Abstract
We report here a series of 16 highly malignant diffuse large B-cell lymphomas of the oral cavity with unique immunohistologic features. Fifteen of these developed in human immunodeficiency virus-positive patients. All cases displayed morphologic features of diffuse large-cell lymphomas but strikingly differed from them in that they showed a minimal or absent expression of the leukocyte common antigen as well as of the B-cell antigen CD20. Instead, the tumor cells showed a constant reaction with the plasma cell characteristic antibody VS38c and a frequent reaction with the CD79a antibody. This, in conjunction with a variable expression of cytoplasmic Ig and a monoclonal rearrangement of the Ig heavy chain gene in all of the three tested cases confirmed the B-cell nature, the clonal origin, and the plasmacellular differentiation of these neoplasms. The majority of these tumors were negative for the BCL-6 protein, with the remaining cases showing only a partial and weak expression of this antigen. An association with the Epstein-Barr virus (EBV) was found in 9 of 15 tested cases showing abundant EBV-encoded nuclear RNA transcripts in the absence of EBNA-2. Five of the EBV-positive cases variably expressed LMP-1. We propose to name these tumors plasmablastic lymphomas, in accordance with their morphologic and immunohistologic features. Knowledge of this lymphoma entity is important to avoid confusion with nonlymphoid malignancies due to the lack of commonly used lymphoid markers.
INFECTION WITH the human immunodeficiency virus (HIV) dramatically increases the risk of developing non-Hodgkin's lymphomas (NHL)1 (see also Levine2 for a review). In contrast to the lymphoproliferations observed in primary immune deficiencies and in transplanted patients, HIV-related lymphomas have been morphologically classified according to the Working Formulation. Even if numerous different types of lymphomas have been reported to occur in HIV-infected patients, the majority of them fall into three categories: small noncleaved (Burkitt's lymphomas), large cleaved or noncleaved cell lymphomas (centroblastic lymphoma), and immunoblastic lymphoma with plasmacytoid differentiation.3 These tumors display a marked propensity to involve extranodal anatomic sites, in particular the gastrointestinal tract and the central nervous system.3
In this report, we present a series of 16 tumors of the oral cavity and the jaw that were referred to us during the last 4 years for consultation because their nature stayed unclear, even after routine immunohistologic studies. Interestingly, 15 of our 16 oral cavity tumors developed in HIV-infected individuals. They were diffusely growing large-cell tumors with a high proliferative index. These tumors resisted typing according to antigen profile because they lacked common cell type-specific/characteristic antigens, including epithelial, melanocyte, myocyte, and lymphoid cell markers. However, the detectability of cytoplasmic Ig (CIg) in some of these cases pointed towards a relationship of these tumors to cells of the plasma cell series. Thanks to the recent availability of two new plasma cell-reactive antibodies (VS38c and JCB117/CD79a), we were able to show that all of our cases of oral cavity and jaw large cell tumors, including those that lack CIg, expressed immunophenotypic features of plasma cells.
Most of the HIV-related lymphomas were reported to be of B-cell origin, because they were found to express the B-cell–specific antigen CD204 in contrast to the tumors described here. Thus, our tumor series represents a new subcategory of HIV-related high-grade lymphomas. We propose to designate them as plasmablastic lymphomas (PBL) in reference to their blastoid morphology and immunophenotypic features.
MATERIALS AND METHODS
Tissue samples.Sixteen cases of lymphomas of the oral cavity with unusual phenotype were collected from the consultation files of the lymphoma reference center at the Benjamin Franklin Hospital (Berlin, Germany). Formalin-fixed, paraffin-embedded material was available for study for each of the cases. In 1 case (case no. 5), only a limited amount of material was left and the association of this tumor with the Epstein-Barr virus (EBV) antigen could not therefore be tested. Hematoxylin and eosin (H&E)- and Giemsa-stained sections were examined and tumors were classified on the basis of their morphologic features using the REAL classification.5
Immunohistology.Immunohistochemical studies used several monoclonal and polyclonal antibodies that are reactive in paraffin sections. An antigen retrieval method using a pressure cooker was performed before immunostaining.6 Conventional indirect immunohistochemistry was applied following standard protocols.7 The staining consisted of a first-stage incubation with one of the following primary monoclonal antibodies: MIB1 (dilution 1:100; Ki-67), L26 (dilution 1:400; CD20), JCB117 (dilution 1:50; CD79a), VS38c (dilution 1:100), LC (dilution 1:300; CD45), CS1-4 (dilution 1:100; LMP-1), PE2 (dilution 1:100; EBNA-2), BZ-1 (dilution 1:20; BZLF-1), BCL-2 (dilution 1:100; clone 124), and PG-B6p (1:4; BCL-6).8 Polyclonal antibodies were used to test the expression of the CD3 antigen and of the Ig chains κ (dilution 1:10,000), λ (dilution 1:10,000), γ (dilution 1:2,000), μ (dilution 1:600), δ (dilution 1:400), and α (dilution 1:100). The bound antibodies were made visible with an indirect immunoperoxidase method for the antibodies to the Ig heavy and light chains, whereas the alkaline phosphatase antialkaline phosphatase (APAAP) method was used to show the binding of the remaining antibodies. All antibodies were obtained from DAKO (Glostrup, Denmark), except for MIB 1, JCB117, and the BCL-6–specific antibody, which were kindly provided by Dr J. Gerdes (Borstel, Germany), Dr D. Mason (Oxford, UK), and Dr B. Falini (Perugia, Italy), respectively.
In situ hybridization.EBV infection was detected by in situ hybridization using the EBV-encoded small RNAs (EBER) 1 and 2 probes that are specific to the viral nontranslated small RNAs. Because lymphoid cells infected by EBV express the mentioned nontranslated small RNAs at a high rate,9 besides the polymerase chain reaction (PCR), this method is the most sensitive procedure for the detection of an EBV infection.10,11 In situ hybridization was performed as previously described.12 Briefly, digoxigenin-labeled EBER sense and antisense RNA probes were incubated overnight with deparaffinized tissue sections. After stringent washings, hybridization was visualized by incubation of the slides with Fab fragments of an antibody specific to digoxigenin coupled with alkaline phosphatase.
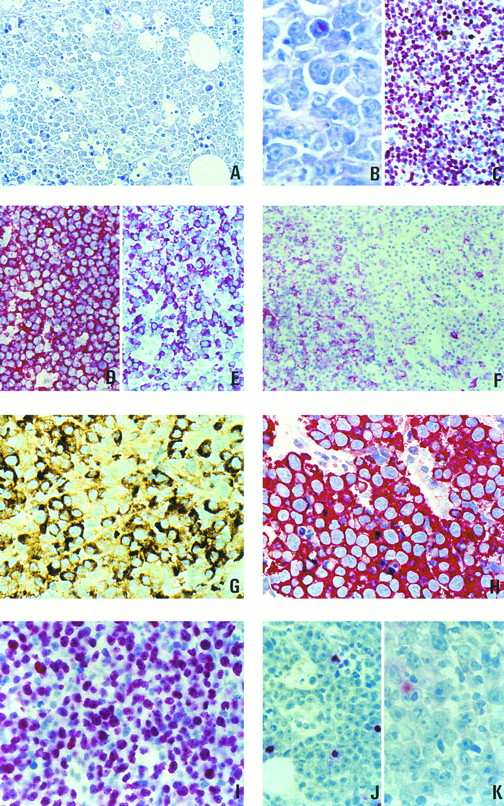
(A through C) Morphology and proliferation index of 1 case of plasmablastic lymphoma. (A) Giemsa staining, original magnification × 400. (B) Giemsa staining, original magnification × 800. (C) Staining with the Ki-67–specific antibody MIB-1. (D through F ) Paraffin sections of lymph nodes from patients with large-cell lymphomas stained for B-cell–specific antigens. (D) Large-cell lymphoma of the common type, L26 antibody, strong membrane staining in all cells. (E) Plasmablastic lymphoma, strong staining for CD79a (JCB117). (F ) Plasmablastic lymphoma, with an unusually high number of cells showing membranous staining with the L26 antibody. (G) Paraffin section from a plasmablastic lymphoma. Intense staining for IgG. (H) Paraffin section from a plasmablastic lymphoma. All neoplastic cells are strongly labeled by the antibody VS38c. (I through K) Association of plasmablastic lymphomas with EBV and expression of viral latent proteins. (I) In situ hybridization for EBER transcripts. (J) Immunostaining with an antibody specific to the viral BZLF-1 protein. (K) Immunostaining with an antibody specific to viral LMP-1.
(A through C) Morphology and proliferation index of 1 case of plasmablastic lymphoma. (A) Giemsa staining, original magnification × 400. (B) Giemsa staining, original magnification × 800. (C) Staining with the Ki-67–specific antibody MIB-1. (D through F ) Paraffin sections of lymph nodes from patients with large-cell lymphomas stained for B-cell–specific antigens. (D) Large-cell lymphoma of the common type, L26 antibody, strong membrane staining in all cells. (E) Plasmablastic lymphoma, strong staining for CD79a (JCB117). (F ) Plasmablastic lymphoma, with an unusually high number of cells showing membranous staining with the L26 antibody. (G) Paraffin section from a plasmablastic lymphoma. Intense staining for IgG. (H) Paraffin section from a plasmablastic lymphoma. All neoplastic cells are strongly labeled by the antibody VS38c. (I through K) Association of plasmablastic lymphomas with EBV and expression of viral latent proteins. (I) In situ hybridization for EBER transcripts. (J) Immunostaining with an antibody specific to the viral BZLF-1 protein. (K) Immunostaining with an antibody specific to viral LMP-1.
PCR amplification method.In 3 cases, the formalin-fixed material sufficed for molecular biologic investigations. DNA was purified from 20-μm–thick paraffin sections with an automatic DNA extractor (Applied Biosystems 341A, Weiterstadt, Germany) using a standard phenol extraction method.
A seminested PCR was performed for the determination of the clonality of the 3 tested PBL.13 Five hundred nanograms of genomic DNA was amplified using high performance liquid chromatography (HPLC)-purified consensus oligonucleotides specific to the variable (FR2A; 400 ng per reaction) and joining (LJH; 100 ng per reaction) Ig heavy chain segments14 in 100 μL of reaction mixture (62.5 mmol/L KCl, 12.5 mmol/L Tris HCl, 2 mmol/L MgCl2 , 0.8 mmol dNTP, 2 U of AmpliTaq polymerase; Perkin-Elmer Cetus, Norwalk, CT). The PCR conditions included five initial stringent cycles (96°C for 15 seconds, 63°C for 30 seconds with a ramping time of 45 seconds, and 72°C for 30 seconds) followed by 35 cycles with a lower annealing temperature (96°C for 15 seconds, 57°C for 30 seconds with a ramping time of 45 seconds, and 72°C for 30 seconds) in a GeneAmp PCR system 9600A (Perkin-Elmer Cetus). One microliter of the amplified product was then reamplified using the FR2A oligonucleotide (200 ng per reaction) and an oligonucleotide internal to LJH (VLJH; 200 ng per reaction) in the same buffer, with the exception of the MgCl2 concentration (1.5 mmol/L). For the reamplification, 25 cycles of PCR (96°C for 15 seconds, 63°C for 30 seconds with a ramping time of 45 seconds, and 72°C for 30 seconds) were performed.
For the detection of bcl-2 rearrangements, amplification of the genomic DNA template was performed in 100 μL of PCR buffer (50 mmol/L KCl, 10 mmol/L Tris HCl, 1.5 mmol/L MgCl2 , and 0.2 mmol/L of each of the dNTPs) containing 2 U of the AmpliTaq polymerase (Perkin-Elmer Cetus) and primers (0.5 μmol/L) that span the major breakpoint region 5′ TTAGAGAGTTGCTTTACGTGGCCTG 3′ and the JH region 5′ ACCTGAGGAGACGGTGACCAGGGT 3′.15 Thirty cycles of amplification (denaturation at 94°C for 1 minute, annealing at 55°C for 2 minutes, and synthesis at 72°C for 1 minute) were performed.
The search for viral sequences specific to the Kaposi's sarcoma-associated herpesvirus was performed using the same buffer conditions as for the bcl-2 amplification and with the following specific primers: 5′ TCCGTGTTGTCTACGTCCAG 3′, 5′ AGCCGAAAGGATTCCACCAT 3′.16 Forty cycles of PCR were performed at 94°C for 1 minute, 58°C for 1 minute, and 72°C for 1 minute.
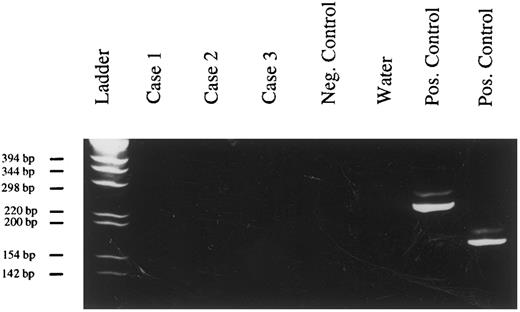
Evaluation of the clonality of PBL by PCR. Amplification products after IgH-PCR and PAGE (6% polyacrylamide gel, staining with ethidium bromide). Case no. 1, tumor of patient no. 12; case no. 2, tumor of patient no. 15; case no. 3, tumor of patient no. 9; positive control, DNA extracted from the Raji cell line.
Evaluation of the clonality of PBL by PCR. Amplification products after IgH-PCR and PAGE (6% polyacrylamide gel, staining with ethidium bromide). Case no. 1, tumor of patient no. 12; case no. 2, tumor of patient no. 15; case no. 3, tumor of patient no. 9; positive control, DNA extracted from the Raji cell line.
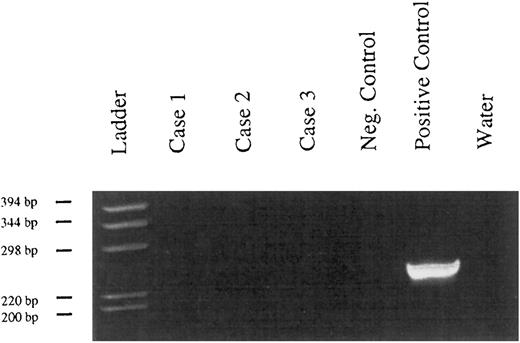
Absence of bcl-2 rearrangements in PBL, (6% polyacrylamide gel, staining with ethidium bromide). Case no. 1, tumor of patient no. 12; case no. 2, tumor of patient no. 15; case no. 3, tumor of patient no. 9; negative control, DNA extracted from a tonsil; positive controls, 2 cases of follicle center lymphomas.
Absence of bcl-2 rearrangements in PBL, (6% polyacrylamide gel, staining with ethidium bromide). Case no. 1, tumor of patient no. 12; case no. 2, tumor of patient no. 15; case no. 3, tumor of patient no. 9; negative control, DNA extracted from a tonsil; positive controls, 2 cases of follicle center lymphomas.
RESULTS
Clinical features.The most relevant clinical features of the patients who presented the malignancies investigated in the present study are listed in Table 1. Fifteen of the 16 patients were infected by HIV. Twelve were homosexual and 2 were intravenous drug abusers. In 1 patient, the mode of contamination could not be identified. In all instances, the tumors were localized in the mucosa of the oral cavity and often involved the gingiva. In 1 patient, the bulk of the tumor involved the palatal mucosa and a minimal infiltration of the adjacent bone could be verified by x-ray analysis. Extension of the lymphomas to the abdomen, to the retroperitoneum, or to other organs such as the bones could be identified in some patients at the time of diagnosis, during staging, or shortly after it. In 1 patient, a minimal infiltration of the bone marrow could be shown histologically. In none of the patients could a serum monoclonal Ig be detected by serum protein electrophoresis or immunoelectrophoresis. The tumors clinically showed a highly malignant behavior and the prognosis was on average poor, even after polychemotherapy with or without combined radiotherapy. However, 3 patients with tumors limited to the oral cavity responded well to radiotherapy alone (patients no. 4, 5, and 15; Table 1).
Morphologic features.The histologic appearance was similar in all cases (Fig 1A and B). The lamina propria of the oral mucosa was infiltrated by a diffusely growing cellular population. Interspersed tingible body macrophages imparted a starry sky appearance in the majority of the cases. The neoplastic cells were large and had more or less eccentrically placed slightly irregular round to oval nuclei with little chromatin and with either a single prominent centrally located nucleolus or several peripherally located nucleoli. The cytoplasm was abundant with a paranuclear hof and appeared deeply basophilic after Giemsa staining. It displayed a squared-off appearance confering a cohesive growth pattern to the proliferation. Apoptotic figures as well as single-cell necroses were numerous, mitotic activity was brisk, and the Ki-67 (proliferative) index attested by the staining with the MIB 1 antibody was high (>90% of the tumor cells; Fig 1C). Follicular dendritic cells were absent from the tumor. No or only a few small reactive cells that proved to be of T-cell origin after staining with a CD3-specific polyclonal antibody were found outside the neoplastic infiltration.
Immunophenotype.Results of the analysis of the immunophenotype are compiled in Table 2. For the investigation of CD20 and CD45 expression in the cases of the present study, sections from a nodal diffuse large B-cell lymphoma were used as controls. The latter tumor displayed an intense membranous immunostaining after incubation with these antibodies (Fig 1D). In contrast, the expression of both antigens was diminished or absent in the reported cases. Two different staining patterns could be distinguished. Eight cases displayed a very homogeneous immunophenotype with complete negativity for CD20 and CD45 markers. The immunophenotypes obtained in the remaining 8 cases were more heterogeneous. These 8 cases displayed a weak cytoplasmic positivity for CD20 and/or CD45 in a variable proportion of the tumor cells. In 3 of these 8 cases there was, in addition to the cytoplasmic staining, a weak expression of CD20 and/or CD45 on the surface membrane in a minority of cells (<5%). Only in 1 case was the proportion of the CD20+ cells around 25% (Fig 1F ).
In all of the cases studied, the neoplastic cells showed a strong immunostaining after incubation with the VS38c antibody (Fig 1H). In 13 of 15 cases, the staining with the antibody specific to CD79a was positive. In 5 cases, CD79a was found to be expressed in most neoplastic cells (Fig 1E), whereas in the remaining 8 cases only 5% to 30% of the cells showed positive immunostaining (Table 2). Eight of the 16 cases contained detectable amounts of intracytoplasmic Ig heavy chain γ (Fig 1G). A monotypic light chain expression could be demonstrated in 5 of the 16 cases. In summary, an expression of CD79a and/or of Ig chains could be detected in all cases.
The expression of the BCL-2 protein was heterogeneous among the 16 cases. Four cases were positive, but the remaining cases were negative. Only a weak and partial expression of the BCL-6 could be detected in 4 of 14 tested cases, whereas the remaining cases were completely negative.
Association with the EBV and expression of latent proteins.Fifteen of the 16 studied cases were tested for an association with EBV using in situ hybridization with the EBER-1 and EBER-2 probes (Table 3). Nine of these 15 cases clearly displayed positive signals in the nuclei of the tumor cells (Fig 1I). To more precisely define the significance of the viral infection, the expression of the latent viral proteins in the tumor cells was analyzed using appropriate monoclonal antibodies (Table 3). None of the EBV-positive cases was found to express EBNA-2, a nuclear viral antigen thought to be central in the virally induced immortalization.17 18 In 7 of the 9 EBV-associated plasmablastic lymphomas, an expression of the BZLF-1 was identified in a minority of the tumor cells, ranging from 2% (Fig 1J) to less than 1 per 1,000 of the neoplastic cells. In 4 cases, staining with an antibody cocktail against LMP-1 was positive, but the percentage of labeled cells was small, ranging from 0.1% to 5.0% (Fig 1K).
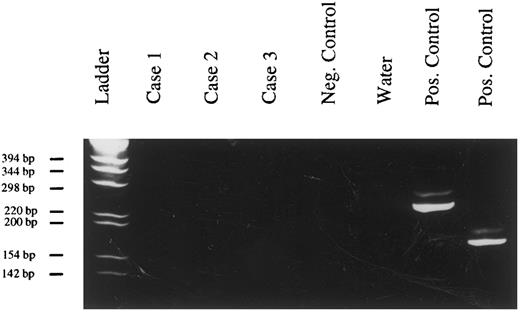
Absence of Kaposi's sarcoma-associated herpesvirus DNA from PBL. (PCR, 6% polyacrylamide gel, staining with ethidium bromide). Case no. 1, tumor of patient no. 12; case no. 2, tumor of patient no. 15; case no. 3, tumor of patient no. 9; negative control, DNA extracted from a tonsil; positive control, Kaposi's sarcoma.
Absence of Kaposi's sarcoma-associated herpesvirus DNA from PBL. (PCR, 6% polyacrylamide gel, staining with ethidium bromide). Case no. 1, tumor of patient no. 12; case no. 2, tumor of patient no. 15; case no. 3, tumor of patient no. 9; negative control, DNA extracted from a tonsil; positive control, Kaposi's sarcoma.
Clonality of PBL.In all 3 tested cases, a single amplification product was obtained after PCR with Ig heavy chain-specific primers, confirming the B-cell origin of PBL and their monoclonality (Fig 2).
Bcl-2 rearrangements.None of the tested cases presented a rearrangement of the bcl-2 gene around the major breakpoint region of the bcl-2 gene (Fig 3).
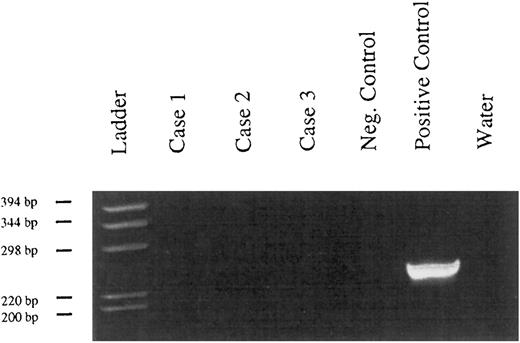
Detection of Kaposi's sarcoma-associated herpesvirus DNA.No viral sequences could be detected in the 3 tested cases by PCR amplification with oligonucleotides specific to Kaposi's sarcoma-associated herpesvirus (Fig 4).
DISCUSSION
Apart from follicle center lymphomas, diffuse large B-cell lymphomas (DLBCL) represent the largest group among non-Hodgkin's lymphomas (NHL). All attempts to identify distinct subcategories within DLBCL failed, with 1 exception: the mediastinal large-cell lymphomas, which exhibit characteristic clinical and patho-anatomical features.5,19 20
In the present report, evidence is provided for the existence of a second biologic entity within the DLBCL. The reported lymphomas were extranodal, with a predilection for the oral cavity and particularly the mucosa of the jaws. They showed a close association with HIV infection (15 of the 16 cases) as well as an unusual immunophenotype. This immunophenotype included a weak or absent expression of CD20 and CD45 and a strong immunostaining with the plasma cell reactive antibody VS38c. The frequent but variable expression of the CD79a antigen combined with the detection of monotypic Ig light and/or heavy chain expression and the presence of clonally rearranged IgH genes in all of the 3 tested cases confirmed a relationship to cells of the B-cell system, a clonal origin of the tumor cells, and a marked plasmacellular differentiation. These tumors showed no or only a partial and faint staining with an antibody specific to the BCL-6 protein. This finding, in combination with the negative or inconstant expression of the BCL-2 protein in the tumor cells and the absence of rearrangement of the bcl-2 gene in the major breakpoint region in the tumor cells in the 3 cases studied, rules out a close relation of PBL to the follicle center lymphomas or to the large-cell lymphomas of follicle center origin. The described blastoid morphology and immunophenotype of these tumors indicate that they are most similar to plasmablasts, ie, cells that still have the blastoid feature of immunoblasts but otherwise have already acquired the antigen profile of plasma cells. In normal lymphoid tissue, the number of plasmablasts is low, which might explain why B cells of this differentiation stage have not yet attracted much attention. However, large numbers of plasmablasts can be encountered in viral lymphadenopathies, eg, caused by rubella virus, or EBV (infectious mononucleosis).21 22 Because of the described similarity between reactive plasmablasts and the large-cell tumors under discussion, we propose to name them plasmablastic lymphomas.
Several tumor entities should be considered in the differential diagnosis of PBL (Table 4). The negative immunostaining for CD20 and CD45 of an undifferentiated large-cell tumor could erroneously exclude a lymphoma from the various diagnostic alternatives. The knowledge of PBL and its features is therefore important and should lead to the application of the plasma cell-reactive antibodies VS38c and anti-CD79a (JCB117) as well as of antibodies to Ig chains when the common lymphoid markers are not demonstrable.
The distinction from a diffuse large B-cell lymphoma of the immunoblastic type with plasmacytoid differentiation is not possible on morphologic grounds alone, although the high degree of cohesiveness and the extremely high apoptotic rate, as observed in our cases, would appear unusual for an immunoblastic lymphoma. The absence of CD20 and CD45 and the strong reactivity of the entire tumor cell population with the VS38c antibody allows the distinction between these two entities. The frequent expression of BCL-6 in DLBCL further helps to distinguish PBL from the majority of DLBCL, because the former do not express these antigens or express them at only a low level.
PBLs may also be confused with plasmacytomas, with which they share a similar immune profile. The absence of serum monoclonal protein and the absence of massive bone marrow involvement in most of the cases argue against a plasmacytoma. Moreover, close attention to cellular details helps further distinguish these related but distinct neoplasms, with PBLs being entirely composed of blasts with a light chromatin and one to a few prominent nucleoli and not containing proplasma cells and mature plasma cells with condensed chromatin and inconspicuous nucleoli, which is typical of plasmacytomas. In addition, the extremely high proliferation index as well as the numerous mitotic figures would be unusual features for a plasmacytoma. Clear separation of PBL from plasmacytoma is reinforced by the high rate of association of PBL with EBV, whereas plasmacytomas have been found to carry the genome of the virus in only 6.7% of the cases.23
The cohesive architecture of PBLs, their extremely high proliferation rate, and the pattern of viral protein expression in EBV-harboring cases are reminiscent of Burkitt's lymphoma (small noncleaved CLL lymphomas). The large size, the plasmacellular differentiation, and the immune profile of the PBL tumor cells easily exclude this latter diagnosis. Burkitt's lymphomas express CD20, CD45, and the membrane-bound Ig heavy chain of the isotype M. In contrast, 50% of the PBL in the series we report here were found to express cytoplasmic IgG and no case expressed IgM. Finally, BCL-6, which is strongly expressed in Burkitt's lymphomas,8 was negative or minimally expressed in all our PBL cases.
It would be interesting to learn if the tumors described in the literature as being intermediate between Burkitt's lymphoma and immunoblastic lymphomas with plasmacytoid differentiation in HIV-positive patients and the Burkitt's lymphoma with intracytoplasmic Igs are more closely related to PBL than to Burkitt's lymphoma or other types of NHLs.24-26
Green and Eversole27 reported on a series of 10 lymphomas of the oral cavity in HIV-positive patients, with 3 cases being negative for CD20. It might well be that the CD20− cases correspond to PBL cases described here.
Because HIV-related lymphomas are frequently associated with EBV infection, the presence of EBV-encoded RNAs was investigated. The identification of EBV-specific RNAs in the neoplastic cells of some of the PBL cases, in the frame of the immune deficiency, may suggest an active role of the virus in the development of these tumors. However, the presence of EBV in only 9 of the 15 tested cases argues against this virus being the sole etiologic agent. Moreover, the viral latent proteins that are thought to mediate the immortalizing potential of EBV, in particular EBNA-2, were not found to be expressed using standard immunohistologic techniques. This indicates that the pattern of EBV-latent protein expression markedly differs from the one observed in most posttransplant lymphomas.28 The recently identified body cavity lymphomas share some similarities with PBL in that they harbor EBV and are often devoid of CD20.29,31 However, in contradistinction to these latter tumors, an infection by the Kaposi's sarcoma-associated herpesvirus could not be detected using a PCR-based approach in the 3 cases tested.16
In conclusion, the morphologic, immunophenotypic, and clinical features described strongly suggest that PBL represents a new distinct subtype among DLBCL. The individualization of this new lymphoma category provides a solid basis for further molecular analysis aimed at defining its pathogenesis.
ACKNOWLEDGMENT
We thank the following clinicians: Dr K. Arasthé (Berlin, Germany), Dr D.H. Pohle (Berlin, Germany), Dr T. Gabrysiak (Hannover, Germany), and Dr R.E. Schmidt (Hannover, Germany) for communication of clinical data.
Supported by a grant from the Deutsche Krebshilfe and by a European community contract (CHRX-CT94-0651).
Address reprint requests to H. Stein, MD, Institute of Pathology, Klinikum Benjamin Franklin, Free University of Berlin, Hindenburgdamm 30, 12200 Berlin, Germany.