Abstract
Thymocytes show differential cytokine responses, depending on the stage of differentiation. Whether these responses are due to preferential cytokine receptor expression or due to downstream signaling mechanisms is unknown. In this study, we examined the relationship between receptor expression and T-cell proliferation or differentiation using thymocytes from transgenic mice constitutively expressing the human granulocyte-macrophage colony-stimulating factor (hGM-CSF ) receptor. Transgenic CD4−CD8−, CD4+CD8−, and CD4−CD8+ cells proliferated when cultured with hGM-CSF in vitro, whereas CD4+CD8+ cells failed to proliferate. To examine the effect of hGM-CSF receptor signaling on T-cell development, we used fetal thymic organ cultures. The addition of exogenous hGM-CSF resulted in the failure of CD4−CD8− cells to differentiate into CD4+CD8+ cells. To more closely identify this maturational inhibition, we reconstituted normal fetal lobes with sorted pro-T–, pre-T–, or post-pre-T–precursor cells from transgenic mice. The addition of hGM-CSF to these cultures led to a block in both pro-T– and pre-T–cell differentiation, whereas the more mature post-pre-T cells differentiated normally. We propose that hGM-CSF receptor signaling during T-cell development results in a stage-specific inhibition of thymic precursor maturation.
THE THYMUS IS THE primary site of T-cell development. During the generation of mature T cells, expression of a variety of molecules, including adhesion molecules, T-cell receptor (TCR), and cytokine receptors, has been implicated in the maturational process. The cytokine response profile of various thymic populations differs depending on the stage of differentiation.1 Immature CD4− CD8− double-negative (DN) thymocytes respond to a variety of cytokines, including interleukin-1 (IL-1), IL-2, IL-7, transforming growth factor-β (TGF-β), and stem cell factor (SCF ). Cytokine responsiveness and cytokine generation are lost as DN cells upregulate CD4 and CD8 to enter the double-positive (DP) stage. During this DP stage, cells undergo positive and negative selection and then mature into CD4 or CD8 single-positive (SP) cells. These mature cells regain both the ability to respond to and to produce cytokines. The mechanisms responsible for this preferential cytokine responsiveness are currently unknown. The expression of several cytokine receptors, including the IL-2 receptor, IL-7 receptor, and SCF receptor (c-kit), is decreased as thymic precursors differentiate into DP cells.2-4 The expression of other cytokine receptors is poorly understood.
In earlier studies, we generated transgenic mice expressing the human granulocyte-macrophage colony-stimulating factor (hGM-CSF ) high-affinity receptor.5 Expression of the receptor is under control of the H-2Ld class I promoter, resulting in expression on numerous hematopoietic cells, including splenocytes, thymocytes, and bone marrow cells. The functional high-affinity GM-CSF receptor (GMR) is composed of two distinct α and β subunits.6 The α subunit confers GM-CSF specificity while binding GM-CSF with low affinity, whereas the β subunit is shared with the IL-3 and IL-5 receptors.7-10 Previous reports have indicated that the high-affinity GMR could be reconstituted by transfecting a pro-B–cell line (BA/F3), a T-cell line (CTLL-2), or a fibroblastic cell line (NIH 3T3) with both α and β subunit cDNAs. The reconstituted GMR was capable of transducing a proliferative signal in all three disparate cell lines in the presence of GM-CSF ligand,10-13 suggesting that signal transduction components associated with the GMR are present in multiple cell lineages and that the response to GM-CSF is regulated at the level of receptor expression. This concept was confirmed when hematopoietic progenitors from hGMR-transgenic (hGMR-tg) were examined. Murine GM-CSF (mGM-CSF ) does not bind to the human receptor; therefore hGMR-tg mice display a normal phenotype in the absence of exogenous hGM-CSF. The addition of hGM-CSF to methylcellulose colony assays examining bone marrow hematopoietic progenitors from hGMR-tg mice resulted in the formation of multiple lineage colonies, including erythrocyte colonies that are normally erythropoietin dependent. In the presence of mGM-CSF, only granulocyte and macrophage colonies were generated. These data suggest that the cytokine responsiveness of both myeloid and erythroid progenitors is regulated at the level of receptor expression rather than through downstream signal transduction machinery. In addition, these data suggest that GM-CSF does not induce differentiation of hematopoietic progenitors to specific myeloid lineages, but rather induces proliferation of multipotent progenitors, the differentiation of which might be regulated by intrinsic mechanisms.
We report here the effects of hGM-CSF signaling on the differentiation of thymocytes from GMR-tg mice. We wanted to evaluate the relationship between receptor expression and the proliferation and differentiation of thymocytes. We show hGM-CSF can transduce proliferation signals to DN and SP thymic subsets when they express the hGMR, whereas DP cells fail to proliferate despite their hGMR expression. We also report that the addition of hGM-CSF to fetal thymic organ cultures (FTOCs) inhibited the differentiation of DN precursor cells into downstream DP cells. To clarify this inhibition of thymic differentiation, we examined specific thymic CD3− CD4− CD8− triple-negative (TN) precursor populations for their ability to differentiate in the presence of hGM-CSF using FTOC. We report that the more immature pro-T cells (CD44+ CD25+ TN) and pre-T cells (CD44− CD25+ TN) failed to differentiate into DP cells when placed into FTOC in the presence of hGM-CSF. In contrast, the more mature post-pre-T cells (CD44− CD25− TN) appeared to differentiate normally. All these data indicate that hGM-CSF transduces signals into immature thymocyte precursor cells through the hGMR and that these signals are capable of inducing a stage-specific block in differentiation.
MATERIALS AND METHODS
Mice.Generation of hGMR transgenic mice has been described in detail elsewhere.5 The transgenic line H2-81 was used throughout the present experiments. Heterozygous hGMR transgenic mice or wild-type C3H/HeN (Simonsen Laboratories, Gilroy, CA) mice 3 to 6 weeks of age were used for sorting thymic populations. For the FTOC experiments, timed pregnant mice were generated by mating wild-type or homozygous transgenic males with wild-type females.
Culture medium and cytokines.Culture medium (CM) consisted of RPMI 1640 (JRH BioScience, Lenexa, KS) containing 10% fetal calf serum (FCS), 200 mmol/L L-glutamine, 5 × 10−5 mol/L 2-mercaptoethanol, minimal essential medium (MEM) amino acids and vitamins, sodium bicarbonate, penicillin, streptomycin, and gentamycin. For FTOC, CM containing 20% FCS was used (FTOC-CM). Recombinant hGM-CSF was kindly provided by R. Kastelein (DNAX, Palo Alto, CA). Recombinant mGM-CSF produced in yeast was kindly provided by A. Miyajima (Tokyo University, Tokyo, Japan).
Antibodies.For FACS analysis of hGMR α and β subunits, mouse anti-hGMR α antibody (clone GMA1) and rat anti-hGMR β antibody (clone 5A5) were used.14 Goat-antimouse IgG-fluorescein isothiocyanate (FITC) or goat-antirat IgG-FITC (Sigma, St Louis, MO) were used to detect primary antibodies. All other antibodies were purchased from PharMingen (San Diego, CA), unless otherwise specified. For isolation of CD4 and CD8 thymocyte populations, anti-CD4-phycoerythrin (PE) (clone RM4-5) and anti-CD8-FITC (clone 53-6.7) were used. For isolation of thymocyte TN precursor populations, the following antibodies were used: anti-CD3-biotin (clone 144-2C11), anti-CD4-biotin, anti-CD8-biotin, anti-B220-biotin (clone RA3-6B2), anti-Mac-1-biotin (clone M1/70), anti-Gr-1-biotin (clone RB6-8C5), anti-CD25-FITC (clone 7D4), anti-CD44-PE (clone IM7), and Streptavidin-TriColor (Caltag Laboratories, South San Francisco, CA). For analysis of in vitro repopulation of fetal lobes, the following antibodies were used: anti-CD4-PE, anti-CD8α-biotin, anti-CD8β-biotin (clone 53-5.8), anti-TCRαβ-FITC (clone H57-597), or anti-TCRγδ-FITC (clone GL3), and streptavidin-TriColor (Caltag). Before staining, cells were incubated with anti-CD32 (anti-FcgRII/III, clone 2.4G2) to reduce nonspecific antibody binding.
Sorting and multiparameter analysis.Thymocytes from hGMR transgenic heterozygous mice or wild-type controls were stained with anti-CD4-PE and anti-CD8-FITC. The four CD4/8 subsets were sorted using a FACStar Plus flow cytometer (Becton Dickinson, San Jose, CA). Identification and isolation of TN thymocyte precursor populations was reported by Godfrey et al.15 Briefly, thymocytes were depleted with anti-CD4 (clone RL172, used as culture supernatant) and anti-CD8 (clone AD4; Cedarlane Laboratories, Hornby, Ontario, Canada) antibodies followed by treatment with low-tox M rabbit complement (Cedarlane) and 20 mg/mL DNase I (Sigma). Viable cells were isolated with Histopaque 1083 (Sigma) and then stained as follows: a panel of lineage antibodies directed against CD3, CD4, CD8, B220, Mac-1, Gr-1 (all biotinylated), anti-CD25-FITC, and anti-CD44-PE. After washing, cells were incubated with streptavidin-TriColor. Cells were sorted using a FACStar Plus or FACS Vantage flow cytometer (Becton Dickinson). Sort purities were routinely greater than 95%. For analysis of T-cell repopulation in FTOC, single-cell suspensions were prepared for staining. To reduce nonspecific staining, cells were incubated with anti-CD32 (clone 2.4G2) before staining for specific antigen expression. FTOC were stained with anti-TCRαβ-FITC or anti-TCRγδ-FITC, anti-CD8α-biotin or anti-CD8β-biotin, anti-CD4-PE followed by streptavidin-TriColor. Analysis was performed using a FACScan flow cytometer (Becton Dickinson) using CellQuest software (Becton Dickinson).
Proliferation analysis of thymic subsets.Thymic CD4/8 subsets were sorted from hGMR-tg mice or from wild-type controls. Cells were cultured at a concentration of 2 × 105 cells/mL in 96-well plates with CM supplemented with hGM-CSF (10 ng/mL), mGM-CSF (5 ng/mL), or concanavalin A (5 ng/mL). After 2 days of culture, the wells were pulsed with 3H-TdR (1 μCi/well) for 18 hours and then harvested.
FTOC.For conventional FTOC, fetal thymic lobes were removed at day 14 to 16 of gestation and cultured under standard FTOC conditions, as described.16 Briefly, thymic lobes were removed from plug-timed pregnant mice and cultured at the air-liquid interface, resting on 0.45-μm pore size filters (Millipore, Bedford MA), supported by small pieces (10 × 10 × 5 mm) of gelfoam sponge (Upjohn, Kalamazoo, MI). Cultures were performed in 6-well plates containing 2.5 mL FTOC-CM. Selected cultures were supplemented with purified recombinant hGM-CSF. Cultures were refed every 6 days. For fetal thymic lobe repopulation studies, lobes were removed at day 15 of gestation and depleted of endogenous T-cell progenitors by culturing in FTOC medium containing 1.35 mmol/L deoxyguanosine for 5 days, as described.17 Depleted lobes were then individually plated with 1 × 103 pro-T or 1 × 104 pre-T or post-pre-T cells in a 30 μL volume of FTOC-CM alone or FTOC-CM containing 50 ng/mL of hGM-CSF in Terasaki plates (Nunc, Kamstrup, Denmark). Plates were then inverted to allow lobe and cells to combine at the bottom of a hanging drop.18 After 24 to 48 hours, repopulated lobes were transferred back into FTOC and refed with FTOC medium every 6 days. At the indicated time points, lobes were gently pressed under a glass coverslip in 100 mL phosphate-buffered saline containing 2% FCS to release the thymocytes, which were then phenotyped as described above.
RESULTS
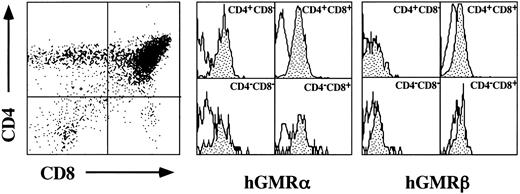
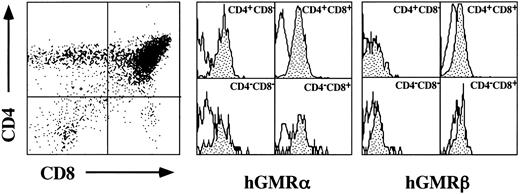
Proliferative responses of hGMR-tg CD4 and CD8 thymic subsets to hGM-CSF.The hGMR transgenes are under the control of the H-2Ld class I promoter, resulting in widespread expression of the receptor.5 We first confirmed thymic expression of both the hGMR α and β subunits by examining their expression on the four thymic subsets delineated by CD4 and CD8 expression. As shown in Fig 1, all four CD4/8 subsets express detectable levels of both hGMR subunits.
Cell surface expression of hGMR α and β subunits on thymocyte CD4 and CD8 subsets. Thymocytes from hGMR-tg mice were stained with anti-CD4, anti-CD8, and anti-hGMR α or β antibodies. CD4 and CD8 expression is indicated by dot plot with quadrant shown. Cells within each quadrant were further analyzed for hGMRα subunit (middle group of histograms) and hGMRβ subunit (right-hand group of histograms) expression. Staining profile from wild-type mice is used as background (white), whereas specific receptor staining is indicated by the shaded area.
Cell surface expression of hGMR α and β subunits on thymocyte CD4 and CD8 subsets. Thymocytes from hGMR-tg mice were stained with anti-CD4, anti-CD8, and anti-hGMR α or β antibodies. CD4 and CD8 expression is indicated by dot plot with quadrant shown. Cells within each quadrant were further analyzed for hGMRα subunit (middle group of histograms) and hGMRβ subunit (right-hand group of histograms) expression. Staining profile from wild-type mice is used as background (white), whereas specific receptor staining is indicated by the shaded area.
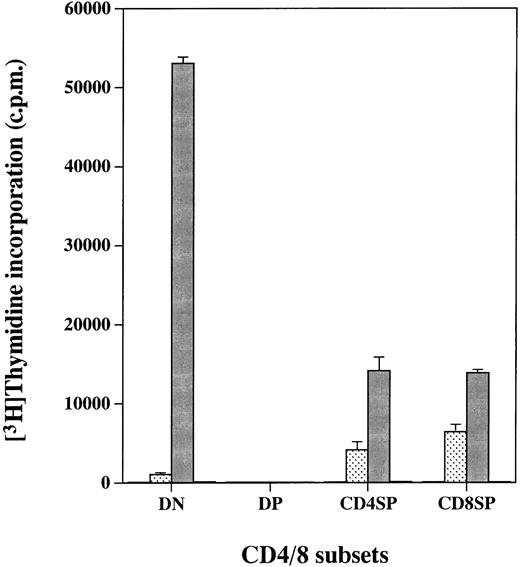
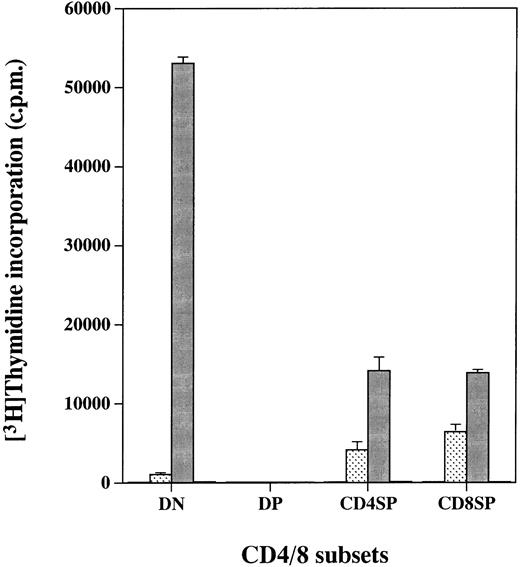
We reported previously that cell lines expressing the hGMR gained the proliferative ability in response to hGM-CSF.10-13 We therefore examined whether expression of the hGMR on thymocytes would confer hGM-CSF responsiveness. The four CD4/8 thymocyte populations from hGMR-tg mice were sorted and cultured for 2 days in the presence of hGM-CSF, followed by a 3H-TdR pulse to measure proliferation (Fig 2). DN, CD4+ SP, and CD8+ SP cells displayed high proliferative capacity when cultured with hGM-CSF. However, DP cells, despite expression of the hGMR, did not proliferate in response to hGM-CSF. As a control, thymocytes from wild-type control mice did not proliferate in response to hGM-CSF (data not shown).
Proliferation of hGMR-expressing thymocyte subpopulations induced by hGM-CSF. Thymocyte subpopulations were sorted as described in the Materials and Methods. Cells were cultured with or (□) without 50 ng/mL of () hGM-CSF, (▪) mGM-CSF, or (▧) ConA for 2 days and then pulsed with 3H-TdR.
Proliferation of hGMR-expressing thymocyte subpopulations induced by hGM-CSF. Thymocyte subpopulations were sorted as described in the Materials and Methods. Cells were cultured with or (□) without 50 ng/mL of () hGM-CSF, (▪) mGM-CSF, or (▧) ConA for 2 days and then pulsed with 3H-TdR.
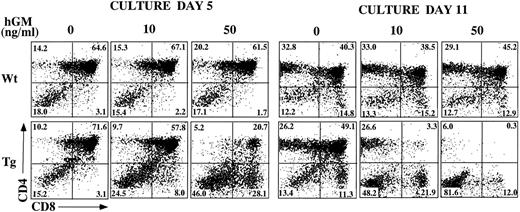
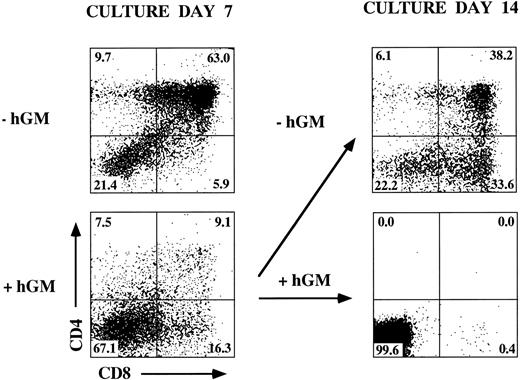
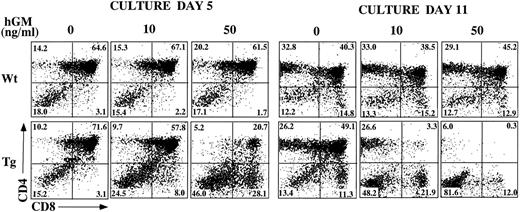
Inhibition of T-cell development in hGMR-tg FTOC by exogenous hGM-CSF.In other studies, we noted that hGM-CSF supports the growth of hematopoietic progenitor cells from hGMR-tg bone marrow cells, without altering differentiation potential.5 To examine the effect of hGMR signaling on thymic T-cell development, we established FTOC using hGMR-tg fetal lobes and examined thymic differentiation by phenotyping the cultures for CD4 and CD8 expression. FTOC were established using fetal lobes taken at day 15 of gestation and culturing them in the presence or absence of 10 ng/mL or 50 ng/mL hGM-CSF for 5 or 11 days. At both timepoints, the cell yields within the lymphoid sized scatter gate were twofold to fivefold lower with the addition of exogenous hGM-CSF. Treatment of wild-type control lobes with hGM-CSF did not affect cell yield (data not shown). On day 5 of culture, the frequency of DP cells was reduced and a corresponding increase in DN cell frequency was observed in a dose-dependent response to hGM-CSF (Fig 3). This decrease in DP and increase in DN frequencies was even more pronounced by day 11 of culture, such that DP and SP cells were essentially absent from FTOC cultured with 50 ng/mL of hGM-CSF. These data suggest a dose-dependent inhibition in the differentiation of DN cells into the DP population in response to exogenous hGM-CSF. However, this inhibition is not complete; even at the higher dose of hGM-CSF, a small percentage of DP cells was seen on day 5 and SP cells were observed on day 11.
Effect of hGM-CSF on FTOC development. Day-15 fetal thymic lobes were harvested from hGMR-tg and normal control embryos and placed into FTOC. Lobes were cultured in FTOC-CM alone or with the addition of 10 ng/mL or 50 ng/mL hGM-CSF. FTOC were harvested on days 5 and 11 and examined for expression of CD4 and CD8, as described in the Materials and Methods.
Effect of hGM-CSF on FTOC development. Day-15 fetal thymic lobes were harvested from hGMR-tg and normal control embryos and placed into FTOC. Lobes were cultured in FTOC-CM alone or with the addition of 10 ng/mL or 50 ng/mL hGM-CSF. FTOC were harvested on days 5 and 11 and examined for expression of CD4 and CD8, as described in the Materials and Methods.
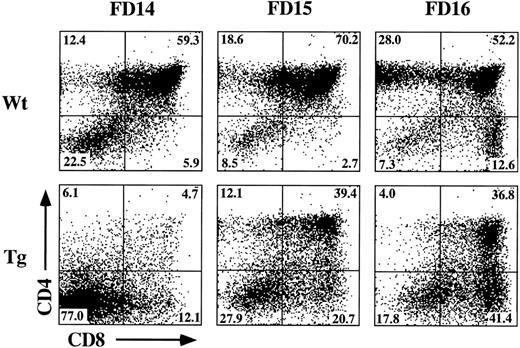
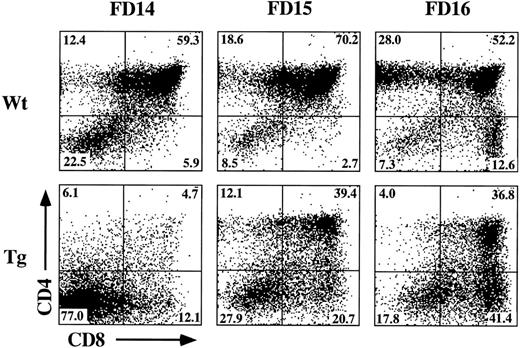
The incomplete block in differentiation may be related to the heterogeneous thymic precursor cell composition of day-15 fetal lobes. If inhibition of differentiation is stage-specific, then day-15 lobes may contain precursor populations downstream of the putative block point. To examine this possibility, we established FTOC from hGMR-tg and wild-type control mice using day-14, -15, and -16 gestational fetal lobes cultured in the presence or absence of 50 ng/mL hGM-CSF. Cultures were then harvested on day 7 and phenotyped for CD4 and CD8 expression (Fig 4). Confirming an effect on thymic differentiation, both day-15 and day-16 hGMR-tg FTOC contained fewer DP cells and a corresponding increase in DN cells when compared with age-matched control FTOC. Day-14 hGMR-tg FTOC contained a sharp decrease in the frequency of DP cells when compared with either wild-type controls or day-15/16 hGMR-tg FTOC. These data support the hypothesis that signaling through the hGMR induces a stage-specific inhibition in T-cell differentiation. The more gestationally mature day-15/16 fetal lobes likely contain thymic precursors downstream of the induced block point, whereas the majority of day-14 fetal precursors are upstream of this block point. Day-15/16 hGMR-tg FTOC contained an increased frequency of CD8+ SP cells, the majority of which were mature αβ- or γδ-TCR–expressing cells (data not shown). In agreement with our earlier data, the cell yields within the lymphoid sized scatter gate from hGMR-tg FTOC were threefold to fivefold lower than those of wild-type controls, regardless of gestation age.
Effect of hGM-CSF on various gestational ages of fetal thymic lobes. Fetal thymic lobes from fetal day (FD) 14, 15, and 16 embryos were harvested from hGMR-tg and normal control fetuses and placed into FTOC with or without 50 ng/mL hGM-CSF. After 7 days of culture, the cells were harvested and analyzed for the expression of CD4 and CD8. FTOC established from day-14 transgenic lobes showed marked inhibition in the generation of DP cells, whereas day-15 and -16 FTOC generated increasing numbers of DP cells.
Effect of hGM-CSF on various gestational ages of fetal thymic lobes. Fetal thymic lobes from fetal day (FD) 14, 15, and 16 embryos were harvested from hGMR-tg and normal control fetuses and placed into FTOC with or without 50 ng/mL hGM-CSF. After 7 days of culture, the cells were harvested and analyzed for the expression of CD4 and CD8. FTOC established from day-14 transgenic lobes showed marked inhibition in the generation of DP cells, whereas day-15 and -16 FTOC generated increasing numbers of DP cells.
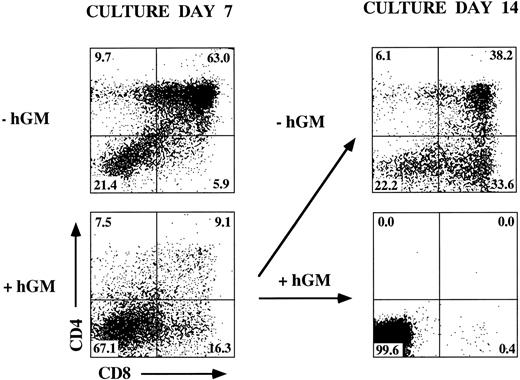
Removal of hGM-CSF from hGMR-tg FTOC results in T-cell differentiation.The data presented indicate a stage-specific block in T-cell differentiation by hGMR signaling. We then asked whether this block would be an irreversible event leading to the loss of T-cell developmental potential of upstream precursors. To examine this possibility, we determined the effect of hGM-CSF withdrawal from transgenic FTOC containing hGM-CSF for 7 days. The addition of hGM-CSF to day-14 hGMR-tg FTOC for 7 days led to a dramatic decrease in the frequency of DP cells. When hGM-CSF was removed from these cultures on day 7 and cultured for an additional 7 days, generation of DP cells was observed (Fig 5). These data indicate that hGMR signaling results in a block in differentiation of immature thymic precursors and that this block does not alter the differentiation potential of these precursors because removal of hGM-CSF results in continued T-cell differentiation.
Release of inhibition with removal of hGM-CSF from FTOC. Day-14 fetal thymic lobes were harvested from hGMR-tg embryos and placed into FTOC with or without 50 ng/mL of hGM-CSF. After 7 days of culture, a set of lobes were harvested and analyzed for the expression of CD4 and CD8. The lobes cultured with hGM-CSF were further divided to two groups. One group of lobes was cultured without hGM-CSF, whereas the other group was left in 50 ng/mL of hGM-CSF. After 7 more days of culture, cultures were harvested and analyzed for the expression of CD4 and CD8.
Release of inhibition with removal of hGM-CSF from FTOC. Day-14 fetal thymic lobes were harvested from hGMR-tg embryos and placed into FTOC with or without 50 ng/mL of hGM-CSF. After 7 days of culture, a set of lobes were harvested and analyzed for the expression of CD4 and CD8. The lobes cultured with hGM-CSF were further divided to two groups. One group of lobes was cultured without hGM-CSF, whereas the other group was left in 50 ng/mL of hGM-CSF. After 7 more days of culture, cultures were harvested and analyzed for the expression of CD4 and CD8.
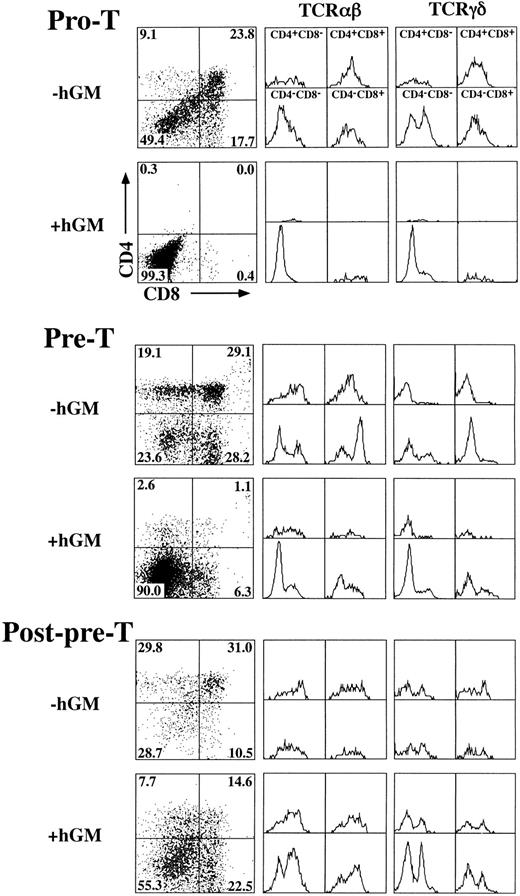
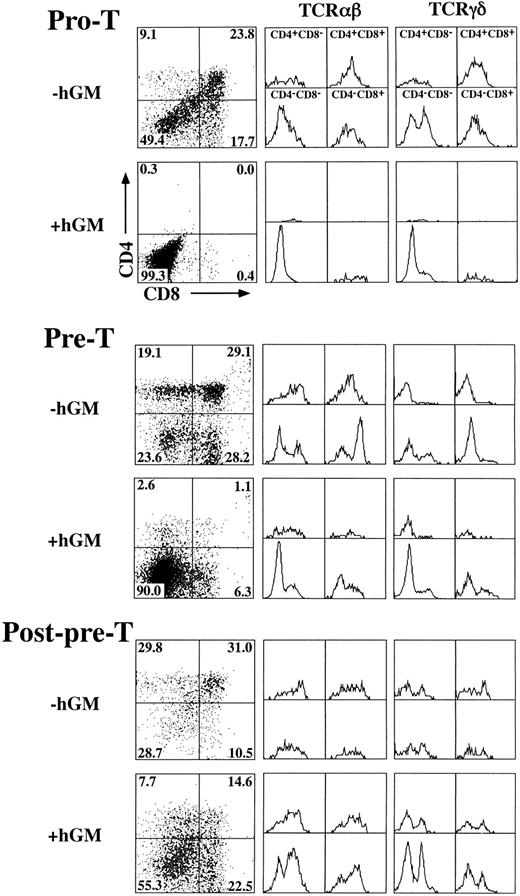
hGM-CSF inhibits hGMR-tg T-cell development at the pre-T–cell stage.To define the specific developmental block induced by hGM-CSF, we sorted specific TN precursor populations from adult hGMR-tg mice and repopulated deoxyguanosine-depleted fetal lobes. Because transgene expression is under the control of a class I promoter, it is possible that both thymic stromal cells as well as thymocytes express hGMR. To exclude any potential effects of stromal cell activation via hGM-CSF, we repopulated wild-type fetal lobes with transgenic sorted thymocyte precursors. With this approach, any effect seen from the addition of hGM-CSF to the repopulated fetal lobes would be due to a direct effect on the thymocyte precursors and not from stromal cells. Immature TN thymocytes can be subdivided into four distinct phenotypic precursor populations by the differential expression of CD44 and CD25 through the following pathway: CD44+25− → CD44+25+ (pro-T) → CD44−25+ (pre-T) → CD44−25− (post-pre-T).15 19 We sorted the latter three subsets from hGMR-tg adult mice and repopulated fetal thymic lobes from day-15 gestation wild-type mice. Analysis of hGMR-tg pro-T–cell repopulated FTOC 23 days after hGM-CSF culture indicated a complete block in the generation of DP and SP mature cells (Fig 6, top). No mature DN αβ-T cells were seen; however, DN γδ-T cells were detected, suggesting that these cells arise without the need to go through a DP intermediate stage. A similar inhibition was observed with hGMR-tg pre-T–cell repopulated FTOC analyzed after 14 days of culture in hGM-CSF (Fig 6, middle). However, this inhibition of pre-T cells appeared to be less complete than that seen with pro-T cells; a small percentage of DN and SP cells expressing αβ-TCR was observed. In contrast to pro-T– and pre-T–repopulated FTOC, post-pre-T–cell repopulated lobes contained DP and SP cells 9 days after hGM-CSF culture (Fig 6, bottom). In fact, the higher frequency of DN and SP cells expressing either αβ- or γδ-TCR may be due to the proliferation of these mature T cells induced by hGMR expression and exogenous hGM-CSF added to the cultures. Cell yields within the lymphoid sized scatter gate were increased threefold to fivefold for post-pre-T populations when hGM-CSF was added. Interestingly, cell yields within the lymphoid sized scatter gate were also increased slightly for the pro-T population and threefold for the pre-T population in the presense of hGM-CSF. The increased lymphoid cell yield from pro-T– and pre-T–cell repopulated FTOC suggests that these cells, although unable to differentiate past the pre-T–cell stage, are capable of proliferating within the fetal thymic environment in response to hGM-CSF. Incubation of pro-T, pre-T, and post-pre-T cells with hGM-CSF in single-cell suspension cultures resulted in an increase in cell numbers for all three transgenic precursor populations (data not shown), thereby confirming the ability of these cells to proliferate in response to hGM-CSF. Taken together, these data indicate that hGMR signaling in pro-T cells and the majority of pre-T cells block the normal differentiation of these precursors into DP and SP cells expressing αβ-TCR. The more developmentally mature post-pre-T cells appear to be downstream of this hGM-CSF–induced block in differentiation, as seen by their relatively normal differentiation in the presence of hGM-CSF.
Effect of hGM-CSF on differentiation of TN thymocyte precursor subsets. Pro-T (CD44+25+), pre-T (CD44−25+), and post-pre-T (CD44−25−) cells were sorted from hGMR-tg mice and transferred into 2-deoxyguanosine depleted wild-type fetal lobes, as described in the Materials and Methods, and then cultured with or without 50 ng/mL of hGM-CSF. Pro-T–cell cultures were harvested on day 23, pre-T–cell cultures on day 14, and post-pre-T–cell cultures on day 9. Harvested cells were stained for expression of CD4, CD8, and αβ- or γδ-TCR. CD4 and CD8 expression is indicated by dot plot with quadrant percentages shown. Cells within each quadrant were further analyzed for TCRαβ (middle group of histograms) and TCRγδ expression (right-hand group of histograms).
Effect of hGM-CSF on differentiation of TN thymocyte precursor subsets. Pro-T (CD44+25+), pre-T (CD44−25+), and post-pre-T (CD44−25−) cells were sorted from hGMR-tg mice and transferred into 2-deoxyguanosine depleted wild-type fetal lobes, as described in the Materials and Methods, and then cultured with or without 50 ng/mL of hGM-CSF. Pro-T–cell cultures were harvested on day 23, pre-T–cell cultures on day 14, and post-pre-T–cell cultures on day 9. Harvested cells were stained for expression of CD4, CD8, and αβ- or γδ-TCR. CD4 and CD8 expression is indicated by dot plot with quadrant percentages shown. Cells within each quadrant were further analyzed for TCRαβ (middle group of histograms) and TCRγδ expression (right-hand group of histograms).
DISCUSSION
We have recently generated transgenic mice expressing both the α and β subunits of the hGMR.5 We report here on the effects of signaling through the hGMR on T-cell proliferation and differentiation. All four thymic subsets delineated by CD4 and CD8 expressed detectable levels of both hGMR α and β subunits. Proliferation assays showed DN and SP populations proliferated in response to hGM-CSF in vitro, although they did not respond to mGM-CSF. These results indicate that GM-CSF alone transduces proliferation signals to thymocytes when they express its receptor. The result is consistent with our previous report that hGM-CSF supports growth of erythroid precursor cells in the absence of erythropoietin when they express hGMR.5 However, DP cells failed to proliferate in response to exogenous hGM-CSF, despite a detectable hGMR expression. It has been reported that DP cells fail to respond to a variety of cytokines. This failure of DP cells to respond to cytokines has not been characterized, although it has been suggested that the lack of response may be due to the lack of cytokine receptor expression on these cells. Our data indicate that the failure of DP cells to respond to cytokines is not only due to the lack of receptor expression but may also be due to the lack of signal transducing machinery. Alternatively, DP cells can transduce hGM-CSF signal normally but cannot proliferate because of growth suppression or an apoptosis that overcomes the hGM-CSF signal. Studies are currently underway to elucidate the mechanism of DP cell unresponsiveness.
Signaling through the hGMR not only induces thymocyte proliferation but also has pronounced effects on thymocyte development. Culture of FTOC established with day-14, -15, or -16 gestational lobes with hGM-CSF resulted in a decrease in the generation of DP cells that was dependent on gestational age. Fetal day (FD) 15 and 16 lobes produced a low level of DP cells, whereas the more gestationally immature FD 14 lobes were severely inhibited in generating DP cells. These observations suggested that the inhibition induced by hGM-CSF occurred at a discrete stage of immature thymocyte maturation. The gestationally more mature lobes likely contained precursor populations downstream of the block induced by hGM-CSF. Confirmation was obtained by sorting TN thymocyte precursor populations from hGMR-tg mice and repopulating wild-type FTOC. Pro-T and most pre-T cells were unable to differentiate into DP cells when placed back into FTOC in the presence of hGM-CSF. However, the more mature post-pre-T cells were able to differentiate into DP and SP cells in FTOC. When pro-T–cell repopulated FTOC were phenotyped after culture, CD44− CD25+ pre-T cells were present, indicating that the presence of hGM-CSF during culture did not prevent pro-T cells from differentiating into pre-T cells (data not shown). Pre-T–cell repopulated cultures contained a small percentage of DP and SP cells. The most likely explanation for this observation is that pre-T cells themselves are heterogeneous and that a small number of these cells had already progressed past the block point induced by hGM-CSF.
Previous studies examining the effects of cytokines on T-cell development in FTOC reported similar effects.20,21 In particular, the addition of IL-7 to FTOC resulted in a decrease in DP cell frequency and a decrease in the number of αβ-TCR–expressing T cells with a corresponding increase in the generation of γδ-T cells.22 Although these findings are similar to our findings, the addition of IL-7 affected T-cell differentiation at an earlier point than seen by signaling via the hGMR, occurring at the CD44+ CD25− or CD44+ CD25+ stages. Other studies including the generation of IL-7–deficient mice have also suggested a role for IL-7 at an earlier point in maturation than that seen in our model system.23-25 Together, these studies indicate that cytokines play a critical role in the differentiation of thymocyte precursors before the expression of CD4 and CD8. The main regulator of proliferation and differentiation of thymocytes might shift from cytokines to TCR with progression of maturation, and normal thymocyte maturation may perhaps be regulated by the controlled expression of cytokine receptors at defined stages of differentiation. Our results might indicate that inappropriate cytokine signaling during early development inhibits differentiation.
The hGM-CSF–induced block in differentiation occurs at a later point in development than that induced by other cytokines. However, there are similarities between the hGM-CSF block point and the thymocyte maturation block point seen in gene-deficient mice affecting pre-TCR expression and/or selection. Pre-TCR consists of a rearranged β-TCR chain in association with the pre-Tα chain that form a receptor capable of transducing a signal required for continued maturation of αβ-T cells, in combination with CD3ε and p56lck. Mice lacking expression of recombinase-activating gene (RAG), TCR-β, pre-Tα, CD3ε, or p56lck have a defect in their ability to generate αβ-T cells and have an arrested thymic differentiation at the pre-T to post-pre-T transition stage.15 26-31 These findings are similar to those we report here; it is interesting to speculate that hGMR signaling either inhibits β-TCR gene rearrangement or β-selection via pre-TCR expression.
Another possibility that may explain the thymocyte inhibition is that hGM-CSF alters the lineage fate of thymic progenitors. We reported that bone marrow-derived hematopoietic progenitor cells from hGMR-tg mice differentiated in vitro into various hematopoietic lineages, including erythroid cells, under the influence of hGM-CSF.5 These results suggest that hGM-CSF signaling supports the proliferation of these progenitor cells without altering their ability to differentiate into various lineages. However, in our model of T-cell development presented here, signaling through the hGMR inhibits T-cell differentiation. We observed large granulated cells along with lymphoid-sized cells in hGMR-tg FTOC. Preliminary analysis indicated that these cells expressed Mac-1 and contained granules that were not stained by May-Grünewald-Giemsa staining (data not shown). Interestingly, hGMR-tg pro-T–cell repopulated FTOC contained a small number of large granulated cells also, although we have not phenotyped this population. These data would suggest a limited myeloid lineage differentiation; however, further studies are needed. Regardless, the majority of thymic progenitors inhibited at the pre-T–cell stage retain their ability to differentiate into mature T cells because removal of hGM-CSF after 7 days of culture relieved the inhibition of T-cell development.
We are currently examining the relationship between cell proliferation and the inhibition of differentiation induced by hGM-CSF. Total DN cells showed proliferative activity in response to hGM-CSF and the addition of hGM-CSF to hGMR-tg pro-T, pre-T, or post-pre-T–cell repopulated FTOC resulted in an increase in total cell numbers. In contrast, the addition of hGM-CSF to conventional hGMR-tg FTOC resulted in decreased cell yields. These divergent results may be due to secondary effects of hGM-CSF on transgenic stromal cells. The addition of hGM-CSF to pro-T–, pre-T–, or post-pre-T–cell single-cell suspensions resulted in cellular proliferation as well (data not shown). Recently, it has been shown that, although pro-T cells are in cell cycle, a portion of pre-T and DP cells do drop out of cell cycle. This finding suggests a correlation between pre-TCR or TCR selection and cell cycle status.32 It is possible that the disregulated proliferation of pre-T cells induced by hGM-CSF might result in the failure or inability to differentiate into downstream populations. Studies are underway to examine these possibilities.
ACKNOWLEDGMENT
We thank Dr J. Cupp, E. Callas, V. Hong, D. Polakoff, and M. Takizawa for expert assistance in cell sorting; Drs N. Arai, T. Yokota, S. Habu, K. Ikuta, A. Kudo, K. Kurata, and R. Nishinakamura for advice and help; and Drs A. Koch and M. Ohara for comments on the manuscript.
Supported in part by Grant-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sports and Culture of Japan. The DNAX Research Institute of Molecular and Cellular Biology is supported by Schering-Plough Corp.
Address reprint requests to Ken-ichi Arai, MD, PhD, Department of Molecular and Developmental Biology, Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108, Japan.