IT WAS 25 YEARS AGO that the war against cancer was officially launched by President Nixon. The ensuing investment in the study of cancer has been a major engine driving the current revolution in cell and molecular biology. Yet, the clinical rewards have been less than remarkable. This runs the danger of raising the level of skepticism among the public and may erode the level of support, just when it is needed most to effect a translation of the accomplishments of basic research into the fruits of clinical medicine.
Over the last 3 to 5 decades, the treatment of cancer has relied primarily on the use of various forms of cytotoxic chemotherapy and radiation therapy. These interventions have had profound positive results on many hematologic malignancies and a few solid tumors, especially germ cell and some childhood malignancies. However, the most prevalent of malignancies have proved to be more or less resistant to these interventions. Dose escalation using high-dose chemotherapy may have resulted in a modest improvement in responses but has not constituted a breakthrough. In all these cases, the effectiveness of cytotoxic treatments has been limited by the side effects of these agents on normal tissues and cells, even with the successful attempts of reducing these toxicities using various forms of supportive measures (such as bone marrow rescue, antibiotics, antiemetics, and growth factor support).
On the other hand, recent accomplishments in the understanding of mechanisms of growth regulation, stress response, and the action of several cytotoxic agents may allow us to begin to develop a conceptual framework for pursuing cancer chemotherapy at the cell and molecular level. This may then translate into a goal-directed and rational approach to cancer treatment.
Indeed, accruing evidence obtained in the last few years is beginning to establish that many (and perhaps all) agents of cancer chemotherapy effect tumor cell killing in vitro and in vivo through launching the mechanisms of apoptosis (or programmed cell death). These lines of investigation have provided significant insight into the mechanisms involved in cell death in general and tumor cell death more specifically. Such insight may allow the identification of novel targets and the development of more specific chemotherapeutic agents that are designed to launch specifically the apoptotic machinery of the cell. Paradoxically, the realization that chemotherapeutic agents (and possibly ionizing irradiation) are effective primarily because they activate apoptosis raises the concern that tumors that are intrinsically resistant to chemotherapy are unable to activate the apoptotic machinery and may therefore be fundamentally resistant to chemotherapeutic cell death.
This review will highlight the recent developments in the field of apoptosis in general (especially at the mechanistic level), provide evidence linking chemotherapy-induced cell death and apoptosis, formulate a hypothesis for cancer chemotherapy, and conclude with a discussion of the ramifications of such a formulation for the future development of cancer chemotherapy.
APOPTOSIS: DEFINITIONS AND MECHANISMS
The History and Biology of Apoptosis
Apoptosis has become one of the hottest areas of cell biology research, probably because of the belated realization that cell death is a biochemically regulated process that may be as complex as other fundamental biological processes. The existence of various forms of cell death involving tissues and cells was recognized in the 19th century, although it never received primary attention.1 Programmed forms of cell death have also been recognized in the field of botany, but it has been labeled mostly as senescence. In 1970, Wyllie and Kerr formalized the existence of a human form of cell death distinct from necrosis that they termed apoptosis.2,3 Apoptosis received its primary boost with the identification of internucleosomal DNA breakdown during apoptosis and not necrosis.2 Because this form of DNA breakdown suggested the action of an endonuclease, this singular finding may have convinced many investigators that apoptosis is the manifestation or outcome of biochemical processes.
Apoptosis is now recognized as a mechanistically driven form of cell death that is either developmentally regulated,3 launched in response to specific stimuli (such as the cytokines tumor necrosis factor α or the fas ligand),4,5 or activated in response to various forms of cell injury or stress.6,7 In developmental biology, programmed cell death is responsible for eliminating superfluous or redundant precursor or mature cells. For example, in immunobiology, apoptosis accounts for the elimination of self-reacting lymphocytes.8 Apoptosis also appears to play an important role in tissue remodeling and reaction to the environment whereby unnecessary cells may undergo cell death to allow the growth and differentiation of cells that are better geared to deal with the changing environmental demands.3 In cancer biology, it is becoming increasingly apparent that many cancer cells circumvent the normal apoptotic mechanisms to prevent their self-destruction, which would have been indicated because of the many mutations they may harbor.9 10 Indeed, disarming apoptosis and other surveillance mechanisms may be of fundamental significance in allowing the development of the malignant and metastatic phenotype.
Mechanisms of Apoptosis
In the 1980s, two major endogenous regulators of apoptosis were identified. Although initially identified as an oncoprotein, it soon became clear that the wild-type p53 protein functioned as an inducer of cell death, especially in response to DNA damaging events.11-13 Reciprocally, studies on the Bcl-2 oncogene led to the identification of an important antiapoptotic function for this protein that therefore gave survival advantage to lymphomas that overexpressed the protein product.14,15 Bcl-2 is now appreciated to belong to a family of related and interacting molecules such as Bax, Bcl-x, Bad, Bag, Bak, and Bik, some of which are antiapoptotic, whereas other members of the family, such as Bax, display pro-apoptotic function.15-17
In independent lines of investigation, investigators determined the existence of specific genes that regulate cell death during the development of Caenorhabditis elegans, including a homologue of Bcl-2 and a protease that is a member of the interleukin-converting enzyme (ICE) family of cysteine proteases.18,19 These key developments appear to have convinced the scientific community that apoptosis and cell death are regulated outcomes. As a consequence, an intensive effort has been applied in the recent past aimed at defining mechanisms involved in the initiation and execution of cell death. These intensive efforts have resulted in a flurry of activity and a wealth of information as well as the realization that this is a complex process with many as yet undiscovered components and processes that ultimately regulate apoptosis. There are numerous reviews2,3,6-10,14,15 20-28 that cover various aspects of apoptosis, its historical development, components involved in apoptosis, and mechanisms regulating apoptosis.
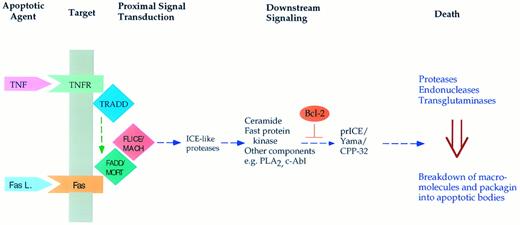
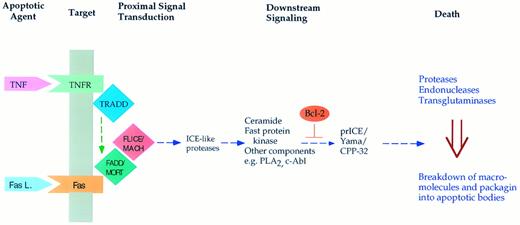
Mechanisms regulating cell death are perhaps best exemplified in cytokine-induced apoptosis (Fig 1). For example, both tumor necrosis factor α and the Fas ligand cause the death of certain malignant and normal cells. Activation of the receptors for these cytokines launches an intracellular pathway through specific proteins29,30 that interact with these receptors (FADD, TRADD, and others). These proteins appear to couple to a protease (MACH/FLICE), and activation of this protease propagates the apoptotic signal.31,32 A component of this pathway includes sphingomyelinases that act on membrane sphingomyelin and cause the release of the lipid mediator ceramide.33,34 Ceramide may then cause the activation of downstream proteases that, in turn, seem to launch the final phase of apoptosis. This phase involves the breakdown of several macromolecules including DNA and proteins, fragmentation of organelles, and packaging of the cellular debris into apoptotic bodies that are then engulfed by phagocytic cells. Through as yet unidentified mechanisms, Bcl-2 appears to interfere with the activation of the distal proteases,35 36 whereas p53 seems to launch a more proximal component of this pathway. Obviously, major gaps exist in connecting the different components of this scheme and in defining the relative contribution of these components. Nevertheless, this is a very promising beginning towards unraveling the regulation of this important process.
Scheme for induction of apoptosis in response to the cytokines tumor necrosis factor α and the Fas (APO-1) ligand. Both cytokines bind to specific membrane receptors (such as TNFR and Fas) and allow for the interaction of the receptors with specific proteins (such as TRADD and FADD/MORT). This may result in activation of a protease (Mach/Flice) that somehow launches the apoptotic response. Downstream mediators of this response may include sphingomyelinase causing the accumulation of ceramide, phospholipase A2 with accumulation of arachidonate, and other mediators such the Fast kinase.94 Eventually, this results in activation of downstream proteases such as prICE/CPP-32, which cleaves PARP and possibly other protein substrates. This then appears to induce the terminal and irreversible phase of apoptosis characterized by macromolecular breakdown and cellular fragmentation.
Scheme for induction of apoptosis in response to the cytokines tumor necrosis factor α and the Fas (APO-1) ligand. Both cytokines bind to specific membrane receptors (such as TNFR and Fas) and allow for the interaction of the receptors with specific proteins (such as TRADD and FADD/MORT). This may result in activation of a protease (Mach/Flice) that somehow launches the apoptotic response. Downstream mediators of this response may include sphingomyelinase causing the accumulation of ceramide, phospholipase A2 with accumulation of arachidonate, and other mediators such the Fast kinase.94 Eventually, this results in activation of downstream proteases such as prICE/CPP-32, which cleaves PARP and possibly other protein substrates. This then appears to induce the terminal and irreversible phase of apoptosis characterized by macromolecular breakdown and cellular fragmentation.
CANCER CHEMOTHERAPY AND APOPTOSIS
Chemotherapeutic Agents Kill Susceptible Cells by Apoptosis
In a landmark study investigating the mechanism of action of etoposide (an inhibitor of topoisomerase II) and other chemotherapeutic agents, it was found that etoposide, early on, induced internucleosomal DNA fragmentation.37 This observation raised the possibility that etoposide caused apoptotic cell death. Since then, the spectrum of chemotherapeutic agents causing apoptosis has expanded progressively, and the evidence supporting the role of apoptosis in chemotherapy action continues to accumulate. The chemotherapeutic agents that have thus far been identified as apoptosis-inducing include etoposide, VM26, m-AMSA, dexamethasone, vincristine, cis-platinum, cyclophosphamide, paclitaxel, 5′-fluoro-deoxyuridine, 5′-fluorouracil, and adriamycin37-41 (Table 1). These apoptotic effects have been observed in several cell lines in tissue culture, including normal thymocytes, lymphoma cells, ovarian epithelial tumors, leukemia cells, adenocarcinoma cells, and others. In addition, tumor hypoxia, ionizing radiation, and hormone withdrawal in hormone-dependent tumors have been shown to cause apoptosis.42-47 The occurrence of apoptosis has been documented by morphologic criteria, the occurrence of endonucleosomal DNA breakdown, flow cytometric analysis of DNA content, and other criteria. The reader is referred to multiple reviews that document the ability of chemotherapeutic interventions to cause apoptosis.9,14 48-50
Other studies are also beginning to provide evidence that chemotherapeutic agents induce apoptotic tumor cell death in vivo. For example, a retinoic acid-treated T-cell lymphoma was shown to undergo apoptosis in vivo.51 In a study of esophageal squamous cell carcinoma, it was shown that both radiation and chemotherapy (5-fluorouracil, cis-platinum, and bleomycin) induced apoptotic cell death in vivo, as determined by examination of biopsy specimens.52 In an experimental study in murine tumors, evidence was also provided that cis-platinum, cyclophosphamide, and other chemotherapeutic agents caused apoptosis in several in vivo tumors, including adenocarcinoma, lymphoma, sarcomas, and squamous cell carcinomas.53 (Although not all agents caused apoptosis in all the tumors.) Similarly, in a study of murine mammary adenocarcinoma and ovarian carcinoma, it was observed that cyclophosphamide treatment increased apoptosis in these tumors.54 Antileukemic therapy (including etoposide, m-AMSA, and cytosine arabinoside) caused apoptotic cell death in patients undergoing chemotherapy for acute leukemia.55
Mechanisms of Chemotherapy-Induced Apoptosis
With the developing understanding of mechanisms regulating apoptosis, it is becoming increasingly clear that chemotherapeutic agents operate through similar mechanisms. Indeed, some of the insight into mechanisms regulating apoptosis has come from the examination of chemotherapy-induced death. This is best illustrated again with the case of etoposide in which Kaufmann et al56 identified proteolytic cleavage of poly(ADP-ribose) polymerase (PARP) in response to etoposide. Subsequent studies indicated that this was a result of activation of a specific protease and that it preceded endonuclease activation and DNA fragmentation.57 This PARP protease has evolved as a centerpiece in the study of apoptotic mechanisms, and it has been cloned by several investigators and has been given several names, including CPP-32, prICE, Yama, and apopain.58-60 It now appears that many inducers of cell death, including cytokines and other chemotherapeutic agents, ultimately converge on the activation of this and related proteases, which then appear to launch the terminal and execution stages of apoptosis (Fig 1).
Another convincing set of data supporting the role of apoptosis in chemotherapy action has come from studies on the interaction of chemotherapeutic agents with modulators of apoptosis. Bcl-2 overexpression has been shown to inhibit apoptosis in vitro in response to several chemotherapeutic agents, including etoposide, methylmethanesulfonate, N′-methyl-N′-nitrosourea, dexamethasone, camptothecin, doxorubicin, 4-hydroxyperoxy-cyclophosphamide, vincristine, and actinomycin D.14,61-67 In addition, the level of expression of Bcl-2 in clinical tissue samples has been correlated with disease prognosis in non-Hodgkin's lymphoma,68 but not in childhood acute lymphoblastic leukemia.69 On the other hand, reduced expression of Bax, a pro-apoptotic homologue of Bcl-2, was found to be associated with poor response to combination chemotherapy and worse survival in patients with metastatic breast carcinoma.70 In addition, many chemotherapeutic agents, such as cytosine arabinoside, vincristine, daunorubicin, and ionizing radiation, have been shown to cause accumulation of ceramide.71-74 In the case of ionizing radiation, it has been shown recently that this may involve activation of an acidic sphingomyelinase and that mice lacking this enzyme acquire resistance to radiation-induced apoptosis in the lung.75
Other modulators of apoptosis have also been shown to interact with chemotherapy-induced cell death. For example, the cytotoxicity of several chemotherapeutic agents, including vincristine, adriamycin, cytosine arabinoside, and cyclophosphamide, has been shown to be inhibited by hematopoietic growth factors such as granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, or interleukin-6.76 Disruption of p53 protects breast carcinoma cells from platinum-induced death,77,78 and BCR-ABL protects from apoptosis induced by a variety of chemotherapeutic agents.79 The Epstein-Barr viral protein BHRF1, which shows structural80 and functional81 homology to Bcl-2, was shown to protect against cell death induced by etoposide and cis-platinum,82 and the expression of bcl-x was shown to modulate drug sensitivity of breast cancer neuroblastoma83,84 and prolymphocytic85 cells. The oncogenes H-ras and MDM2 were shown to confer resistance to chemotherapy-induced apoptosis in rhabdomyosarcoma and glioblastoma, respectively.86,87 Safingol, an inhibitor of protein kinase C (which is usually associated with a viability response), was found to potentiate the ability of Mitomycin C to kill gastric cancer cells.88
These in vitro and in vivo studies, coupled with the mechanistic insight evolving on the regulation of chemotherapy-induced cell death, clearly show the induction of apoptosis by several chemotherapeutic agents in different cell lines and tumors. On the other hand, it has not yet been ascertained what fraction of tumor cell death is effected through apoptotic mechanisms. Determining this fraction may not be trivial, but it could underscore the extent of apoptosis and its significance in chemotherapy-induced death. (It is anticipated that the more we learn about chemotherapy-induced cell death, the more we will appreciate the extent and significance of apoptosis in mediating chemotherapy-induced cytotoxicity.)
HYPOTHESIS AND RAMIFICATIONS
If, indeed, various agents of cancer chemotherapy act primarily through the induction of apoptotic cell death in susceptible cancer cells, then how can we reconcile the existence of distinct targets for these disparate chemotherapeutic agents with the more or less unifying concept of apoptosis?
Hypothesis
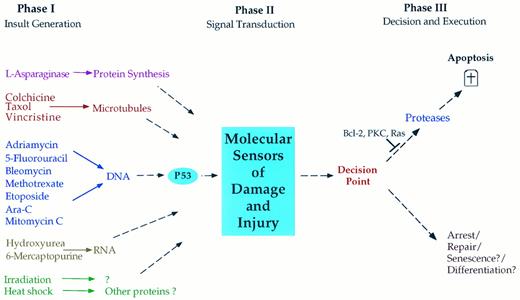
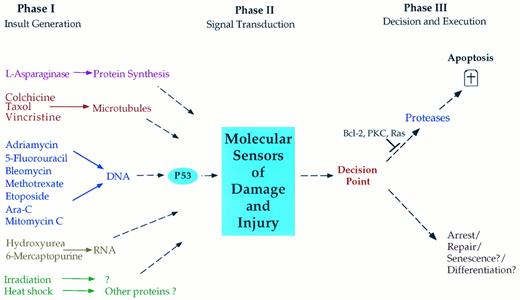
The simplest, yet most encompassing, hypothesis (Fig 2) proposes that each chemotherapeutic agent interacts with a specific target causing dysfunction and injury, which is then interpreted by susceptible cancer cells as an instruction to undergo apoptosis. Thus, we may consider that chemotherapy-induced cell death proceeds through three distinct general phases. (1) Phase I: an insult-generating mechanism. In this phase, each class of chemotherapeutic agents interacts with a specific target such as DNA, RNA, and microtubules and the action of these agents on their respective targets causes target injury or dysfunction. (2) Phase II: signal transduction. In this phase, the cell, through yet poorly defined mechanisms, is able to decipher and assess the specific injury to the chemotherapy target. For example, DNA-damaging agents may use the c-Abl tyrosine kinase to induce cell cycle arrest in a p53-dependent mechanism.89 Treatment of some T-cell leukemia lines with doxorubicin results in upregulation of the ligand to the Fas receptor, which may mediate the ability of doxorubicin to kill these cells.90 Such a mechanism may account for the sensitivity of leukemia cells to chemotherapy. As described in the previous section, the existence of such mechanisms for transducing cell death in response to cytokines is becoming increasingly appreciated and defined, and further studies should clarify the mechanisms involved in transducing the apoptotic responses to chemotherapeutic agents. (3) Phase III: induction of apoptosis. In the third and final phase, a decision point may exist such that susceptible cells react to the signals generated in response to chemotherapy-induced injury as a go-ahead for the execution phase of apoptosis. For example, γ-radiation causes the induction of the pro-apoptotic Bax in radiosensitive but not in radio-resistant cells. This may drive cells to apoptosis.91 In this phase, the cells undergo the orderly breakdown of macromolecules through the operation of proteases, endonucleases, transglutaminases, and possibly lipases. Other cell types may preferentially execute programs of cell cycle arrest and damage repair in response to these same signals. Although such a decision point appears critical in imparting selectivity of responses to similar stimuli, little is known concerning its existence, components, or regulation.
Hypothesized phases in the induction of apoptosis in response to chemotherapeutic agents. In phase I, cytotoxic agents impart damage to a critical component of the cell such as DNA or microtubules. In phase II, the cell recognizes the damage and its degree of severity through poorly characterized signaling mechanisms. In phase III, the cell assesses the extent of damage and decides on the appropriate response. In many cancer cells, the preferred response is the induction of apoptosis, whereas in most normal cells and in many cancer cells, the response may involve growth arrest to allow for repair. It is also possible that certain cells may react to damage by undergoing senescence or terminal cell differentiation. Cancer cells may acquire resistance to apoptosis at several points in this pathway. For example, mutant p53 may impart resistance to DNA-damaging agents; mutations may exist in the signaling phase (phase II) or in the apoptotic phase III such as with mutant Bcl-2, mutant ras, or hyperactive protein kinase C (PKC).
Hypothesized phases in the induction of apoptosis in response to chemotherapeutic agents. In phase I, cytotoxic agents impart damage to a critical component of the cell such as DNA or microtubules. In phase II, the cell recognizes the damage and its degree of severity through poorly characterized signaling mechanisms. In phase III, the cell assesses the extent of damage and decides on the appropriate response. In many cancer cells, the preferred response is the induction of apoptosis, whereas in most normal cells and in many cancer cells, the response may involve growth arrest to allow for repair. It is also possible that certain cells may react to damage by undergoing senescence or terminal cell differentiation. Cancer cells may acquire resistance to apoptosis at several points in this pathway. For example, mutant p53 may impart resistance to DNA-damaging agents; mutations may exist in the signaling phase (phase II) or in the apoptotic phase III such as with mutant Bcl-2, mutant ras, or hyperactive protein kinase C (PKC).
Analogy: The Cell as a Highly Organized Factory Owned by a Large Multinational Company
A useful analogy would be to think of the cell as a highly efficient production facility with different components charged with specific functions (such as engines, batteries, software, etc). For the sake of high efficiency, the activities of these different components must be highly coordinated so that the final product is not seriously limited by any one component. This also ensures that energy is not wasted on overproduction of other components. This necessitates that the function and status of each component be monitored closely and that these monitoring systems interact and feed back into each other. According to this analogy, if one component receives an insult (such as a fire affecting the battery-producing facility), then the factory has to make a decision as to whether to slow down other production facilities to allow repair (analogous to cells undergoing cell cycle arrest to take care of DNA damage). If the injury is severe and leads to irreversible damage (complete burn down), then the decision may be to permanently close down the production facility and move viable functions to other existing and better-suited production units so as to minimize the losses of the mother company (cells altruistically undergoing irreversible apoptosis for the sake of the tissue or organism).
Ramifications
This formulation suggests a number of ramifications that ultimately impact not only on how we perceive of cancer chemotherapy, but also, more importantly, on what we need to do to achieve a new level of success in cancer therapeutics.
New targets for chemotherapy.As discussed above, most chemotherapeutic agents in current practice appear to induce damage to a major component in the cell, ie, they appear to act in phase I of the proposed scheme (Fig 2). The first ramification suggested by the apoptosis hypothesis stipulates that cancer cell death may be effected by bypassing the initial targets of currently existing chemotherapeutic agents. That is, agents could be developed that either target phase II (ie, falsely signaling damage) or phase III (instructing the cell to undergo apoptosis directly). An advantage to this approach is that it may bypass many of the current hurdles and obstacles facing chemotherapeutic agents. This may be considered the mechanistic approach to cancer chemotherapy. Its development requires significantly more understanding of the various components of phases II and III and how they can be modulated therapeutically.
Why are some cancer cells prone to apoptosis?This component of the hypothesis suggests that most normal cells in the human body are intrinsically much more resistant to apoptosis than several cancer types. Indeed, some experimental evidence shows that diploid fibroblasts are more resistant to apoptosis and actually undergo cell cycle arrest in response to many of the agents that would cause leukemia and lymphoma cells to undergo apoptosis (M. Smyth and Y. Hannun, unpublished observations). This is also supported by the observation that activation of p53 fails to induce apoptosis in normal diploid fibroblasts; however, transformation of these cells renders them susceptible to apoptosis.92 Why are then some cancer cells more susceptible to apoptosis? Unfortunately, at this point in time very little is known concerning the mechanisms that determine whether a cell undergoes apoptosis, cell cycle arrest, or other responses to agents of injury. Understanding the biochemical and molecular bases for this decision making by the cell (Fig 2) could allow a better therapeutic window to drive more cancer cells into apoptosis while preserving normal cells. This would result in better selectivity for cancer chemotherapy.
It should also be noted that conceptualizing the rationale for the increased susceptibility of cancer cells to apoptosis is somewhat counterintuitive. Current understanding of molecular cancer pathogenesis suggests that cancers should be more resistant to apoptosis as a mechanism to escape self-elimination. This problem is revisited in the next section.
Why are most cancers resistant to apoptosis?The third ramification of this hypothesis concerns the issue of resistance. Why are certain tumor cells more intrinsically resistant to apoptosis? Are they more resistant than normal cells? And what can we do about it? The major contention of this hypothesis is that most solid tumors are intrinsically resistant to apoptosis in general and to chemotherapy-induced apoptosis in particular. These cancers may very well have disarmed regulatory mechanisms that survey damage or injury. This is supported by the frequent mutations in p53, which appears to play a critical role in the response to DNA damage. Other mutations may exist that also allow these cancer cells to escape suicide. If chemotherapeutic agents act primarily to induce apoptosis, then it becomes obvious that these cancer cells may be a priori resistant to cell death in response to these agents. If this is the case, then the hematologic and germ cell malignancies may be the exceptional malignancies. For some reason these cancers may have traded the increased susceptibility to apoptosis for some survival or growth advantage. What the era of cytotoxic chemotherapy may have accomplished is to segregate cancers into two groups: those that are prone to apoptosis and those that are resistant!
At this point in time it is not known if the resistant cancers have defects solely in phase II or also defects in phase III. By having defects in either phase II or III, these cells would be very resistant to any agent that acts in phase I. Therefore, pursuing classical cytotoxic chemotherapy would be a futile endeavor.
THE DILEMMA
Addressing these issues of resistance could either provide us with a rational approach to cancer therapy or it could present us with the insurmountable dilemma: many cancer cells are at least as intrinsically resistant to apoptosis as normal cells. There are no windows of opportunity to selectively kill cancer cells without killing the host tissues!
Let us consider a few possibilities as to what could make cancer cells resistant to chemotherapy (Fig 2).
(1) A cancer cell has a defect in one arm of induction of apoptosis, such as a mutation in p53, and the cell becomes resistant to activators of that arm (such as DNA-damaging agents). All other components of the apoptotic pathways are intact. This resistance could be overcome by using agents that either act on another arm in phase I or bypass the defect by acting further downstream. This is probably not a very common scenario because the cancers we need to deal with most are the ones that appear to be resistant to a variety of apoptosis-inducing agents.
(2) A cancer cell has one or more mutations or defects in the common signaling components of phase II, but otherwise the downstream apoptotic machinery is intact. This resistance can be overcome by developing agents that act later in phase II or in phase III. This would result in therapy based on apoptosis only.
(3) A cancer has a mutation or a change in a gene that provides a selective survival advantage (eg, overexpression of Bcl-2). If these blocks are undone or bypassed (eg, agents that downregulate or act downstream of Bcl-2), then the cancer cells can be killed. Indeed, antisense approaches to Bcl-2 (which attenuate the expression of Bcl-2) have been shown to relieve the block to apoptosis in leukemia cells with elevated expression of Bcl-2.93 Mutations in ras, which may confer resistance to chemotherapy,86 may be overcome by specific approaches that target the function of ras (such as inhibitors of prenylation of ras). Such approaches may result in combination chemotherapy with a cytotoxic agent coupled to a specific agent designed mechanistically for the particular cancer.
(4) The cancer cells have either a defect in the apoptotic machinery or are unable to respond to damage by undergoing apoptosis. The existence of such a defect may allow the selective killing of cancer cells if the defect is repaired or overcome. On the other hand, these cells may resemble normal cells in their intrinsic resistance to apoptosis: their decision process may be more similar to normal cells in that they may preferentially undergo cell cycle arrest or other nonapoptotic responses to damaging insults. In this case, selectivity over normal cells may not be achievable. Alternative strategies have to target treatments to the cancer cells specifically to avoid toxicity to normal cells (eg, antibody- or receptor-based targeting). A recent and promising example is the use of an engineered adenovirus that selectively replicates in and kills cells lacking a functional p53.95
UNANSWERED QUESTIONS
There is an emerging realization that cancer chemotherapeutic agents act primarily by inducing cancer cell death through the mechanisms of apoptosis. This review has attempted to provide the foundation for this concept and develop it further with the aim of generating the next level of hypotheses and questions: (1) Does apoptosis account for all, or at least most, of the action of chemotherapeutic agents? (2) What are the intracellular mechanisms responsible for transducing the apoptotic signals generated by chemotherapeutic agents? (3) What are the general mechanisms operating in the induction of apoptosis (the mechanisms that operate with cytokines, injury, stress, irradiation, starvation, etc)? (4) Can we target directly the machinery of apoptosis in cancer cells and effect cancer cell death? (5) What makes some cancers (primarily leukemias and lymphomas) very prone to apoptosis? Is this peculiar to these malignancies or does it extend to other curable cancers? Can we use this knowledge to render resistant cancers sensitive? (6) What are the mechanisms that bestow on most solid tumors resistance to chemotherapy? Is it because of a generalized resistance to apoptosis? Can this be bypassed? (7) Can we find experimental windows of opportunity that allow us to induce cancer cell death selectively without killing normal cells?
These questions and hypotheses should provide a foundation for a rational approach towards cancer chemotherapy based on the premise that cancer cell death is a biochemically driven outcome that is subject to scientific understanding as well as rational targeting.
ACKNOWLEDGMENT
The author thanks Drs Miriam Smyth and Lina Obeid for their suggestions and the careful review of the manuscript and Rita Fortune for expert secretarial assistance.
Supported by the following grants: NIH-GM 43825, ACS CB-122, and DoD AIBS-516.
Address reprint requests to Yusuf A. Hannun, MD, Division of Medical Oncology, Program in Molecular Medicine, Box 3355, Departments of Medicine and Cell Biology, Duke University Medical Center, Durham, NC 27710.