Abstract
Phosphatidylserine (PS), a class of acidic phospholipids, normally localizes on the internal surface of cellular plasma membranes. The internal PS is externalized when cells undergo apoptosis; however, the mechanism for this is largely unknown. To study the mechanism of PS externalization during development of apoptosis, we examined the correlation between the activation of interleukin-1β–converting enzyme (ICE) family protease and PS externalization in human monocytic leukemia U937 cells and in their apoptosis-resistant variants, UK711 and UK110, after treatment with etoposide and anti-Fas antibody. We found that PS externalization accompanied the development of apoptosis and the activation of ICE family proteases in these cell lines. Furthermore, inhibitors of ICE family proteases, Z-Asp and Z-VAD, prevented apoptosis and PS externalization in etoposide-treated U937 cells. These results indicate that PS externalization is a downstream event of ICE family protease activation during apoptosis development. Because ICE family proteases play a crucial role in apoptosis, PS externalization could be a rational and useful marker for the development of apoptosis.
IN CELLULAR PLASMA membranes, phosphatidylserine (PS), a class of acidic phospholipids, is normally located on the internal leaflet of the lipid bilayer.1-4 The molecular mechanism of the asymmetric distribution of PS is not well understood, but it is assumed that a translocase specific to aminophospholipids, such as PS and phosphatidylethanolamine (PE), preferentially mediates translocation of PS from the outer to the inner leaflet in a pH-dependent manner.5-7 This results in the asymmetric distribution of PS and PE.
The interior PS is exposed to the external surface of the plasma membrane under some physiologic conditions. When platelets are activated, the phospholipid asymmetry is lost in the plasma membrane and PS is externalized.8,9 On the activated platelet membranes, a coagulation cascade is initiated by factor Va binding and the subsequent generation of prothrombinase activity by factor Xa binding, which depends on Ca2+ and the externalized PS.8,10 The externalized PS is also involved in cellular recognition by macrophages. Erythrocytes expose PS on their surfaces when they become senescent.11 PS is also exposed in some pathologic erythrocytes, eg, sickle cells.12 In addition, cells that undergo apoptosis were reported to expose PS on their surfaces.13-15 Macrophages recognize the PS externalized cells through a specific PS receptor, resulting in an accelerated engulfment.13 16-18 Thus, PS externalization is a physiologically important phenomena, although the molecular mechanism is largely unknown.
Apoptosis is a physiologic process by which cells undergo controlled cell death accompanied by nuclear condensation and fragmentation before loss of membrane integrity.19,20 In a hematopoietic system, apoptosis is generally observed during the development of lymphocytes and neutrophils21 and when neoplastic cells are treated with antitumor drugs.22,23 As of now, the molecular mechanism of apoptosis is not well understood, yet we know that interleukin-1β–converting enzyme (ICE) family proteases play an important role in initiating apoptosis in a variety of cell systems because (1) ICE family proteases were activated during apoptosis,24-28 (2) overexpression of the proteases induced the cells to undergo apoptosis,29-36 and (3) inhibitors of ICE family proteases prevented apoptosis of the cells.24,25,37,38 Because PS was externalized in apoptotic cells,13-15 we hypothesized that the PS externalization is initiated by ICE family protease activation. The aim of this study is to clarify the relationship between PS externalization and ICE family protease activation during the development of apoptosis. In fact, we found that PS was externalized after the activation of ICE family protease during apoptosis of human monocytic leukemia cells.
MATERIALS AND METHODS
Materials.Annexin V-fluorescein isothiocyanate (FITC) was purchased from Nexins Research B.V. (Maastricht, The Netherlands). Anti-CPP32 and anti-Fas (clone CH-11) monoclonal antibodies were from Transduction Laboratories (Lexington, KY) and MBL (Nagoya, Japan), respectively. Etoposide was kindly provided by Bristol Meyers-Squibb (Tokyo, Japan). Benzyloxycarbonyl-Asp-CH2OC(O)-2,6-dichlorobenzene (Z-Asp) and benzyloxycarbonyl-Val-Ala-Asp-CH2OC(O)-2,6-dichlorobenzene (Z-VAD) were generous gifts from Dr H. Kawai (Kirin Brewery, Takasaki, Japan).
Cell lines and cell culture.Human monocytic leukemia U937 cells were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan). Apoptosis-resistant variants of U937 cells, UK71139 and UK110,40 41 were isolated in our laboratory. The cells were grown in RPMI 1640 medium (Nissui Co, Ltd, Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum and 100 μg/mL kanamycin in a humidified atmosphere of 5% CO2 and 95% air at 37°C. All of the experiments were performed using cells in their exponential growth phase and were repeated at least three times.
Measurement of apoptosis by flow cytometer.Cells (5 × 105 cells/mL) were treated with the indicated concentrations of etoposide or anti-Fas antibody for 4 hours. After incubation, 5 × 105 cells were harvested, washed in ice-cold phosphate-buffered saline, and fixed in 70% ethanol for 30 minutes on ice. The cells were then washed, treated with 1 mg/mL RNase A for 1 hour at 37°C, and stained in propidium iodide solution (50 μg/mL in 0.1% sodium citrate, 0.1% NP-40). The stained cells were analyzed in a FACScan flow cytometer (Becton Dickinson, Braintree, MA).
Measurement of PS externalization by annexin V binding.After treatments, 2 × 105 cells were harvested, washed in ice-cold phosphate-buffered saline, and resuspended in 200 μL of annexin binding buffer (10 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2 , and 2 mmol/L CaCl2 ). The cells were stained with annexin V-FITC for 10 minutes on ice in the dark, according to the manufacturer's instructions, and analyzed in a FACScan flow cytometer.
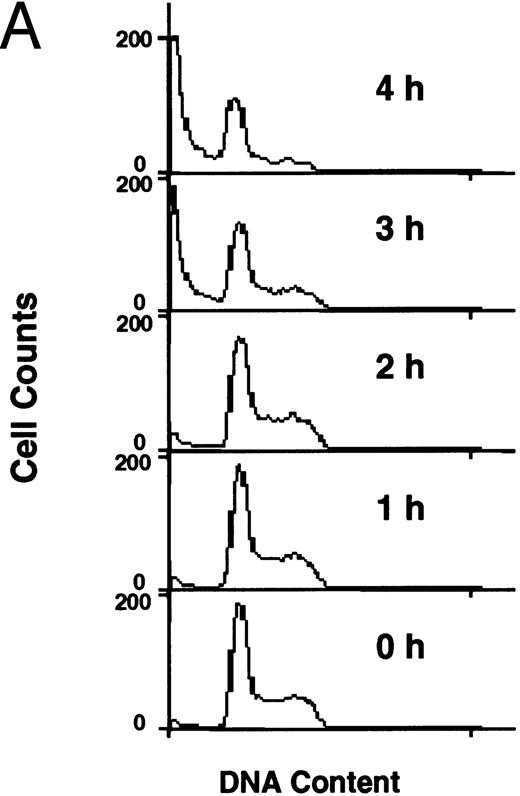
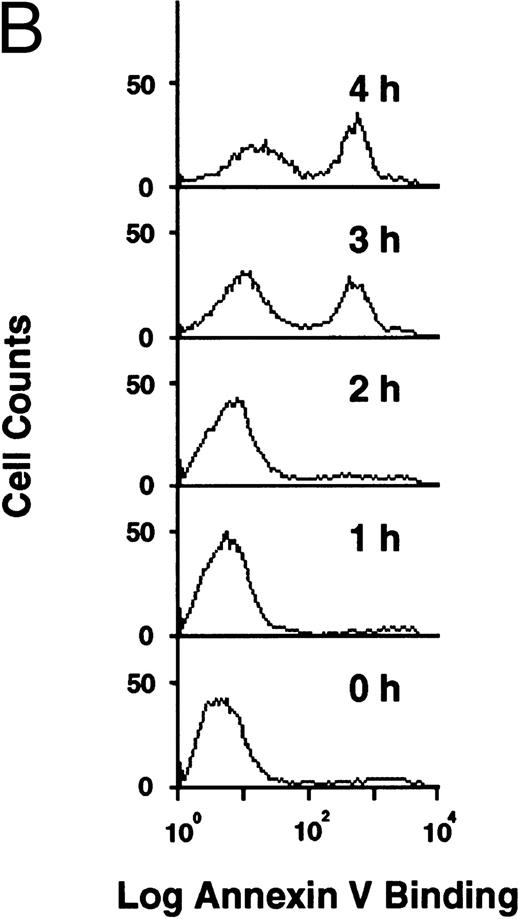
Flow cytometric analysis of development of apoptosis (A) and PS externalization (B) in U937 cells treated with 10 μg/mL etoposide.
Flow cytometric analysis of development of apoptosis (A) and PS externalization (B) in U937 cells treated with 10 μg/mL etoposide.
Immunoblot analysis.The treated cells (1.5 × 106 cells) were harvested, washed, and solubilized in 50 μL of lysis buffer (25 mmol/L Tris-HCl, pH 7.5, 50 mmol/L NaCl, 2% NP-40, 0.5% sodium deoxycholate, 0.2% sodium dodecyl sulfate [SDS], 1 mmol/L phenylmethylsulfonyl fluoride, 0.1 U/mL aprotinin, and 4 mmol/L iodoacetamide) for 15 minutes on ice. After centrifugation, the supernatants were collected as cell lysates. The protein concentration was determined using BCA protein assay reagents (Pierce, Rockford, IL). The cell lysates (20 μg/lane) were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto a nitrocellulose membrane. Immunoblot analysis of CPP32 was performed according to the manufacturer's instruction, and the protein bands were detected using enhanced chemiluminescence (ECL) reagents exposed to reflection autoradiography film (Du Pont-NEN, Boston, MA).
Actin cleavage assay.Actin cleavage activity was measured as described previously.26 Briefly, biotinylated actin (0.2 μg/assay) was incubated with cytosolic fractions from U937, UK711, and UK110 cells for 2 hours. The reaction mixtures were separated by 15%/25% gradient polyacrylamide gel (Daiichi Chemical, Tokyo, Japan) and transblotted onto a nitrocellulose membrane, and the biotinylated proteins were detected with peroxidase-conjugated avidin and ECL reagent.
RESULTS
PS externalization during apoptosis.Human monocytic leukemia U937 cells underwent apoptosis when cells were treated with 10 μg/mL of etoposide, an inhibitor of DNA topoisomerase II with prominent antitumor activity. Figure 1A shows the flow cytometric analysis of the etoposide-treated cells after staining DNA with propidium iodide. Apoptotic cells with DNA content less than G1 dramatically increased at 3 and 4 hours after drug treatment (Fig 1A). PS externalization was measured using FITC-labeled annexin V,14 15 which specifically binds to PS in the presence of Ca2+. As shown in Fig 1B, annexin-positive cells also appeared at 3 and 4 hours after etoposide treatment. Because more than 90% of the apoptotic cells maintained plasma membrane integrity as determined by dye exclusion test (not shown), annexin V bound to the externalized PS on these cells.
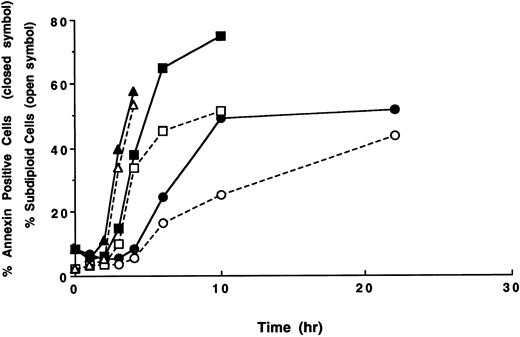
Time course of apoptosis and PS externalization in U937 cells. U937 cells were treated with 1 (circles), 3 (squares), and 10 (triangles) μg/mL etoposide for the indicated times. After the treatments, the development of apoptosis (open symbols) and PS externalization (solid symbols) were analyzed using a flow cytometer, as described in the Materials and Methods. The results are representative of three independent experiments.
Time course of apoptosis and PS externalization in U937 cells. U937 cells were treated with 1 (circles), 3 (squares), and 10 (triangles) μg/mL etoposide for the indicated times. After the treatments, the development of apoptosis (open symbols) and PS externalization (solid symbols) were analyzed using a flow cytometer, as described in the Materials and Methods. The results are representative of three independent experiments.
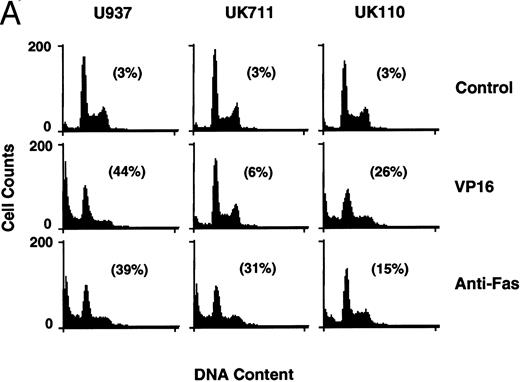
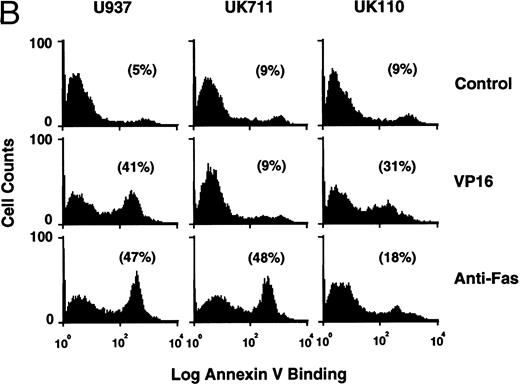
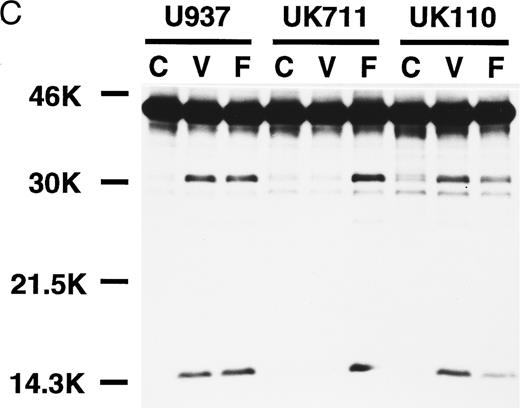
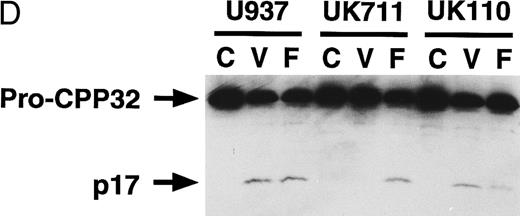
Correlation of (A) apoptosis, (B) PS externalization, and (C) ICE family protease activation determined by actin cleavage assay and (D) CPP32 processing in U937 and its apoptosis-resistant variant cell lines, UK711 and UK110. Cells were treated without (C) or with 10 μg/mL VP16 (V) or 150 ng/mL anti-Fas antibody and 1 μg/mL cycloheximide (F ) for 4 hours. Numbers in parentheses in (A) and (B) are the percentages of apoptotic cells and PS externalized cells, respectively. The experiments were repeated four times and similar results were obtained.
Correlation of (A) apoptosis, (B) PS externalization, and (C) ICE family protease activation determined by actin cleavage assay and (D) CPP32 processing in U937 and its apoptosis-resistant variant cell lines, UK711 and UK110. Cells were treated without (C) or with 10 μg/mL VP16 (V) or 150 ng/mL anti-Fas antibody and 1 μg/mL cycloheximide (F ) for 4 hours. Numbers in parentheses in (A) and (B) are the percentages of apoptotic cells and PS externalized cells, respectively. The experiments were repeated four times and similar results were obtained.
From the histograms of the flow cytometric analysis, we quantitated percentages of apoptotic cells and PS externalized cells (Fig 2). When U937 cells were treated with 10 and 3 μg/mL of etoposide, the populations of PS externalized cells and apoptotic cells rapidly increased after 3 and 4 hours of drug treatment, respectively. The PS externalization and the apoptosis were observed to occur more slowly when cells were treated with 1 μg/mL etoposide. In all cases, PS externalization accompanied the progression of apoptosis in the etoposide-treated U937 cells.
PS externalization and ICE family protease activation in U937 cells and their apoptosis-resistant variants.To study the relationship between PS externalization and ICE family protease activation during apoptosis, we induced apoptosis with etoposide and anti-Fas antibody in U937 cells and their apoptosis-resistant variant cell lines UK711 and UK110. In U937 cells, both anti-Fas antibody and etoposide induced apoptosis (Fig 3A) and PS externalization (Fig 3B). Enzyme activity that cleaves actin to generate a 15-kD fragment by an ICE family protease26 appeared in the cytosolic fractions of these apoptotic cells (Fig 3C). CPP32, an ICE family protease involved in apoptosis of U937 cells,41 was consistently processed to the p17 fragment (Fig 3D), indicating that CPP32 was activated by etoposide and anti-Fas antibody treatment in U937 cells.
In UK711 cells, which show resistance to etoposide-induced apoptosis but not to anti-Fas antibody-induced apoptosis (Fig 3A),39 PS was externalized only after anti-Fas antibody treatment (Fig 3B). The anti-Fas antibody treatment induced the actin cleavage activity (Fig 3C) and the processing of CPP32 (Fig 3D) in UK711 cells, whereas etoposide caused neither. Conversely, etoposide could induce apoptosis (Fig 3A), PS externalization (Fig 3B), actin cleavage activity (Fig 3C), and CPP32 processing (Fig 3D) in another apoptosis-resistant variant, UK110, which shows selective resistance to Fas-mediated and p55-TNFR–mediated apoptosis.40 41 Anti-Fas antibody caused all of the apoptotic features in significantly reduced levels in the UK110 cells. These results indicate that PS externalization correlates well with the activation of ICE family proteases during apoptosis.
Inhibition of PS externalization by inhibitors of ICE family proteases.To study further the relationship between PS externalization and ICE family protease activation, we examined the effect of Z-Asp and Z-VAD, inhibitors of ICE family proteases,37 42 on PS externalization. When U937 cells were treated with etoposide in the presence of graded concentrations of Z-Asp, morphologic apoptosis was blocked in a dose-dependent manner. Z-Asp at 100 μg/mL completely inhibited PS externalization, but at less than 30 μg/mL, it could not inhibit PS externalization (Fig 4A). In the presence of 100 μg/mL Z-Asp, CPP32 was processed to the p20 fragment, but further processing to the p17 fragment was completely inhibited (Fig 4B). Z-Asp at 30 and 10 μg/mL partially inhibited the processing from the p20 to the p17 fragment.
Inhibition of apoptosis, PS externalization, and CPP32 processing by inhibitors of ICE family proteases. U937 cells were treated with 10 μg/mL etoposide in the presence of indicated concentrations of Z-Asp (circles) and Z-VAD (squares) for 4 hours. The development of apoptosis (open symbols) and PS externalization (solid symbols) were analyzed using a flow cytometer (A), and CPP32 processing was examined by Western blot analysis (B). CPP32 processing to the p20 and p17 fragments is presented. Lane 1, no inhibitor; lanes 2 through 4, Z-Asp at 10, 30, and 100 μg/mL, respectively; lanes 5 through 7, Z-VAD at 10, 30, and 100 μg/mL, respectively. The experiments were repeated three times and similar results were obtained.
Inhibition of apoptosis, PS externalization, and CPP32 processing by inhibitors of ICE family proteases. U937 cells were treated with 10 μg/mL etoposide in the presence of indicated concentrations of Z-Asp (circles) and Z-VAD (squares) for 4 hours. The development of apoptosis (open symbols) and PS externalization (solid symbols) were analyzed using a flow cytometer (A), and CPP32 processing was examined by Western blot analysis (B). CPP32 processing to the p20 and p17 fragments is presented. Lane 1, no inhibitor; lanes 2 through 4, Z-Asp at 10, 30, and 100 μg/mL, respectively; lanes 5 through 7, Z-VAD at 10, 30, and 100 μg/mL, respectively. The experiments were repeated three times and similar results were obtained.
Z-VAD was a more potent inhibitor of apoptosis than Z-Asp, and it inhibited morphologic apoptosis efficiently even at 10 μg/mL (Fig 4A). However, PS externalization was not effectively inhibited at 10 μg/mL. Z-VAD at 30 and 100 μg/mL effectively inhibited the PS externalization. Again, CPP32 was processed to the p20 fragment but not to the p17 fragment in the presence of Z-VAD at 30 and 100 μg/mL (Fig 4B). The PS externalization and CPP32 processing to p17 fragment were inhibited by 100 μg/mL Z-VAD for longer time periods up to 9 hours (data not shown).
DISCUSSION
We showed that PS was externalized on the plasma membrane when ICE family proteases were activated in the cells irrespective of the sensitivity to etoposide- and Fas-induced apoptosis. Inhibitors of the ICE family proteases, Z-Asp and Z-VAD, prevented apoptosis and PS externalization. These results indicate that PS is externalized after the ICE family protease activation during apoptosis of U937 cells. Notably, PS externalization was observed in the presence of low concentrations of ICE family protease inhibitors, by which morphologic apoptosis was effectively prevented and CPP32 processing to the p17 fragment was partially inhibited. Although the protease responsible for CPP32 processing is as yet unidentified, an ICE family protease is likely to process the p20 to the p17 fragment. These results suggest that PS externalization is more sensitive to ICE family protease activation than are apoptotic morphologic changes. Because the activation of ICE family protease is a crucial event for apoptosis in a variety of cell systems,24-38,43 PS externalization could be a rational and useful indicator of apoptosis. However, results should be carefully interpreted because PS is also externalized when platelets and erythrocytes are activated by calcium ionophore A23187.44,45 Furthermore, erythrocytes from pathologic patients with diabetes46 and chronic myeloid leukemia47 expose PS on their surfaces. Thus, PS externalization could also occur independently of apoptosis.
The mechanism of PS externalization after activation of ICE family protease is not yet clear. The asymmetric distribution of PS is attributed to a translocase specific to aminophospholipids,5-7 which translocates PS and, less effectively, PE from the outer to the inner leaflet of the plasma membranes. Because the charged lipids generally exhibit a slow passive transmembrane diffusion,3 inactivation of the aminophospholipid translocase cannot explain the rapid PS externalization during apoptosis. Recently, it was suggested that downregulation of the aminophospholipid translocase and activation of a nonspecific scramblase were both responsible for PS externalization in T lymphocytes undergoing apoptosis.48 It is possible that these enzymes are processed by an activated ICE family protease during apoptosis.
Recently, several ICE family proteases have been isolated that could be involved in apoptosis.24,29,31-36,38,49 The activation mechanism of ICE family proteases has not been fully elucidated, but it is suggested that a family member (ICE) activates another member (CPP32) in some cells.50 Thus, sequential proteolytic activation was assumed in the ICE family proteases during apoptosis. However, in U937 cells, ICE is not activated during etoposide- and Fas-induced apoptosis,26 indicating that another protease is responsible for CPP32 activation in this cell line. Interestingly, precursor CPP32 was processed to the p20 fragment but not to the p17 fragment in the presence of ICE family protease inhibitors in U937 cells (Fig 4B). This result suggests that CPP32 is processed by at least two steps in this cell line. First, precursor CPP32 is cleaved to p20 (and probably p12) by a protease unaffected by the presence of Z-Asp and Z-VAD; subsequently, the p20 is further processed to the p17 by an ICE family protease inhibitable by Z-Asp and Z-VAD. As of now, the proteases responsible for CPP32 processing in U937 cells have not been determined. Further studies are needed to clarify the mechanism of ICE family protease activation and subsequent PS externalization.
Peroxidase-positive lysosomes (arrow) in a peripheral blood monocyte. Original magnification × 16,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, Boston, MA 02215.)
Peroxidase-positive lysosomes (arrow) in a peripheral blood monocyte. Original magnification × 16,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, Boston, MA 02215.)
ACKNOWLEDGMENT
We are grateful to Drs A. Tomida and N. Fujita for helpful discussions. We also thank Drs H. Kawai and S. Kataoka for generous gifts of ICE family protease inhibitors.
Supported by a special grant for Advanced Research on Cancer and Grants-in-Aid for Cancer Research and Scientific Research from the Ministry of Education, Science and Culture, Japan.
Address reprint requests to Takashi Tsuruo, PhD, Institute of Molecular and Cellular Biosciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113, Japan.