Abstract
1,25-Dihydroxyvitamin D3 [1,25-(OH)2D3 ] induces the differentiation of monocytes into macrophage-like cells in vitro. To identify the genes expressed during this process, we performed differential display polymerase chain reaction on RNA extracted from cord blood monocytes (CBMs) treated with 1,25-(OH)2D3 . Treated CBMs expressed type-I 15-hydroxyprostaglandin dehydrogenase (type-I 15-PGDH), the key enzyme of prostaglandin E2 (PGE2 ) catabolism and a 15-PGDH–related mRNA (15-PGDHr). This newly described 15-PGDH–related mRNA was constitutively expressed in adult monocytes. 15-PGDH gene(s) transcription was accompanied by the appearance of the 15-PGDH activity in treated CBMs. In addition, the cyclooxygenase 2 mRNA level was decreased and PGE2 levels in the culture mediums were lowered (50%). Our results stress that 1,25-(OH)2D3 , at least in neonatal monocytes, can exert, directly or indirectly, a dual control on key enzymes of PGE2 metabolism. In conclusion, we suggest that modifications in prostaglandin metabolism, induced by the expression of type-I 15-PGDH and the downregulation of cyclooxygenase 2, could be involved in monocytic differentiation.
1,25-DIHYDROXYVITAMIN D3 acts on several cellular processes, including cellular growth and differentiation.1 The steroid hormone [1,25-(OH)2D3 ] acts primarily on cells in intestine,2 kidney,3 and bone.4 As with other steroid hormone systems, its mechanism of action involves a specific cytosolic receptor (VDR)5,6 that is able to elicit transcriptional modulation.7,8 This active metabolite of vitamin D is a potent stimulator of normal monocyte-macrophage differentiation9,10 and induces the appearance of macrophage-like cells in the rodent murine myeloid leukemia line (M-1)11 and in the human promyelocytic leukemia line HL-60.12,13 To study the expression of genes modulated by this steroid, we used human umbilical cord blood monocytes (CBMs) as a source of pro-monocytes.14 These nonspecific esterase-positive (>90%) cells contain large numbers of colony-forming units for granulocytes and macrophages (CFU-GM) and colony-forming units for granulocytes-erythrocytes-macrophages and megakaryocytes (CFU-GEMM) precursor cells.15,16 When cultured for 3 weeks in the presence of 1,25-(OH)2D3 (10−8 mol/L), these cells present morphologic features of macrophage and express specific markers of monocytes/macrophages such as Leu-M5 (63% ± 6%) and a small proportion of HLA-DR (30% ± 10%).17 A significant level of cells also express vitronectin (41% ± 2%) and calcitonin receptors,17 18 which are strongly expressed by osteoclasts.
We performed differential amplification19 of total RNA extracted from neonatal monocytes cultured in the presence or absence of 1,25-(OH)2D3 (10−8 mol/L) to detect and identify gene expression that could be modulated by this metabolite. We show here that 1,25-(OH)2D3 induces in CBMs the expression of type-I 15-hydroxyprostaglandin dehydrogenase (type-I 15-PGDH [EC.1.1.1.141]), a key enzyme of prostaglandin E2 (PGE2 ) catabolism.20 This enzyme inactivates this PG by catalyzing oxidation of the (S) C-15 hydroxyl group of this molecule.21 We extended our studies to the expression of the glucocorticoid-inducible cyclooxygenase 222 (COX 2), an enzyme responsible for the synthesis of PGs. We report that 1,25-(OH)2D3 decreases COX 2 mRNA levels. Furthermore, we showed that a 15-PGDH highly related mRNA (15-PGDHr) previously identified in HL-60 cells23 was also induced in treated CBMs. The presence of type-I 15-PGDH has also been established by polymerase chain reaction (PCR) and Western blot analysis in human peripheral monocytes.24
A previous study reported that indomethacin (an inhibitor of cyclooxygenases) potentiates the induction of the human promyelocytic cell HL-60 differentiation to monocytes by 1,25-(OH)2D3 .25 In addition, PGE2 decreases the transcription of c-Fms (53%) and tumor necrosis factor (TNF ) genes that are increased during the U-937 cell tetradecanoyl phorbol acetate (TPA)-induced monocytic differentiation.26 Consistent with these observations, we suggest that induction of type-I 15-PGDH activity, followed by a downregulation of COX 2, could play a role at an early stage of normal monocytes/macrophage 1,25-(OH)2D3 –induced differentiation.
MATERIALS AND METHODS
PCR Primers
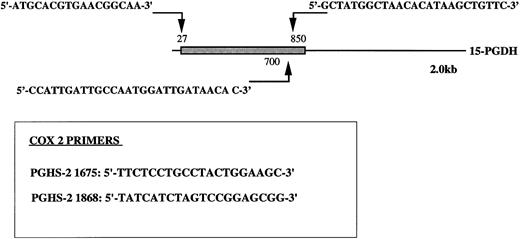
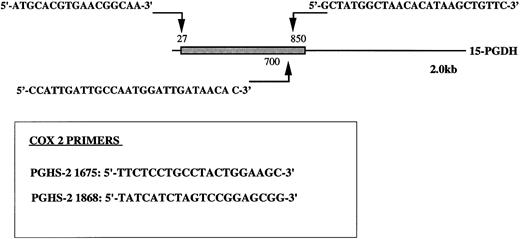
Oligonucleotide primers 5′-T11CA-3′ and Ltk3 (5′-CTTGATTGCC-3′) previously used for differential display19,27; type-I 15-PGDH–specific oligonucleotide primers 15-PGDH sense P27, sense P700, and antisense P850; COX 2-specific oligonucleotide primers sense PGHS-2 1675 and antisense PGHS-2 1868 (Fig 1); and β-actin primers Act sense 383 Act antisense 1404 were synthesized by Genosys (Cambridge, UK).
Position of type-1 15-PGDH primers. The 15-PGDH coding sequence of the 2.0 kb mRNA is represented as a box and the resting noncoding sequence is represented as a solid line. The positions of the primers are noted with arrows. Primers specific to PGHS-2 (COX2) nucleic sequence are boxed. Numbers noted in labeling correspond to the positions of the primers in original sequences.
Position of type-1 15-PGDH primers. The 15-PGDH coding sequence of the 2.0 kb mRNA is represented as a box and the resting noncoding sequence is represented as a solid line. The positions of the primers are noted with arrows. Primers specific to PGHS-2 (COX2) nucleic sequence are boxed. Numbers noted in labeling correspond to the positions of the primers in original sequences.
Cell Separation and Culture
Human fetal and adult monocytes.Informed consent was obtained from all subjects included in the study. Blood was drawn from the umbilical cord from normal parturient volunteers. Adult blood was obtained by venipuncture from normal human volunteers. Heparinized blood was diluted with Eagle's minimal essential medium (αMEM), layered over lymphocyte separation medium (Ficoll-Hypaque), and centrifuged at 300g for 20 minutes.
Mononuclear leukocyte suspension was collected from the Ficoll-αMEM interface and plated in tissue culture plates (Costar, Brumath, France). After an overnight incubation, the cell cultures were washed carefully to remove the nonadherent cells. Viability, as assessed by trypan blue exclusion, was greater than 98%. The adherent cells were cultured with fresh medium supplemented with 20% heat-inactivated horse serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L glutamine, and 4 mmol/L HEPES buffer, with and without 10−8 mol/L 1,25-(OH)2D3 . The cultures were maintained in a humidified atmosphere of 5% CO2 -95% air at 37°C for 1, 2, and 3 weeks. Culture medium was changed every 4 days.
HL-60 cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) and 2 mmol/L glutamine. These cells were stimulated by 10−8 mol/L 1,25-(OH)2D3 for 48 hours, 1 week, and 2 weeks.
Differential Display
Total RNA were extracted by guanidinium thiocyanate28 and differential display was performed on 0.2 μg aliquots of total RNA extracted from neonatal monocytes after 3 weeks of culture. Reverse transcription (RT) was performed in a 20 μL reaction volume with 300 U of Moloney murine leukemia virus (Mo-MLV) reverse transcriptase (GIBCO-BRL, Cergis, France) in the presence of 2.5 μmol/L of T11CA as primer and 200 μmol/L dNTP (Pharmacia-Biotech, Cergis, France) for 60 minutes at 37°C. After heat inactivation of the reverse transcriptase at 95°C for 7 minutes, 2 μL of the sample was added to 18 μL of PCR labeling mix containing 2.5 U of Taq DNA polymerase, 0.5 μmol/L of sense primer Ltk3, 2.5 μmol/L of T11CA, and 1 μL of α-(35S)dATP (1,200 Ci/mmol; Dupont, NEN, les Ulis, France). Amplification was performed during 40 cycles as follows: 94°C for 30 seconds, 42°C for 2 minutes, 72°C for 30 seconds, and 72°C for 5 minutes at the end of the 40 cycles. The amplified cDNAs were then separated on a 6% DNA sequencing gel, transferred to a Whatmann 3-mm paper (Maldstone, France), and exposed with a Hyperfilm βmax film (Amersham, les Ulis, France).
Recovery and reamplification of cDNA probes.cDNA bands of interest were located by cutting through the autoradiogram film. The gel slice along with the 3-mm paper were incubated in 100 μL ddH2O for 10 minutes and then the cDNA was diffused out by boiling the gel slice for 15 minutes. cDNA was recovered by ethanol precipitation in the presence of 0.3 mol/L sodium acetate and 1 μL of glycogen (10 mg/mL; Boehringer Mannheim, Meylan, France) and resuspended in 10 μL of ddH2O. Five microliters of the eluted cDNA was reamplified in a 40 μL reaction volume using the same primer set and PCR conditions as used in the mRNA display without isotope. Twenty microliters of PCR samples was run on a 1% agarose gel stained with ethidium bromide. DNA was purified using QIAEX kit (QIAGEN) and labeled with α-(32P)dCTP using a random-priming DNA labeling kit (Promega, Charbonnières, France). The labeled cDNA were used to screen the cloned cDNA library. The remaining samples were stored at −20°C for subcloning.
Cloning and DNA sequencing.Reamplified cDNA were cloned into the PCR-II vector using the TA-cloning system from Invitrogen (San Diego, CA). Clones were screened with labeled cDNA probe, and both strands of interesting cDNA fragments were sequenced with the double-strand cDNA cycle sequencing system kit (GIBCO-BRL) using M13 reverse and M13-40 forward primers labeled with γ-(33P)ATP and Taq DNA polymerase to generate a single-stranded template for DNA sequence analysis by the dideoxynucleotide chain termination method.29 Amplification was performed for 20 cycles (95°C for 30 seconds, 55°C for 30 seconds, and 70°C for 60 seconds) followed by 10 cycles (95°C for 30 seconds and 70°C for 60 seconds).
15-PGDH–Specific RT-PCR and Analysis of PCR Products
RT-PCR cDNA was synthesized from 1 μg of total RNA in a 20 μL volume reaction with 200 U of superscript reverse transcriptase (GIBCO-BRL) in the presence of 1 mmol/L of each dNTP and 50 pmol of a 3′-oligo-dT primer for 60 minutes at 42°C. The reaction was then diluted to 95 μL with the same buffer containing 50 pmol of specific primers (Fig 1) 15-PGDH P27 with 15-PGDH P850 (to amplify the complete open reading frames [ORFs]) and 2.5 U of Taq DNA polymerase. After 5 cycles of only 15-PGDH amplification, β-actin primers (Acts 383 and Acts 1404) were added to each reaction tube and amplification was continued for another 30 cycles. The parameters of PCR were 94°C for 40 seconds, 55°C for 30 seconds, and 72°C for 2 minutes (5 cycles) and then 94°C for 40 seconds, 55°C for 30 seconds, and 72°C for 2 minutes (30 cycles).
Time course 15-PGDH amplification was performed with primers 15-PGDH 27 and 15-PGDH 850 (to amplify the complete ORFs) during 30 cycles as follows: 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute and 72°C for 5 minutes at the end of the 30 cycles. We also used primers 15-PGDH 700 combined with 15-PGDH 850 (Fig 1) to amplify the two 15-PGDH mRNA isomers without distinction.
The amplification parameters were established by plotting the amount of amplification products obtained in respect to the amplification cycle numbers. We used 10 separate RT reactions using 1 μg of total RNA in the previous condition. We pooled these reaction mixtures and used 10 aliquots of 20 μL for amplification in the reaction mixture previously described. We then stopped the amplification in a tube every 2 or 5 cycles by freezing.
Amplified products were analyzed by electrophoresis in 1.5% agarose gel, visualized by ethidium bromide (BET), and transferred to nylon membrane (Genescreen; NEN). The amplified cDNA were alternatively hybridized with a 15-PGDH– and a β-actin–specific probe labeled with α-(32P)dCTP using a random priming method (specific activities of 108/109 cpm/μg DNA). The membranes hybridized at 42°C in the presence of 50% formamide were then washed in 2× SSC for 10 minutes at room temperature at 55°C and then washed twice with 2× SSC containing 1% sodium dodecyl sulfate (SDS) for 20 minutes and twice in 0.2× SSC containing 1% SDS for 20 minutes. Autoradiography was performed at −80°C with intensifying screen.
COX 2-Specific RT-PCR
First-strand cDNA was synthesized from 1 μg of total RNA in a 20 μL volume reaction with 200 U of Mo-MLV RT (GIBCO-BRL) in the presence of 1 mmol/L of each dNTP and 50 pmol of a 5′ oligo-dT primer for 60 minutes at 37°C. The reaction was then diluted to 100 μL with the same buffer containing 50 pmol of each specific set of COX 2 primers (Fig 1) and 2.5 U of Taq DNA polymerase. Amplification was performed as described above. Linear phases of these amplifications were determined as described before.
Amplified products were analyzed by electrophoresis in a 2% agarose gel visualized by ethidium bromide. The amplified cDNA was then cloned into TA-cloning and the amplicon was sequenced (data not shown) with the double-strand cDNA cycle sequencing system kit using M13 reverse and M13-40 forward primers labeled with γ-(33P)dATP and Taq DNA polymerase to generate single-stranded template for DNA sequence analysis by the dideoxynucleotide chain termination method.29
Cloning and Expression of the 15-PGDH–Related mRNA
To test the stability and the presumed activity of the 15-PGDH–related protein (15-PGDHr), we cloned the coding sequence (from ATG to TGA) in a frame with a nucleic sequence coding for a 13-kD peptide biotinylated in Escherichia coli (Pin point expression system; Promega). Briefly, expression was induced by isopropyl-b-thiogalactopyranoside (IPTG) (100 μmol/L). The recombinant protein was purified on an avidin affinity column in Tris-HCl buffer, pH 7.4, 1 mmol/L dithiothreitol (DTT). Digestion with factor Xa was performed to remove the biotinylated peptide to obtain the 178-kD expected recombinant protein (from Met1). We then tested the ability of this new protein to convert PGE2 into 15-Keto PGE2 using thin-layer chromatography experiments as described below for CBM 15-PGDH assays.
15-PGDH Activity
The ability to convert PGE2 into 15-Keto-PGE2 by the 1,25-(OH)2D3 –treated neonatal monocytes and the 15-PGDHr recombinant protein was determined by using thin-layer chromatography.30 (5,6,8,11,12,14,15(n)-3H)-PGE2 (specific activity, 171 Ci/mmol) was purchased from DuPont de Nemours, (NEN). After 1 and 2 weeks of culture, neonatal monocytes were scraped in 50 mmol/L Tris-HCl, pH 7.4, 1 mmol/L DTT and then sonicated for 20 seconds at 4°C. Homogenates were centrifuged for 5 minutes at 1,500g. We used the soluble phases to test the presence of type-I 15-PGDH activity. Reactions were performed in the presence of 1 μmol of NAD(+) (Sigma, St Quentin, France), 10 μg of PGE2 (Sigma), and 0.2 μCi of tritiated PGE2 . Reaction mixtures were completed to 1 mL with a 50 mmol/L Tris-HCl, pH 7.4, buffer and placed for 30 minutes at 37°C. After the removal of proteins by methanol precipitation (75% vol:vol), water was added to dilute the methanol to 10%. Soluble phase extractions were performed using octadecyl 18-C silica cartbridges (J.T. Baker, Deventer, Holland). The dried extracts were run on 20 × 20 60A silica plate (Prolabo, Fontenay, France) using the organic phase of ethyl acetate/acetic acid/iso-octane/water (11:2:5:10).31 Authentic PGE2 , 15-Keto-PGE2 , and 13,14 dihydro-15-Keto PGE2 were comigrated on separate lanes. After localization of the compounds using phosphomolybdic spray, we scraped the silica and determined the respective amounts of PGE2 and 15-Keto PGE2 by radioactivity counting.
We used the same experimental approach to test the ability of the recombinant 15-PGDHr to convert PGE2 into 15-Keto PGE2 . We used 1, 5, or 10 μg of PGE2 containing 0.1 μCi of (5,6,8,11,12,14,15(n)-3H)-PGE2 (specific activity, 171 Ci/mmol). Because this new protein presents a type-I 15-PGDH N-terminal domain, presumed to be implicated in NAD(+) binding, we used NAD(+) as a cofactor. Incubations were performed with 25, 50, 100, or 200 ng of the purified recombinant protein (>90%) for 20 or 40 minutes at 37°C.
PGE2 Assays
PGE2 levels in culture medium from three separate blood cultures were estimated using an enzyme-linked immunosorbent assay (ELISA) kit (Boehringer Mannheim) with a specific antibody for PGE2 . Duplicate aliquots from unstimulated and 1,25-(OH)2D3 –stimulated CBMs after 1, 2, and 3 weeks of culture were assayed according to the manufacturer's protocol (Boehringer Mannheim).
RESULTS
Monocytes and Differential Display
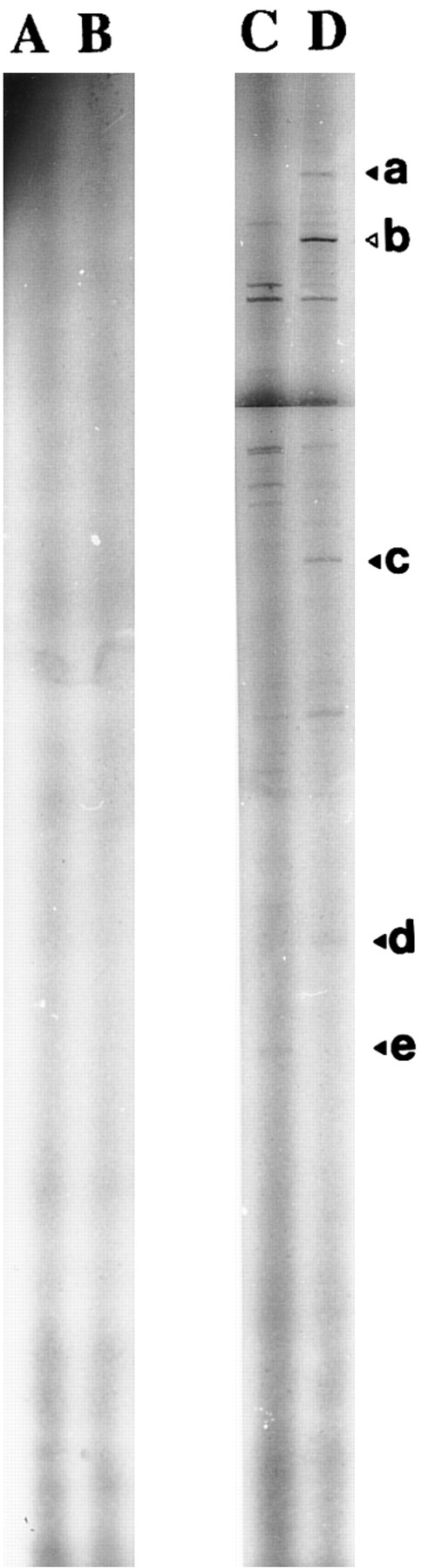
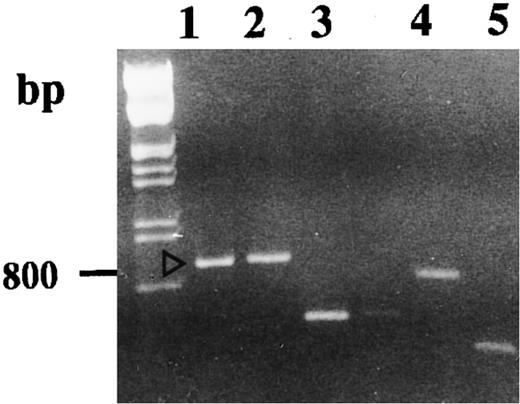
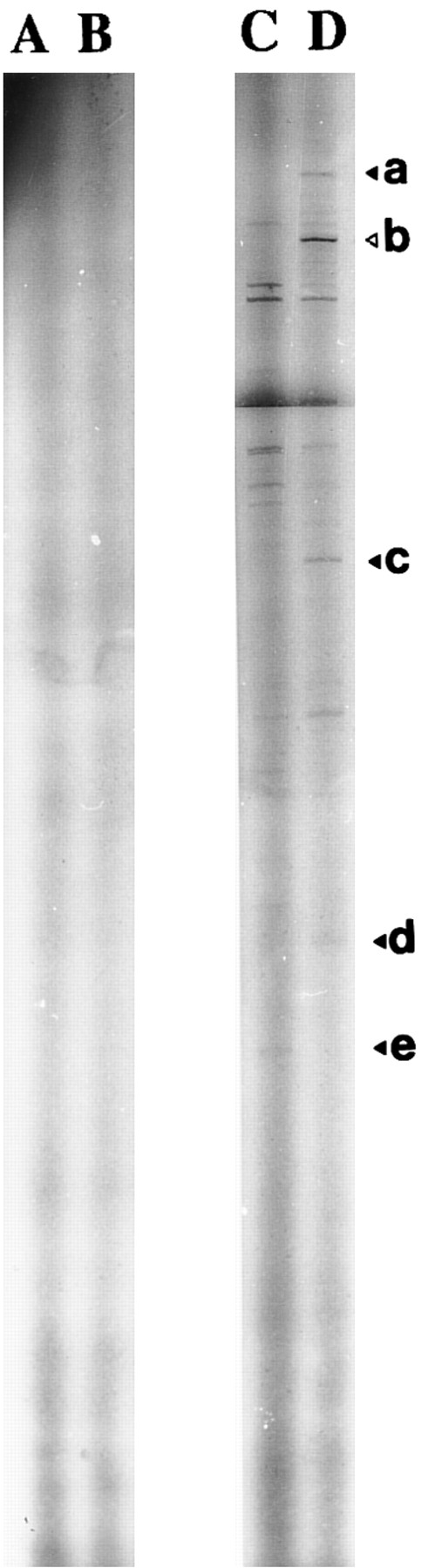
Autoradiography of amplified (35S)dATP-labeled products showed differences in the electrophoretic profile from unstimulated and 1,25-(OH)2D3 –stimulated cells with one set of primers (Fig 2). Differences considered as significant (bands a, b, c, d, and e) were excised and reamplified (Fig 3) with the same primers and cloned (TA-cloning; Invitrogen). Comparison of the sequences of the amplified double-stranded DNAs with sequences recorded in genomic and protein banks (Genebank, EMBL, and Swiss prot data bank) showed that four of these were unknown sequences (probably PCR artifacts, because we did not detect the corresponding mRNA on Northern blot experiments) and that one coded for the type-I 15-PGDH enzyme.32
Identification of 1,25(OH)2D3 -modulated genes transcription by DD-RT-PCR. DD-RT-PCR was performed on total RNA from unstimulated (lane C) and 1,25-(OH)2D3 –stimulated neonatal monocytes (lane D) as described in the Materials and Methods using primers T11CA and Ltk3 . Arrows indicate interesting labeled DNA products. Amplified double-stranded DNA corresponding to the amplification of 15-PGDH is noted as “b.” Total RNA samples pretreated by RNase A were used in the same experimental conditions to confirm the absence of genomic DNA contamination (lanes A, untreated cells; lane B, 1,25-(OH)2D3 –treated cells).
Identification of 1,25(OH)2D3 -modulated genes transcription by DD-RT-PCR. DD-RT-PCR was performed on total RNA from unstimulated (lane C) and 1,25-(OH)2D3 –stimulated neonatal monocytes (lane D) as described in the Materials and Methods using primers T11CA and Ltk3 . Arrows indicate interesting labeled DNA products. Amplified double-stranded DNA corresponding to the amplification of 15-PGDH is noted as “b.” Total RNA samples pretreated by RNase A were used in the same experimental conditions to confirm the absence of genomic DNA contamination (lanes A, untreated cells; lane B, 1,25-(OH)2D3 –treated cells).
Agarose gel electrophoresis of reamplified excised DNA. Reamplifications of bands we retained to study were performed as described in the Materials and Methods. Lanes 1, 2, 3, 4, and 5 correspond, respectively, to the DD-PCR–excised bands b, a, d, c, and e.
Agarose gel electrophoresis of reamplified excised DNA. Reamplifications of bands we retained to study were performed as described in the Materials and Methods. Lanes 1, 2, 3, 4, and 5 correspond, respectively, to the DD-PCR–excised bands b, a, d, c, and e.
RT-PCR Confirmation of 15-PGDH Induction in Neonatal Monocytes
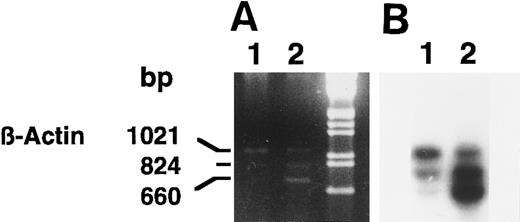
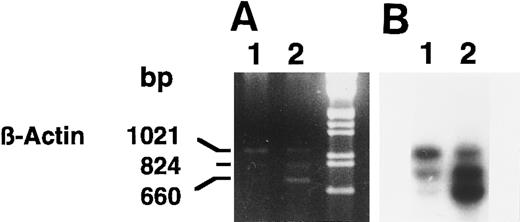
We confirmed the induction of 15-PGDH mRNA(s) in neonatal 1,25-(OH)2D3 –stimulated monocytes by using semiquantitative RT-PCR (Fig 4A and B) and several sets of 15-PGDH–specific primers. Semiquantitated experiments performed with 15-PGDH P27 and P850 primers showed the presence of 15-PGDH mRNA(s) in 1,25-(OH)2D3 –treated CBMs. The 824-bp amplicon corresponded to the size expected for type-I 15-PGDH and the 660-bp amplicon corresponded to the size expected for the 15-PGDH–related mRNA recently described in HL-6023 (Fig 4A and B, lanes 2). These products were detected as faint traces in the lane corresponding to the untreated cells, after hybridization with the 15-PGDH radiolabeled probe (Fig 4B, lane 1). The 1,021-bp amplicon corresponded to the β-actin mRNA amplification. The β-actin internal coamplified control (1,021 bp) showed the specific increase in 15-PGDH mRNA levels in the treated monocytes (Fig 4A and B, lanes 1 and 2).
Agarose gel electrophoresis (A) and autoradiography (B) of Southern blot of 15-PGDH and β-actin products from total RNA extracted from neonatal monocytes as described in the Materials and Methods. RT-PCR using 15-PGDH primers P27 and P850 and β-actin primers Acts 383 and Acts 1404, in the same tube, was performed on total RNA from unstimulated and 1,25-(OH)2D3 –stimulated cells after 1 week (lanes 1 and 2).
Agarose gel electrophoresis (A) and autoradiography (B) of Southern blot of 15-PGDH and β-actin products from total RNA extracted from neonatal monocytes as described in the Materials and Methods. RT-PCR using 15-PGDH primers P27 and P850 and β-actin primers Acts 383 and Acts 1404, in the same tube, was performed on total RNA from unstimulated and 1,25-(OH)2D3 –stimulated cells after 1 week (lanes 1 and 2).
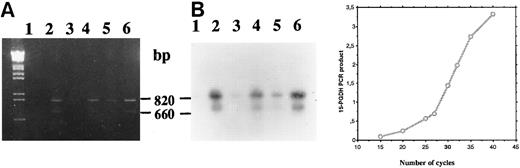
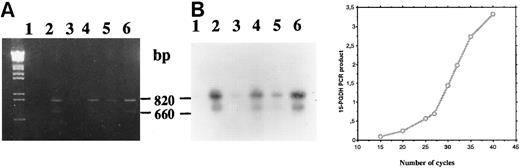
Time course analysis of the amplification products (primers P27/P850) confirmed the presence of amplified cDNA corresponding to two 15-PGDH mRNAs at 1 and 2 weeks of culture in 1,25-(OH)2D3 –stimulated CBMs (Fig 5A and B, lanes 2 and 4), whereas no products were visualized from unstimulated CBMs at these times of culture (lanes 1 and 3). In addition, we sequenced this double-stranded DNA (data not shown) and confirmed the sequence we have reported from HL-60 cells excepted for the E97K punctual mutation. A faint band on the exposed film was observed after amplification of total RNA extracted from unstimulated CBMs cultured for 3 weeks (Fig 5A and B, lane 5).
Agarose gel electrophoresis (A) and autoradiography (B) of Southern blot of 15-PGDH RT-PCR products from total RNA extracted from neonatal monocytes as described in the Materials and Methods. RT-PCR using primers 15-PGDH primers P27 and P850 was performed on total RNA from unstimulated and 1,25-(OH)2D3 –stimulated cells after 1 week (lanes 1 and 2), 2 weeks (lanes 3 and 4), and 3 weeks of culture (lanes 5 and 6).
Agarose gel electrophoresis (A) and autoradiography (B) of Southern blot of 15-PGDH RT-PCR products from total RNA extracted from neonatal monocytes as described in the Materials and Methods. RT-PCR using primers 15-PGDH primers P27 and P850 was performed on total RNA from unstimulated and 1,25-(OH)2D3 –stimulated cells after 1 week (lanes 1 and 2), 2 weeks (lanes 3 and 4), and 3 weeks of culture (lanes 5 and 6).
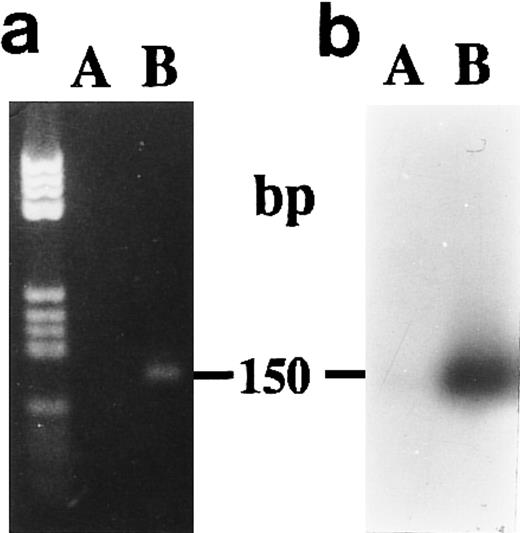
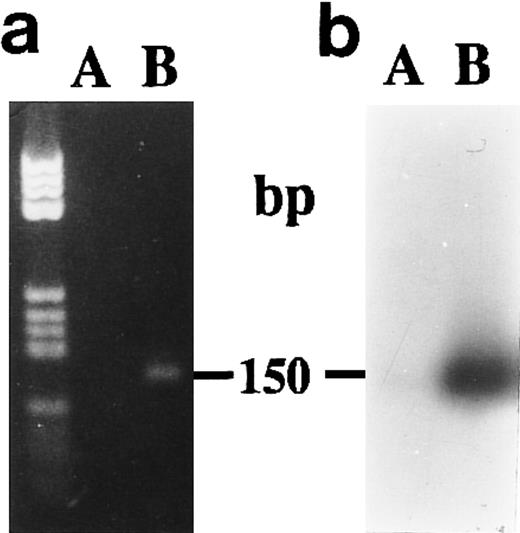
We did not obtain amplification products from unstimulated CBMs after 1 week of culture when we used P700 and P850 primers (Fig 6A and B) to amplify without distinction the two 15-PGDH mRNA isoforms.
Agarose gel electrophoresis (a) and autoradiography (b) of Southern blot of RT-PCR products obtained from total RNA extracted from neonatal monocytes. The amplification of both 15-PGDH and iso-15-PGDH RNA(s) using 15-PGDH primers P700 and P850 from 1 μg of total RNA was performed after 1 week of culture on RNA from unstimulated (lane A) and 1,25-(OH)2D3 –stimulated cells (lane B).
Agarose gel electrophoresis (a) and autoradiography (b) of Southern blot of RT-PCR products obtained from total RNA extracted from neonatal monocytes. The amplification of both 15-PGDH and iso-15-PGDH RNA(s) using 15-PGDH primers P700 and P850 from 1 μg of total RNA was performed after 1 week of culture on RNA from unstimulated (lane A) and 1,25-(OH)2D3 –stimulated cells (lane B).
RT-PCR of 15-PGDH mRNAs From Adult Peripheral Monocytes
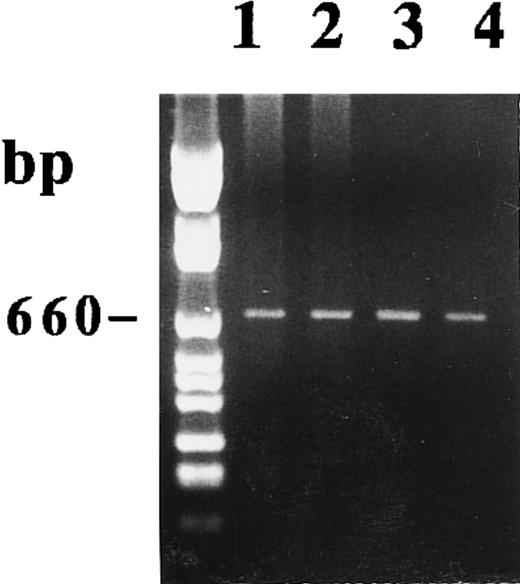
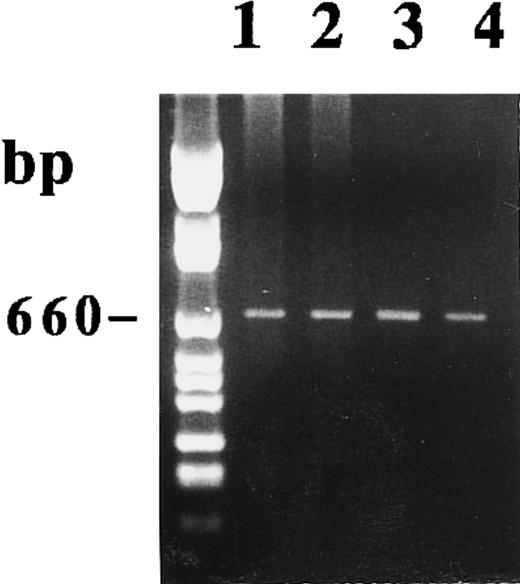
As shown in Fig 7, we amplified, using the P27 and P850, 15-PGDH mRNA from 1 μg of total RNA extracted from unstimulated and 1,25-(OH)2D3 –stimulated adult monocytes after 1 week of culture. We detected the 660-bp band corresponding to the 15-PGDH–related mRNA by staining with BET. In contrast to treated CBMs, this amplicon was the only amplification product we detected in the two adult peripheral monocyte samples treated or not treated by 1,25-(OH)2D3 . This result is consistent with and confirms a previous study that showed the presence of 15-PGDH mRNA in peripheral blood monocytes by using an RT-PCR experiment.24
Agarose gel electrophoresis of 15-PGDH RT-PCR products from total RNA extracted from adult monocytes as described in the Materials and Methods. RT-PCR using primers 15-PGDH 27 and 15-PGDH 850 were performed on RNA from two samples of unstimulated and 1,25-(OH)2D3 –stimulated monocytes after 1 week of culture. Lanes 1 and 2 correspond, respectively, to untreated and treated monocytes (sample 1). Lanes 3 and 4 correspond, respectively, to untreated and treated monocytes (sample 2).
Agarose gel electrophoresis of 15-PGDH RT-PCR products from total RNA extracted from adult monocytes as described in the Materials and Methods. RT-PCR using primers 15-PGDH 27 and 15-PGDH 850 were performed on RNA from two samples of unstimulated and 1,25-(OH)2D3 –stimulated monocytes after 1 week of culture. Lanes 1 and 2 correspond, respectively, to untreated and treated monocytes (sample 1). Lanes 3 and 4 correspond, respectively, to untreated and treated monocytes (sample 2).
15-PGDH Activity Assays
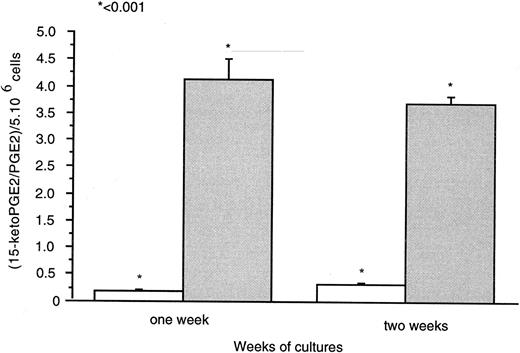
Thin layer chromatography (T.l.c.) experiments (Fig 8) showed 15-PGDH activity by 1,25-(OH)2D3 –treated neonatal monocytes. Radioactivity counting showed the presence of (3H)-15-Keto PGE2 generated with the cell lysates treated with 1,25-(OH)2D3 for 1 and 2 weeks. We did not detect this metabolite in untreated cell lysates as a source of 15-PGDH activity after the same time of culture. PGE2 and 15-keto-PGE2 were separated effectively with retardation factor (RF ) values as follows: PGE2 , 0.28; and 15-keto-PGE2 , 0.53. Results were expressed as the ratios [(15-Keto PGE2 /PGE2 ) × 100] for 5 × 104 cells (Fig 8). 15-PGDH activity was increased 17-fold and 12-fold, respectively, after 1 and 2 weeks of 1,25-(OH)2D3 treatment compared with untreated cells. Ratios for untreated cells were very close to the control ratio (0.024; i.e., in the absence of cell lysates).
Means of neo-synthesized 15-Keto PGE2 on PGE2 are represented for 5 × 106 cells after 1 and 2 weeks of culture in the ( ) presence or (□) absence of 1,25-(OH)2D3 (10−8 mol/L). Experiments were performed on three different neonatal blood samples.
Means of neo-synthesized 15-Keto PGE2 on PGE2 are represented for 5 × 106 cells after 1 and 2 weeks of culture in the ( ) presence or (□) absence of 1,25-(OH)2D3 (10−8 mol/L). Experiments were performed on three different neonatal blood samples.
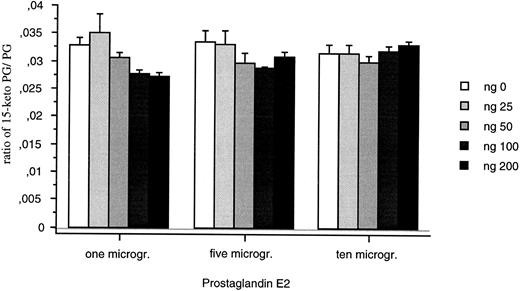
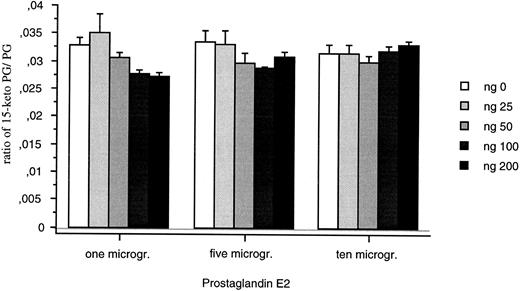
The recombinant protein was detected as both a monomer (21 kD) and a dimer (40 kD) on gel electrophoresis (data not shown) after purification from E coli. The recombinant protein (25 to 200 ng) did not convert PGE2 into 15-Keto PGE2 (Fig 9). Changing incubation times (20 and 40 minutes) or the sensitivity of the assay did not modify this result.
15-PGDH activity assays for the recombinant 15-PGDH C-terminal truncated isoform. Means of neo-synthesized 15-Keto PGE2 on PGE2 are represented for 25, 50, 100, and 200 ng of recombinant protein. All the measures were duplicated and repeated three times.
15-PGDH activity assays for the recombinant 15-PGDH C-terminal truncated isoform. Means of neo-synthesized 15-Keto PGE2 on PGE2 are represented for 25, 50, 100, and 200 ng of recombinant protein. All the measures were duplicated and repeated three times.
COX 2-Specific RT-PCR on Neonatal Monocytes
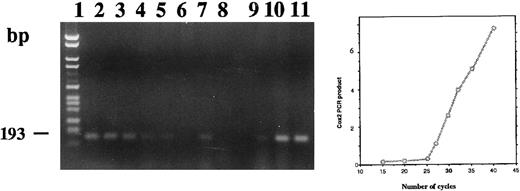
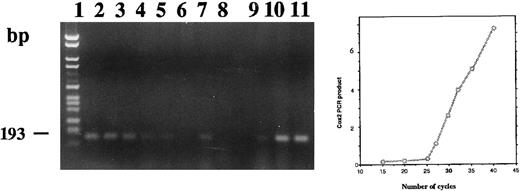
Amplification of total RNA extracted from stimulated or unstimulated CBMs showed the presence of COX 2 mRNA (Fig 10). The intensity of staining of the amplified product was not different in the stimulated or unstimulated cells after 24 hours. At 1 week and thereafter, the amount of amplified product decreased in both groups; however, the decrease was more marked in the treated group. Compared with untreated neonatal monocytes, untreated HL-60 cells expressed a low level of COX 2 mRNA at 24 hours of culture (lane 9). The signal obtained for both the adherent and the floating 1,25-(OH)2D3 –treated HL-60 cells (lanes 10 and 11) were approximately equivalent to those we obtained in the case of the CBMs after 24 hours of culture with or without 1,25-(OH)2D3 .
Agarose gel electrophoresis of COX 2 RT-PCR products from total RNA extracted from neonatal monocytes as described in the Materials and Methods. RT-PCR using primers PGHS-2 1675 and PGHS-2 1868 was performed on RNA from unstimulated and 1,25-(OH)2D3 stimulated cells after 24 hours (lanes 1 and 2), 1 week (lanes 3 and 4), 2 weeks (lanes 5 and 6), and 3 weeks of culture (lanes 7 and 8) and on total RNA extracted from HL-60 unstimulated (lane 9) and 1,25-(OH)2D3 –stimulated adherent (lane 10) and floating cells (lane 11).
Agarose gel electrophoresis of COX 2 RT-PCR products from total RNA extracted from neonatal monocytes as described in the Materials and Methods. RT-PCR using primers PGHS-2 1675 and PGHS-2 1868 was performed on RNA from unstimulated and 1,25-(OH)2D3 stimulated cells after 24 hours (lanes 1 and 2), 1 week (lanes 3 and 4), 2 weeks (lanes 5 and 6), and 3 weeks of culture (lanes 7 and 8) and on total RNA extracted from HL-60 unstimulated (lane 9) and 1,25-(OH)2D3 –stimulated adherent (lane 10) and floating cells (lane 11).
PGE2 Assay in Culture Medium
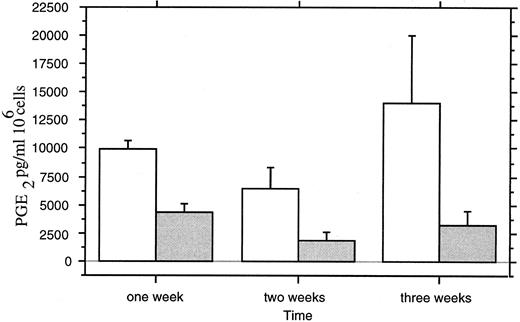
Levels of PGE2 in the culture medium were decreased by 1,25-(OH)2D3 treatment (Fig 11). A maximal decrease (50%) was observed after 3 weeks of culture (P < .0042; ANOVA).
PGE2 assays in culture medium of (□) untreated and ( ) 1,25-(OH)2D3 –treated neonatal monocytes. Measures were performed at 1, 2, and 3 weeks of culture on three separated monocyte cultures with duplicated measures for each point.
PGE2 assays in culture medium of (□) untreated and ( ) 1,25-(OH)2D3 –treated neonatal monocytes. Measures were performed at 1, 2, and 3 weeks of culture on three separated monocyte cultures with duplicated measures for each point.
DISCUSSION
Our results show for the first time that one of the early events during differentiation of immature monocytes by 1,25-(OH)2D3 is an increase in the transcription of the 15-PGDH gene. This transcriptional induction was accompanied by the induction of type-I 15-PGDH expression and activity detected in the 1,25-(OH)2D3 –treated cells. Transcriptional induction was confirmed by PCR amplification of the total RNA extracted from the stimulated CBMs and by sequencing the amplified products. Two isoforms of 15-PGDH mRNA(s) are expressed by CBMs after 1,25-(OH)2D3 stimulation. The first is identical in sequence to that reported by Ensor et al.32 The second corresponds to the form we detected in HL-60, human liver, placenta, and TT cells.23 This 15-PGDH–related mRNA (15-PGDHr) codes for a C-terminal truncated form of type-I 15-PGDH. It was weakly expressed as compared with type-I 15-PGDH mRNA in neonatal monocytes. In HL-60 cells it appears to be the dominant form, although expression of both mRNAs was very low compared with their expression in 1,25-(OH)2D3 –stimulated neonatal monocytes (data not shown). The 15-PGDHr mRNA appeared to be the predominant form expressed in the two samples of adult monocytes we tested. This last observation is consistent with the 15-PGDH–specific RT-PCR study performed on adult peripheral monocytes by Maddox et al.24
We have cloned and expressed this new protein in E coli and tested its ability to convert PGE2 into 15-Keto PGE2 . This recombinant enzyme was not able to catalyze this 15-PGDH reaction in vitro. This isomer could be an inactive form of 15-PGDH or active on another substrate. Serhan et al33 speculated on the existence of a 15-PGDH–like enzyme involved in the oxydation of the 15-C hydroxyl group of LXA4 in peripheral monocytes and in differentiated macrophages. Oxydation of LXA4 C-15 OH by type-I 15-PGDH has been shown using recombinant enzyme.34 This predicted C-terminal truncated form of 15-PGDH is identical to the type-I 15-PGDH for the first 166 N-terminal amino acids. It thus could present the NAD(+) binding domain35 and the doublet Y — K at positions 151-155, which is presumed to be implicated at the active site of the type-I 15-PGDH.36 The inability of 15-PGDHr to oxidize PGE2 suggests that the C-terminal part of the type-I 15-PGDH is important for catalytic activity. This region of the type-I 15-PGDH could be involved in protein folding. Further studies will be necessary to determine if this predicted 15-PGDH isomer can oxidize LXA4 or other C-15 hydroxyl metabolites.
The low levels of both 15-PGDH mRNAs in the unstimulated CBMs could be due to the presence of trace amounts of 1,25-(OH)2D3 in the medium culture. This result is consistent with the observation that monocyte to macrophage-like differentiation occurs spontaneously in vitro.37,38 The induction of 15-PGDH transcription by 1,25-(OH)2D3 in CBMs could be direct or indirect and mediated via a second molecule. In favor of this last hypothesis is the observation that 1,25-(OH)2D3 acts as an enhancer of monocytic differentiation in vitro.37 In addition, we did not detect 15-PGDH(s) transcripts until after 1 week of 1,25-(OH)2D3 treatment.
Although type-I 15-PGDH is an ubiquitous enzyme that is widely distributed in many cell types,39 our results suggest that induction of this enzyme in the immature monocyte could be a key event in the process of differentiation of these cells. In favor of this hypothesis is the fact that biosynthesis of eicosanoids is characteristic of the mature activated monocyte.40 Transcription of the c-Fms gene coding for the CSF-1 receptor is increased when U-937 monocytic differentiation is triggered by TPA. Addition of PGE2 in the culture medium decreased by 53% the transcription of this gene.26 Indomethacin, an inhibitor of cyclooxygenases, potentates the 1,25-(OH)2D3 –induced monocytic differentiation of HL-60 cells.25 In addition, we show here that type-I 15-PGDH transcription was induced in CBMs during the 1,25-(OH)2D3 –induced differentiation of these cells. This mRNA was not inducible in more mature monocytes (adult peripheral monocytes). The apparition of 15-PGDH(s) transcripts in treated CBMs was accompanied by synthesis of fully active type-I 15-PGDH in these cells. This finding suggests a transitional role of this enzyme during the early monocytic differentiation in our culture model.
Most published works on the synthesis and release of eicanosoids during 1,25-(OH)2D3 –induced monocytic differentiation concern tumor cell lines such as U937, HL-60, and THP-1 cells. Studies on U937 and THP-1 showed that 1,25-(OH)2D3 induces a marginal increase in eicasonoid synthesis,41 and Honda et al42 reported that PGE2 metabolism was increased during HL-60 1,25-(OH)2D3 –induced monocytic differentiation. Consistent with this report, we detected an increase of COX 2 mRNA in HL-60 cells after 24 hours of 1,25-(OH)2D3 treatment. In contrast to HL-60 cells, the transcription of COX 2 mRNA in neonatal monocytes was decreased, especially after 2 and 3 weeks of 1,25-(OH)2D3 treatment. The decrease of PGE2 in the medium of these cells provides indirect evidence that the levels of one or both enzymes are modified. This decrease was shown at 1 week of culture in the treated cell medium and thus precedes the apparition of markers such as calcitonin receptors.18
Most studies have focused attention on the regulation of cyclooxygenases and thromboxane synthetase during monocytic differentiation. The regulation of type-I 15-PGDH, the key enzyme of PGE2 catabolism, has not been extensively explored, except in two instances.31,43 Both of these studies suggest a role of this enzyme in monocytic differentiation, because Xun et al31 reported a stimulation of type-I 15-PGDH synthesis in HL-60 cells treated by phorbol ester, an agent known to induce monocytic-macrophage differentiation of these tumor cells. Our results provide evidence for an implication of 15-PGDH activity in the differentiation of normal monocytes and stress the importance of this enzyme in the control of prostaglandin metabolism during this process.
In conclusion, we suggest that modulation by 1,25-(OH)2D3 of the genes encoding for the key enzymes involved in prostaglandin biosynthesis, in particular the induction of cytosolic type-I 15-PGDH enzyme, may play a major role in the differentiation of monocytes toward macrophage lineages.
F.P., S.R., and J.L.F. are the recipients of individual grants from the French Ministry of Research and Education. R.D.M. is a recipient of an individual grant from the Ligue Nationale contre le Cancer.
Address reprint requests to A. Jullienne, PhD, U. 349 INSERM, Hôpital Lariboisière, centre Viggo Petersen, 6 rue Guy Patin, 75475 Paris cedex 10, France.