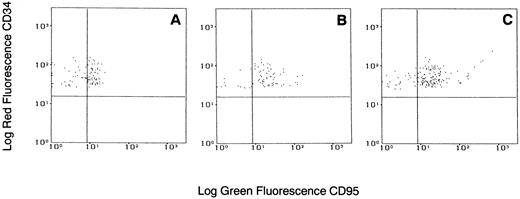
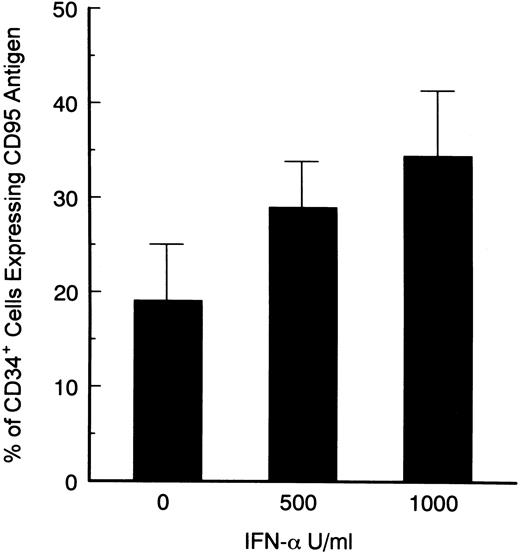
Effects of IFN-α on Fas-R expression on CD34+ cells.IFNs, specifically IFN-γ, have been associated with induction of Fas-R expression,34,35 possibly via the IRF-1 pathway.36 Fas triggering leads to hematopoietic suppression and apoptosis. We investigated whether IFN-α was capable of inducing Fas-R expression on CD34+ cells derived from CML patients. When purified CD34+ cells from CML BM were cultured in the presence of IFN-α levels comparable to those found after administration of IFN-α to patients in vivo, an increased expression of Fas-R mRNA was detected using RT-PCR (Fig 3). IFN-α–induced Fas-R expression was also quantitatively documented by two-color flow cytometric analysis: IFN-α enhanced Fas-R expression on CD34+ cells from CML patients in a dose-dependent manner (Figs 4 and 5). CD34+ cells derived from normal volunteers were less responsive to the stimulatory effect of IFN-α (CD34+CD95+, 7.8 ± 6 and 12.6 ± 10 without and with 1,000 U/mL IFN-α, respectively; P > .05).
Expression of Fas-R mRNA in CD34+ cells from two CML patients after stimulation with IFN-α. Bands represent the product of the RT-PCR for Fas (upper gel) and β-actin (lower gel) from purified CD34+ cells of two patients with CML in chronic phase.
Expression of Fas-R mRNA in CD34+ cells from two CML patients after stimulation with IFN-α. Bands represent the product of the RT-PCR for Fas (upper gel) and β-actin (lower gel) from purified CD34+ cells of two patients with CML in chronic phase.
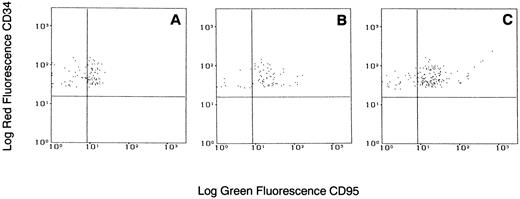
Flow cytometric analysis of Fas-R expression on CD34+ cells from a patient with CML stimulated with various concentrations of IFN-α. Total BM cells were cultured with or without IFN-α for 48 hours, stained with PE-conjugated CD34 and FITC-conjugated CD95 MoAb, and analyzed in the blast gate. (A) Without IFN-α; (B) IFN-α 500 U/mL; (C) IFN-α 1,000 U/mL. Gates were set based on isotypic controls.
Flow cytometric analysis of Fas-R expression on CD34+ cells from a patient with CML stimulated with various concentrations of IFN-α. Total BM cells were cultured with or without IFN-α for 48 hours, stained with PE-conjugated CD34 and FITC-conjugated CD95 MoAb, and analyzed in the blast gate. (A) Without IFN-α; (B) IFN-α 500 U/mL; (C) IFN-α 1,000 U/mL. Gates were set based on isotypic controls.
Dose-dependent induction of CD95 expression on CD34+ cells derived from BM of CML patients. Total BM cells were cultured in the presence of various concentrations of IFN-α for 48 hours, and stained with PE-conjugated CD34 and FITC-conjugated CD95 MoAb. Statistical analysis (paired t test): control v 500 and 1,000 U/mL IFN-α; P < .05 (summary of 7 experiments).
Dose-dependent induction of CD95 expression on CD34+ cells derived from BM of CML patients. Total BM cells were cultured in the presence of various concentrations of IFN-α for 48 hours, and stained with PE-conjugated CD34 and FITC-conjugated CD95 MoAb. Statistical analysis (paired t test): control v 500 and 1,000 U/mL IFN-α; P < .05 (summary of 7 experiments).
Effects of IFN and Fas-R agonist MoAb CH-11 on CML progenitor growth.We tested the influence of IFN-α on colony formation by both total BM cells and purified CD34+ cells from normal subjects and CML patients. Generally, there was more variability in the number of colonies (higher standard deviations) among patients with CML (Table 2). IFN-α at concentrations comparable to those achieved after in vivo administration (15 to 50 U/mL52 ) marginally inhibited colony growth from normal and CML BM cells (Table 2). Higher concentrations of IFN-α consistently inhibited both erythroid and myeloid colony formation from normal and CML BM cells in a dose-dependent manner (Table 2). The ID50 of IFN-α (50% inhibition of colony formation) in normal BM progenitor cells was 770 U/mL and 14.5 U/mL without and with Fas MoAb, respectively. However, when the inhibitory capacity of IFN-α was tested in CML BM progenitor, the ID50 of IFN-α was 1,050 U/mL and 2.5 U/mL without and with Fas mAb, respectively. Using highly purified BM CD34+ cells, we showed that this effect did not require accessory cells, since similar results were generated with CD34+ cells and total BM cells in methylcellulose assays (Table 2). To exclude the possibility of involvement of other known inducers of Fas-R expression, two experiments were performed with neutralizing antibodies to IFN-γ and TNF-α. In the presence of anti–IFN-γ and anti–TNF-α antibodies added to the methycellulose cultures, similar results with regard to the synergistic effects of IFN-α and CH11 on colony inhibition were observed (data not shown).
In previous studies, we have demonstrated that an anti-Fas MoAb (CH11) acts synergistically with IFN-γ and TNF-α to inhibit hematopoietic colony formation from normal hematopoietic progenitors.34 Anti-Fas MoAb (CH11) mimicks the biologic activity of Fas-L on Fas-R–expressing cells, including hematopoietic progenitors. Using this MoAb as a Fas-R agonist, we investigated whether CH11-treated CD34+ cells from CML patients were more sensitive to Fas-R–mediated hematopoietic suppression. There was a correlation between the IFN-α–mediated enhancement of Fas expression and the degree of hematopoietic inhibition seen in the colony cultures. Since normal progenitor cells also express low levels of Fas-R, there was some inhibition of colony formation seen in cultures of normal cells treated with CH11 (without priming with IFN-α). In samples derived from patients, the effect of CH11 was much stronger in comparison to normal BM, since CML progenitors express higher levels of Fas-R (Table 2). In total BM cultures, IFN-α potentiated the effect of anti-Fas MoAb in a dose-dependent manner (Table 2). Similar results were obtained in a 3H-thymidine proliferation assay performed with total BM cells from CML patients (data not shown). To determine whether this effect requires the presence of accessory cells, we performed colony assays with purified CD34+ cells. IFN-α significantly potentiated Fas-R–mediated inhibition of colony formation derived from both CML and normal progenitors (Table 2).
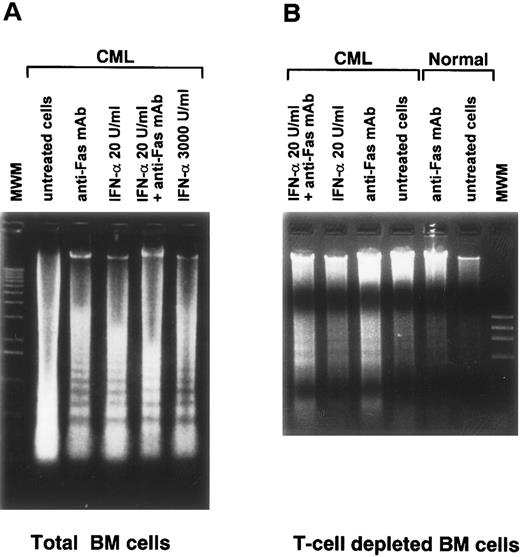
Potentiation of Fas-R–mediated apoptosis by IFN.In experiments with purified CD34+ cells, we have previously demonstrated that Fas-R–mediated inhibition of hematopoiesis is associated with BM progenitor cell apoptosis,34 and this effect is potentiated by cytokines including IFN-γ and TNF-α.33 When BM cells from CML patients and normal subjects were treated with anti–Fas-R MoAb in combination with IFN-α, the Fas-R agonist potentiated IFN-α–mediated induction of apoptosis, as demonstrated by a DNA fragmentation assay (Fig 6). Normal BM cells treated with the same concentration of anti–Fas-R34 or IFN-α (Fig 6) showed no visible apoptosis in agarose gel electrophoresis. A sufficient number of purified CD34+ cells for apoptosis assay could not be obtained from CML patients to test whether apoptosis was a complex phenomenon involving other cell subset. However, similar results were obtained with macrophage- and T-cell–depleted BM, suggesting that under our experimental conditions the induction of apoptosis did not require the presence of accessory cells.
Induction of apoptosis in culture of total and T-cell–depleted BM cells from a normal subject and from a patient with CML stimulated by anti-Fas MoAb. (A) and (B) Ethidium bromide staining of agarose gel after electrophoresis of low–molecular-weight DNA. DNA was extracted from 1 × 106 total BM cells (A) and T-cell–depleted BM cells (B) after culture for 48 hours in the presence of the indicated concentrations of IFN-α. Anti-Fas MoAb was used at a concentration of 1 μg/mL.
Induction of apoptosis in culture of total and T-cell–depleted BM cells from a normal subject and from a patient with CML stimulated by anti-Fas MoAb. (A) and (B) Ethidium bromide staining of agarose gel after electrophoresis of low–molecular-weight DNA. DNA was extracted from 1 × 106 total BM cells (A) and T-cell–depleted BM cells (B) after culture for 48 hours in the presence of the indicated concentrations of IFN-α. Anti-Fas MoAb was used at a concentration of 1 μg/mL.
DISCUSSION
We have investigated the role of Fas-R expression and triggering in the regulation of proliferation of hematopoietic progenitor cells in CML. Our results demonstrate that CML CD34+ cells in the chronic phase of the disease show significantly higher expression of Fas-R and increased sensitivity to Fas-R–mediated inhibition of colony growth. Furthermore, IFN-α enhanced the expression of Fas-R on CML progenitor cells and rendered them more susceptible to apoptosis induced by the Fas-R agonist anti–Fas MoAb CH11. IFN-α at high concentrations directly inhibited CML progenitor cells,22,23 whereas low concentrations (in the range achieved in blood after in vivo administration57 ) showed only a marginal suppressive effect in colony assay. In the presence of a Fas-R agonist, the same low IFN-α concentrations were capable of inducing a profound decrease in CML progenitor cell proliferation. Some selectivity of the Fas agonist–mediated effects on CML versus normal progenitor cells is inferred from the lower IFN-α concentrations required for 50% inhibition of colony formation in the cultures supplemented with Fas MoAb. The mechanism for such selectivity in Fas-R–mediated killing remains to be elucidated.
As inferred from studies on allogeneic BM transplants in CML, the cellular immune response may play an important role in inhibiting Ph+ hematopoiesis (graft versus leukemia [GvL] effect). Although the existence and significance of an autologous GvL effect is a subject of controversy,53 the presence of leukemia-specific T-cell clones has been demonstrated in CML.54 55 Fas expression may render CML progenitors more vulnerable to Fas-R–mediated killing, triggered by membrane-bound Fas-L on leukemia-specific T cells. Secretory Fas-L might also induce apoptosis of CML cells without the need for recognition of targets by specific T cells or direct cell-cell contact.
The effects of IFN-α in CML may be at least partially explained by Fas-R upregulation and the subsequent increased death rate of Fas+ CML progenitor cells. These cells, upon expression of Fas-R, showed low clonogenicity even in the absence of Fas agonist, as demonstrated in the sorting of CD34+CD95+ and CD34+CD95− cells (J.P. Maciejewski, unpublished results). It is possible that Fas-R expression is an event associated with preparation of the progenitor cell for apoptosis. Expression and subsequent triggering of Fas-R could represent the “final hit” in this process. Because increased Fas-R expression has been observed on several leukemic cell lines of both lymphoid and nonlymphoid origin,37,38,43-49 Fas-R induction pathways may not be tissue-specific. Recently, we have documented that IFN-γ and TNF-α induce Fas-R expression on CD34+ cells.34,35 The effects of these cytokines are mediated by intracellular induction of IRF-1.36 Involvement of the Fas-R pathway in IFN-α–induced apoptosis in the Daudi lymphoma cell line suggested that the Fas gene may belong to the IFN-inducible gene family,56 and its transcription may be transactivated through IRF-1 or other IFN-mediated signal transcription factors.57 However, since the promotor region of the Fas gene does not contain any IFN-responsive elements,58 further molecular analyses will be required to clarify the mechanisms of Fas-R induction by IFN-α.
The hybrid bcr/abl gene has been implicated in the suppression of programmed cell death.9-12 Recently, intrinsic kinase activity of bcr/abl was shown to be responsible for activation (phosphorylation) of STAT1 and STAT5, also involved in the signal transduction of several growth factor receptors.59 Fas effects could be due to several mechanisms, eg, the downmodulation of p210 that blocks the physiologic apoptotic pathway in CML progenitor cells. However, a recent report60 has shown that bcr/abl antiapoptotic activity is ineffective against apoptosis induced by natural killer (NK) or lymphokine-activated killer (Lak) cells. The NK-effector mechanism involves the perforin/granzyme pathway, but Lak cells may also use a Fas-R–mediated killing mechanism. In some reports, the sensitivity to apoptosis in CML cells did not differ from that of normal progenitor cells, a result that argues against a role for inhibition of apoptosis in clonal expansion.12
Enhanced Fas-R expression of CML progenitor cells may be intrinsic to the genetic defect in CML and caused by bcr/abl. The absence of Fas-R on, eg, K562 cells carrying bcr/abl does not support this hypothesis (J.P. Maciejewski, unpublished data). Alternatively, increased expression of Fas-R on CML CD34+ cells may also be a result of hypersensitivity to endogenously produced IFN-α. Although the IFN-α effect on Fas-R expression was observed even in a colony assay with purified CD34+ cells, we cannot rigorously exclude the possibility that in vivo Fas-R upmodulation is a result of the action of several other cytokines released as part of the natural response to malignancy. These cytokines may include IFN-γ and TNF-α, which have been previously shown to enhance Fas-R expression on normal CD34+ cells.34 In vitro experiments with highly purified CD34+ cells lacking accessory IFN-γ and TNF-α producers and IFN-γ – and TNF-α–neutralizing experiments did not support this possibility.
The finding of increased CD95 expression on CD34+ cells from CML patients may be restricted to a discrete, phenotypically defined subpopulation of CD34+ cells more abundant in CML patients. Because most immature progenitor and stem cells can only be characterized by functional tests (hematopoietic colony and long-term culture–initiating cell assays), a selective analysis of Fas-R expression in these cells is not possible. In our experiments, elevated Fas-R levels were also observed on phenotypically more mature cells from CML (CD33+, CD13+, and CD3+ cells; data not shown). Since the induction of Fas-R may be part of the normal myeloid maturation process, the comparison of Fas-R expression on these cells in CML patients and normal subjects was less conclusive.
Although the number of CML patients in blastic crisis we studied is small, our findings suggest that during transformation Fas-R expression is elevated. One possible interpretation is that a selective pressure in vivo may confer an advantage on clones with acquired genetic resistance to apoptotic stimuli. Additional investigations now in progress will clarify whether IFN-α–induced Fas-R expression occurs in vivo and is specific for CML cells, as well as whether the increased Fas-R expression in the blastic phase of CML is associated with a loss of sensitivity to Fas-mediated apoptosis.
In the future, a proper understanding of the Fas-R/Fas-L system in the regulation of normal and leukemic hematopoiesis might lead to introduction of novel immunotherapeutic principles for the treatment of CML.
Address reprint requests to Jaroslaw P. Maciejewski, MD, PhD, Department of Internal Medicine, University of Nevada, Reno, Howard Medical Bldg 320, Reno, NV 89557-0046.