Abstract
Graft-versus-leukemia (GVL) and Graft-versus-host (GVH) reactions were compared after systemic transfer of allogeneic antitumor immune T lymphocytes from B10.D2 (H-2d; MIsb) into DBA/2 (H-2d; MIsa) mice. Before immune cell transfer, recipient DBA/2 mice were sublethally irradiated with 5 Gy to prevent host-versus-graft reactivity. Recipients were either bearing syngeneic metastatic ESb lymphomas (GVL system) or were normal, non–tumor-bearing mice (GVH system). We previously reported that this adoptive immunotherapy protocol (ADI) had pronounced GVL activity and led to immune rejection of even advanced metastasized cancer. In this study, monoclonal antibodies were used for immunohistochemical analysis of native frozen tissue sections from either spleen or liver to distinguish donor from host cells, to differentiate between CD4 and CD8 T lymphocytes, and to stain sialoadhesin-positive macrophages at different time points after cell transfer. The kinetics of donor cell infiltration in spleen and liver differed in that the lymphoid organ was infiltrated earlier (days 1 to 5 after transfer) than the nonlymphoid organ (days 5 to 20). After reaching a peak, donor cell infiltration decreased gradually and was not detectable in the spleen after day 20 and in the liver after day 30. The organ-infiltrating donor immune cells were mostly T lymphocytes and stained positive for CD4 or CD8 T-cell markers. A remarkable GVL-associated observation was made with regard to a subset of macrophages bearing the adhesion molecule sialoadhesin (SER+ macrophages). In the livers of tumor-bearing mice, their numbers increased between days 1 and 12 after ADI by a factor greater than 30. Double-staining for donor cell marker and SER showed that the sialoadhesin-expressing macrophages were of host origin. The SER+ host macrophages from GVL livers were isolated by enzyme perfusion and rosetting 12 days after ADI, when they reached peak values of about 60 cells per liver lobule, and were tested, without further antigen addition, for their capacity to stimulate an antitumor CD8 T-cell response. The results of this immunologic analysis suggest that these cells in the liver function as scavengers of the destroyed metastases and as antigen-processing and -presenting cells for antitumor immune T cells.
VARIOUS BIOLOGIC STRATEGIES for treatment of cancer are based on the transfer of cells with particular functions. Two examples of such cell-based therapies are (1) transfer of hematopoietic stem cells for bone marrow reconstitution (bone marrow transplantation [BMT]) after high-dose chemotherapy and (2) transfer of activated tumor-reactive lymphocytes for adoptive cellular immunotherapy (ADI) of primary tumor and metastases.1-3 A combined effect of stem cell and immune cell reconstitution may explain the beneficial effects of allogeneic versus autologous BMT.4 Such graft-versus-leukemia (GVL) effects can be observed for instance in a proportion of chronic myelogenous leukemia (CML) patients that receive allogeneic HLA-matched BMT, provided that the bone marrow cells contain allogeneic T cells.5 Allogeneic GVL reactivity can be transfered with donor buffy coat lymphocytes,6-8 but it is usually associated with a risk for development of graft-versus-host (GVH) disease with significant morbidity and mortality. The difficulty in dissociating GVL from GVH reactivity prevents exploitation of BMT for its full potential. To improve this situation, a better understanding of mechanisms of GVL and GVH reactivity is urgently needed.
We recently established an interesting animal model system for the investigation of GVL and GVH reactivity of immune lymphocytes. Using freshly in situ-activated antitumor immune cells from major histocompatability complex (MHC)-congenic mice, we obtained a strong GVL effect in the absence of severe GVH disease.9 The murine leukemia system consists of various well-defined metastasizing sublines (ESb, ESb-MP, and ESbL-lacZ) that are derived from the chemically induced DBA/2 lymphoma L 5178 Y.9 The ADI effects are achieved by the transfer of in situ-activated tumor-reactive lymphocytes from the tumor-resistant strain B10.D2 (H2d, Mlsb) into susceptible syngeneic tumor-bearing DBA/2 (H2d, Mlsa) mice. A single transfer of 2 × 107 allogeneic antitumor immune spleen cells into 5 Gy pretreated tumor-bearing mice resulted in regression, encapsulation, and rejection of primary cancer and eradication of metastases as well as in significant improvements of overall survival. This was true even when the therapy was administered very late, such as 4 weeks after tumor transplantation.9 The therapy effect could be demonstrated and quantified by (1) counting tumor cells and tumor-infiltrating lymphocytes in frozen tissue sections, (2) FACscan analysis of ESbL-lacZ cells and host cells reisolated from the livers of untreated and treated mice, and (3) high-field nuclear magnetic resonance (NMR)-spectroscopy of phosphor metabolites of primary tumors in individual live animals as a noninvasive method.9
The reasons for the predominantly protective effects of this ADI protocol and a possibly downregulated GVH reactivity are not yet entirely clear. The B10.D2 anti-ESb immune spleen cells used contain ESb tumor antigen-specific as well as DBA/2 host antigen-specific T cells.10 Because we observed in some experiments a late chronic GVH reaction,11,12 a contribution of minor histocompatibility antigen-reactive T cells and thus of a GVH reactivity in this antitumor effect cannot be excluded. B10.D2 immune cells are likely to react against a minor lymphocyte stimulatory antigen from DBA/2 mice (Mlsa) that was discovered more than 20 years ago13 and that is now known to be a superantigen (SAg) encoded by endogenous mouse mammary tumor virus (MMTV) proviruses that have randomly integrated into germ cells.14,15 Mls-1a16-18 represents a viral SAg (vSAG7) that binds to MHC class II molecules. Preliminary findings indicate that vSAG7, which behaves as a self-tolerogen in DBA/2 mice by deleting T cells with certain vβ chains from their repertoire,19 may be associated with ESb lymphoma sensitivity of DBA/2 and resistance of B10.D2 mice (unpublished observations).
The present study was undertaken to compare the kinetics of organ infiltration by donor immune cells in tumor-bearing (GVL system) or non–tumor-bearing (GVH system) mice. For this purpose, we used standard histologic and newly established immunohistologic stainings of frozen tissue sections from livers and spleens. The ability to distinguish donor from host cells by antibodies against an allelic form of β-2 microglobulin (β2Mb) enabled us to detect a new GVL-associated host response after the transfer of allogeneic immune cells. This response will be shown in a subpopulation of macrophages staining with a monoclonal antibody (MoAb) against the adhesion molecule sialoadhesin. The data to be shown will also demonstrate that these cells isolated directly ex vivo can stimulate in vitro antitumor immune memory T cells.
MATERIALS AND METHODS
Animals
DBA/2 mice were obtained from IFFA Credo (Lyon, France) and B10.D2 mice were obtained from Olac (Bicester, UK). They were used at an age of 6 to 12 weeks.
Tumor Lines
ESb cells represent a spontaneous high metastatic variant of the chemically induced T lymphoma L5178Y (Eb) of DBA/2 mice. They arose most likely after fusion of Eb cells with a host macrophage.20 The ESb-MP subline is an adhesion variant of ESb that grows in vitro attached to plastic while ESb cells grow in suspension. In vivo, ESb-MP cells grow progressively but show a less aggressive phenotype, metastasizing more slowly than ESb and involving multiple organs.21
ADI Protocol
On day 0 (d0), DBA/2 recipients were inoculated with 2 × 105 ESb-MP tumor cells intradermally. To generate allogeneic immune effector cells, ESb-MP lymphoma cells were injected intravenously into B10.D2 donor mice at a dose of 105 cells.11 Seven days later, these effector cells were isolated from the spleen and transferred intravenously (3 × 107 cells/200 μL RPMI-1640 medium) into 5 Gy (60Co source; Gammatron F 80S; Siemens München, Germany) sublethally irradiated recipient DBA/2 mice. In the GVH system, the recipients were normal DBA/2 mice, whereas in the GVL system, they were mice bearing ESb-MP tumors and metastases 23 days after tumor transplantation. Tumor-bearing DBA/2 mice of the control group remained untreated.
The tumor burden in the liver at the time of ADI was 5% to 8% of the surface area in the ESb-MP model as determined by quantitative in vivo 1H-NMR microimaging22 and about 5% of total reisolated liver sinusoidal cells in the ESbL-lacZ model as dermined by fluorescence-activated cell sorting (FACS) analysis after FDG loading.23 ESb-MP metastases grew as foci and could therefore be discriminated from normal liver tissue by standard histology, whereas ESbL-lacZ metastases were infiltrating diffusely and scattered and required X-gal staining or FDG loading for quantitative discrimination.
Antibodies and Other Reagents
The following rat MoAbs were used as culture supernatants: antimouse CD4 (clone GK 1.5),24 antimouse CD8 (clone 53-6-72),25 and antimouse sialoadhesin (SER-4).26 The rat MoAbs were visualized by using a biotin-conjugated donkey antirat Ig reagent followed by streptavidin-AP (both from Dianova, Hamburg, Germany). The mouse MoAb Lym 11,27 a hybridoma supernatant, directed allele-specific against β2Mb of B10.D2 mice, was kindly provided by P. Robinson (London, UK). The following antibodies were obtained commercially: rat antimouse CD4 or CD8, biotin-conjugated (Life Technologies, Eggenstein, Germany), polyclonal donkey antimouse IgG (H+L) antibodies, horseradish peroxidase (PO)-linked or alkaline phosphatase (AP) or fluorescein isothiocyanate (FITC)-linked F(ab)′2 fragments (Dianova).
Anti-β2Mb Immune Complex Formation
To find optimal molecular proportions between Lym 11 and antimouse Ig, Lym 11 hybridoma supernatant was used in 10-, 20-, 30-, 40-, and 50-fold dilutions and mixed with PO-, AP-, or FITC-linked antimouse Ig to yield a final concentration of the latter of 1:10, 1:20, 1:30, 1:40, 1:50, and 1:100, respectively. Dilutions were made with 1% normal donkey serum (Dianova) and 1% bovine serum albumin (Sigma, München, Germany) in phosphate-buffered saline (PBS). The complexes were left to form for 1 hour at room temperature. Free secondary antibody binding sites remaining were saturated by adding 1% normal mouse serum to the complexes for the last 20 minutes of their formation.
After extensive washing, the enzyme reactions were developed. Peroxidase was preceded by immersion of the sections in 0.1 mol/L acetate buffer (pH 4.6) and followed by the reaction for alkaline phosphatase after pretreatment with 0.05 mol/L Tris-HCl buffer (pH 8.2).
Flow Cytometric Analysis
Cells (1 × 106) were washed with FACS buffer (5% fetal calf serum, 0.1% NaN3 in PBS) and incubated on ice for 20 minutes with preformed immune complexes from MoAb Lym 11 and FITC-linked polyclonal donkey antimouse Ig antibody. The cells were then extensively washed and stained with 1.5 μmol/L propidium iodide to exclude dead cells. Flow cytometric analysis was performed using a FACScan (Becton Dickinson, Heidelberg, Germany). Excitation laser frequency was 488 nm and fluorescence emission was detected at 575 nm. Typically, 10,000 events were collected and data were expressed as histograms.
Separation of Macrophages from Spleen Cells
Single-cell suspensions from spleens were prepared by mechanical dissociation. After lysis of erythrocytes by a short NH4Cl hypotonic solution treatment, residual cells were washed twice and resuspended in RPMI-1640 medium with antibiotics, 10% heat-inactivated fetal calf serum, and 50 μmol/L 2-mercaptoethanol (Sigma) followed by incubation on tissue culture petri dishes for 2 hours at 37°C in 5% CO2. The nonadherent cells were collected.
Immunohistochemistry
Tissue preparation.Livers and spleens were removed and snap-frozen by immersing in liquid nitrogen. Five-micrometer–thick consecutive cryostat sections were mounted on uncovered glass slides. After drying overnight at room temperature, the sections were fixed in aceton for 10 minutes at room temperature and air dried.
One-step immunolabeling with anti-β2Mb preformed immune complexes.After the fixation, the slides were washed in PBS three times for 5 minutes. To avoid nonspecific binding, Fcγ receptors in the mouse tissue were blocked with different concentrations of normal mouse serum. The sections were incubated for 20 minutes with 5% to 20% normal mouse serum at 37°C. Removal of the serum was followed by incubation with the preformed immunocomplex that was found to provide the best quality immunostaining for 1 hour at room temperature. At the end of the antibody treatment, the sections were washed in PBS three times for 5 minutes and immersed in the same buffer as used in the developer solution for another 5 minutes.
Two-step immunolabeling with rat MoAbs.After washing, the sections were covered with normal serum (1% bovine serum albumin and 1% normal rat serum) and incubated for 30 minutes. Removal of the normal serum was followed by rat MoAb treatment for 1 hour. After washing, the sections were incubated for 30 minutes with secondary antibodies (polyclonal donkey antirat IgG conjugated with AP) and washed again.
Double staining with immune complexes and biotin-conjugated MoAbs.The preformed immunocomplex consisting of monoclonal primary antibody and PO-linked secondary antibody was dripped onto the section and incubated for 1 hour. After washing, the sections were incubated with biotin-conjugated anti-CD4 or anti-CD8 MoAbs for 1 hour and washed again. In the case of SER-4, the bound antibody was visualized by biotin-conjugated donkey antirat Ig reagent. This was followed by 30 minutes of incubation with the streptavidin-AP complex.
Development of enzyme reactions.Peroxidase activity was shown by immersing the sections in a solution made by mixing 6 mg 3-amino-9-ethylcarbazole dissolved in 1.5 mL N, N-dimethylformamide (Merck, Darmstadt, Germany), 28.5 mL of 0.1 mol/L acetate buffer (pH 5.0), and 15 μL 30% hydrogen peroxide for about 8 minutes as described.28 The substrate for the development of alkaline phosphatase consisted of 63 μL 5% Neufuchsin (Sigma), 160 μL 4% natrium nitrite solution, and 16 mg naphthol As-Bi-Phosphate (Sigma) dissolved in 188 μL N,N-dimethylformamide (Merck) and 30 mL of 0.05 mol/L Tris-HCl buffer, pH 8.7. The freshly prepared solution was filtered into the staining jar containing the sections.
Development lasted about 3 to 5 minutes, with regular checking of the reaction intensity by the microscope. All steps detailed above were performed at room temperature.
Control and Data Evaluation
The distribution of the reaction products of the one-step method was compared with the results of conventionally performed indirect immunostainings at the same antibody dilutions as those found to give optimal staining for the complexes. Sections from liver and spleen of B10.D2 mice served as a positive test and those from DBA/2 served as negative controls. To determine nonspecific background staining, further negative controls were required involving the following modifications of the above-described immunoenzymatic staining. (1) The specific primary Igs were substituted with Ig from normal serum used to dilute the immunoreagents or with nonimmune serum of the same animal species that provided the primary antibodies in the two-step or the one-step method. The protein concentration of these solutions was chosen to be that found optimal in the complexes. (2) The primary antibody was interchanged by another mouse antibody (anti–β-galactosidase; Boehringer Mannheim, Mannheim, Germany) in both the one-step and two-step stainings. (3) The immune complexes in all combinations were also applied without the saturation step with mouse serum. Only after a clear discrimination between donor and host tissue staining was achieved was donor cell infiltration in host organs quantified.
Data were evaluated by counting positively stained cells and relating them to the structural units of the respective organs, the liver lobuli, and the white pulp of the spleen. The means and standard deviations of the data obtained from the number of mice and experiments indicated were then calculated and presented in graphics. Finally, quantitative comparisons between GVL and GVH reactivity were made and the data were analyzed statistically as described in the Results.
Immunization of DBA/2 Mice and Isolation of Immune Spleen Cells
DBA/2 mice were immunized three times by subcutaneous injection with 2 × 107 irradiated (200 Gy) ESb cells (batch 289) in 100 μL RPMI-1640 medium. Inactivation of tumor cells was achieved by X-irradiation with 100 Gy from a 137Cs source (Gammacell 1000; Atomic Energy of Canada Ltd, Ottawa, Canada). One week after the last immunization, single-cell suspensions from spleens were prepared by mechanic dissociation, treated with NH4Cl hypotonic solution, washed, and incubated in plastic culture dishes for 2 hours at 37°C in 5% CO2 to remove adherent cells. Spleens from normal animals (nonprimed) were prepared following the same protocol. Only nonadherent cells (lymphocytes) were used for the functional assays.
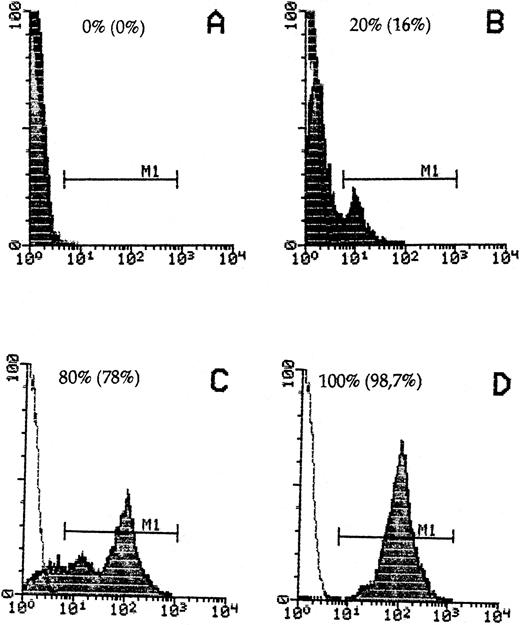
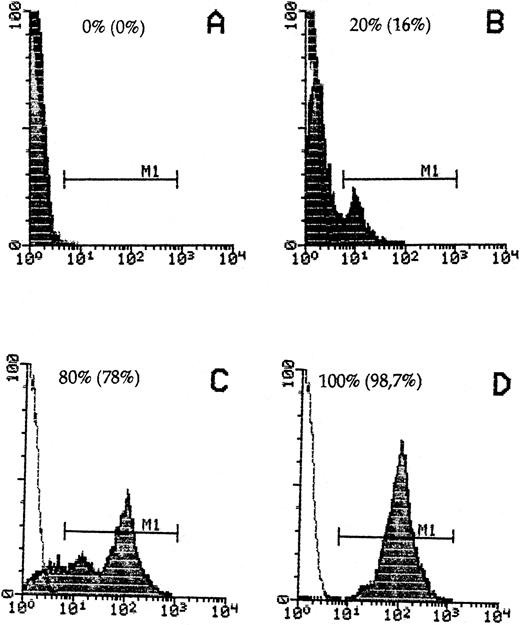
Donor cell detection in host cells by staining with anti-β2Mb immunecomplexes and quantitative FACScan cytometry. Spleen cells from DBA/2 hosts (A) or B10.D2 donors (D) or mixtures of 20% (B) or 80% (C) donor to host cells were stained. The percentage of recovered donor cells in gate M1 is indicated in parentheses.
Donor cell detection in host cells by staining with anti-β2Mb immunecomplexes and quantitative FACScan cytometry. Spleen cells from DBA/2 hosts (A) or B10.D2 donors (D) or mixtures of 20% (B) or 80% (C) donor to host cells were stained. The percentage of recovered donor cells in gate M1 is indicated in parentheses.
Isolation of SER+ Macrophages From the Liver
Liver sinusoidal cells from tumor-bearing mice treated with ADI and from normal mice were isolated by perfusion through the portal vein with pronase and collagenase followed by metrizamide gradient, as previously described.29 Cells were incubated in plastic culture dishes for 2 hours at 37°C in 5% CO2. After the removal of nonadherent cells by washing, Kupffer cells (adherent cells) were scraped with a rubber spatula, washed twice, and resuspended in PBS without divalent cations (Biochrom KG, Berlin, Germany). The SER+ macrophage population was isolated by means of a rosetting technique using unopsonized sheep erythrocytes, as described previously.30 In the case of normal liver, which contains only few SER+ macrophages, we used the whole sinusoidal cell population for the functional assays.
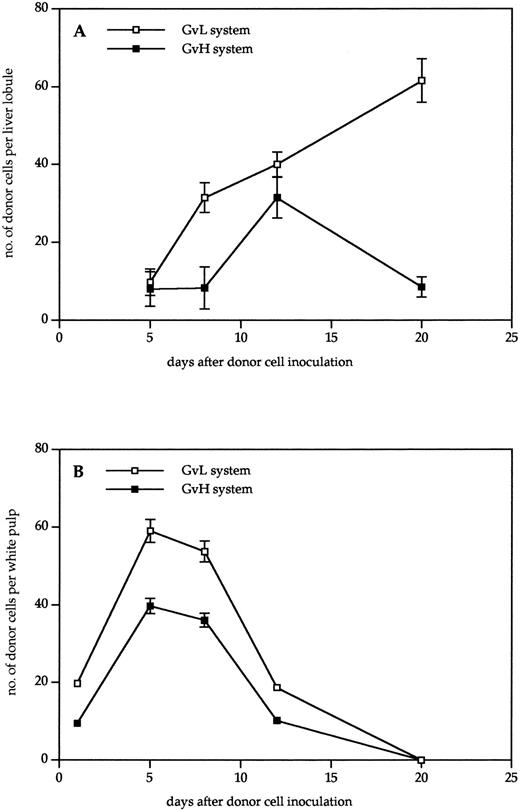
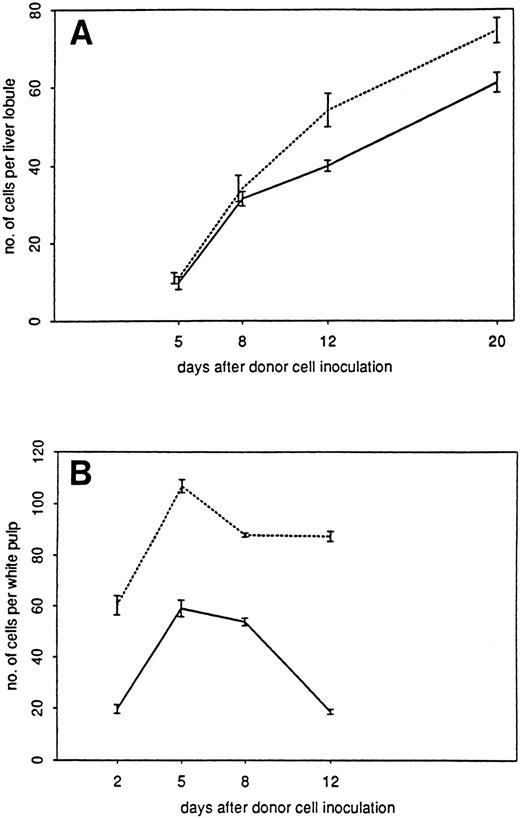
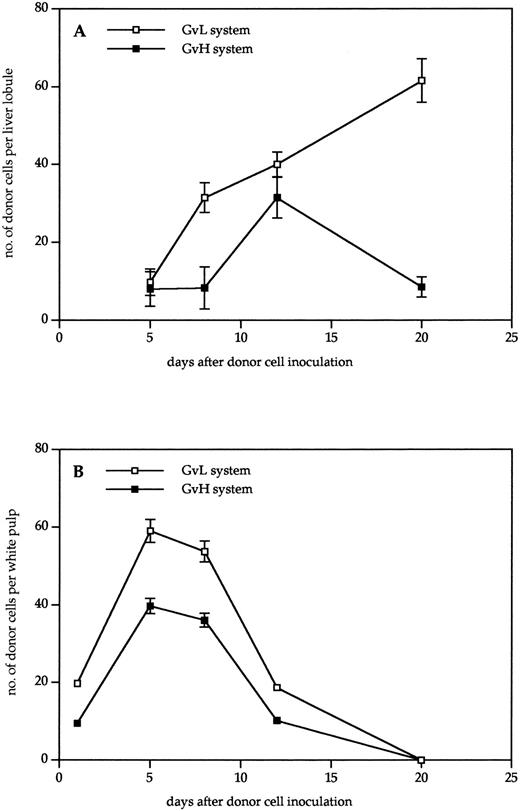
Kinetics of donor cell infiltration of (A) liver and (B) spleen in the GVL (□) or GVH (▪) system. Frozen tissue sections were stained for donor cells with anti-β2Mb immune complexes and analyzed under the microscope. The data indicate the means and standard deviations (SD) from five separate experiments with 5 to 8 animals per time point. Points without SD indicate values for which the SD was smaller than 1.
Kinetics of donor cell infiltration of (A) liver and (B) spleen in the GVL (□) or GVH (▪) system. Frozen tissue sections were stained for donor cells with anti-β2Mb immune complexes and analyzed under the microscope. The data indicate the means and standard deviations (SD) from five separate experiments with 5 to 8 animals per time point. Points without SD indicate values for which the SD was smaller than 1.
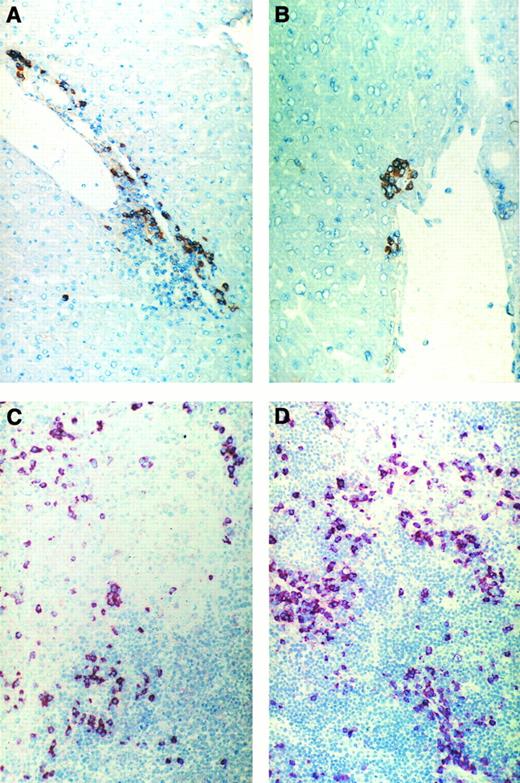
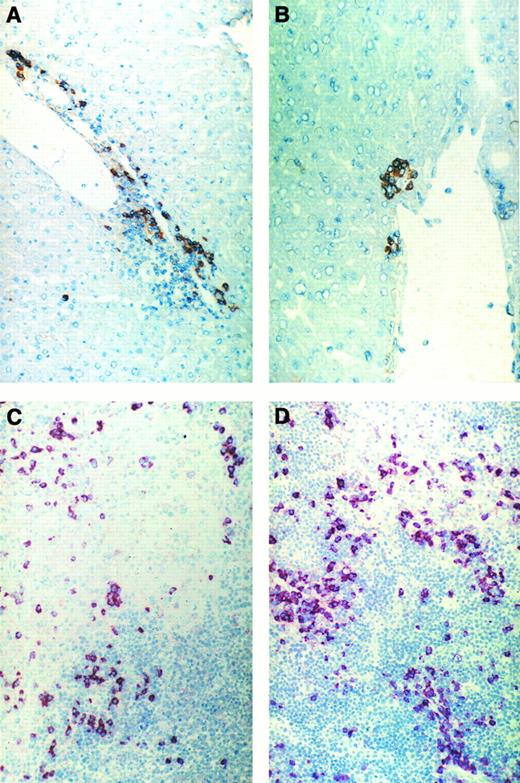
Immunohistochemical pictures of frozen tissue sections showing donor-cell infiltration in the GVL (A and C) or GVH (B and D) system in liver (A and B) and spleen (C and D) 8 days after immune cell transfer. Donor cells were stained with anti-β2Mb immunecomplexes using a one-step immunolabeling method (original magnification × 160).
Immunohistochemical pictures of frozen tissue sections showing donor-cell infiltration in the GVL (A and C) or GVH (B and D) system in liver (A and B) and spleen (C and D) 8 days after immune cell transfer. Donor cells were stained with anti-β2Mb immunecomplexes using a one-step immunolabeling method (original magnification × 160).
Functional Assays to Determine T-Cell Stimulatory Capacity of Liver-Derived Cells
Spleen lymphocytes isolated from normal (nonprimed) and immunized animals were double-stained with antibodies against activation markers and analyzed by flow cytometry (day 0). Another part of lymphocytes (responders) were incubated for 6 days in 24-well plates (Renner, Darmstadt, Germany) with the following stimulator cells (responder:stimulatory cell ratio = 10:1): (1) SER+ liver macrophages, isolated from tumor-bearing DBA-2 mice 12 days after ADI; (2) 200-Gy irradiated ESb289 cells; and (3) liver sinusoidal cells isolated from normal (non–tumor-bearing) animals. The cultured lymphocytes were stained with the same antibodies as at day 0 followed by FACS analysis. The following antimouse antibodies were used: FITC-conjugated anti–interleukin-2 (IL-2) receptor α-chain (CD25) and phycoerythrin (PE)-conjugated CD8a (Ly-29; Pharmingen, San Diego, CA). Cells were incubated at 4°C for 10 minutes with the antibodies, washed, and treated with 1.5 μmol/L propidium iodide (final concentration). FACS analysis was performed using a FACScan (Becton Dickinson). Thirty thousand cells per sample were simultaneously measured for FSC and integrated side scatter as well as green (FL1) and red (FL2 and FL3) fluorescences (expressed as logarithm of the integrated fluorescence light). Recordings were made only on popidium iodide-negative (viable) cells of the red (FL3) fluorescence, excluding aggregates whose FSCs were out of range. Expression of cell surface molecules was analyzed by dot blots of the red fluorescence (FL2) versus green fluorescence (FL1) and data were expressed as the percentage of CD25+ cells (FL1) among the CD8+ T-cell population (FL2).
RESULTS
Detection of Donor Cells in Host Tissue by Anti-β2Mb Immune Complexes
To detect B10.D2 mouse immune spleen cells in DBA/2 host tissue, we used a mouse MoAb against an allelic form of mouse β2M (anti-β2Mb, Lym 11) that is expressed in donor but not host cells and a polyclonal donkey antimouse Ig F(ab)′2 reagent linked to either PO or to AP. To reduce possible interactions of this second antibody with mouse Ig on cells, we first established a new technique of immune complex preformation and determined the optimal molecular proportions between the Lym 11 hybridoma supernatant and the second antibody, as described in the Materials and Methods. FACS flow cytometric analysis was performed to determine the specificity of immune complex binding in a quantitative way and to exclude a possible exchange between donor and host cells. As can be seen from Fig 1, 100% of donor cells were stained by the immune complexes, whereas there was no significant staining of host DBA/2 cells. When donor cells were mixed with host cells, they could be clearly distinguished even when the majority were of host origin. The percentage of positive cells obtained was close to the theoretical expectation, thus excluding significant exchanges of immune complex-bound donor β2Mb with host cell β2Ma chains.
Kinetics of Donor Cell Infiltration in Host Organs
Immune spleen cells from B10.D2 mice that had been immunized against the DBA/2 lymphoma variant ESb-MP were transfered into 5 Gy irradiated ESb-MP tumor-bearing DBA/2 mice, in which they exerted strong GVL effects associated with only mild GVH reactivity. To distinguish between GVL and GVH reactivity, we performed comparative studies in either tumor-bearing mice (GVL situation) or non–tumor-bearing mice (GVH situation). At different times after intravenous immune cell transfer, spleens and livers of the recipients were removed and frozen to be later analyzed by immuno-histochemistry. The amount of detected donor cells was related to the structural units of the respective organs, the liver lobuli, and the white pulp of the spleen.
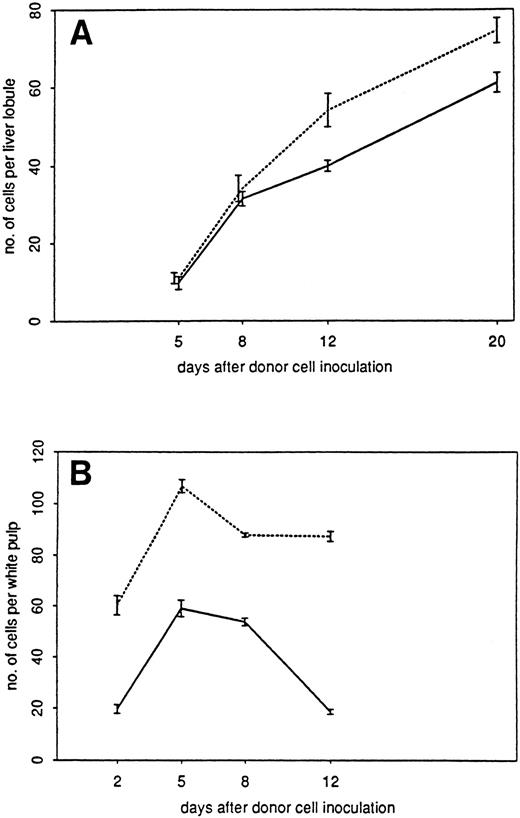
Kinetics of infiltration of liver (A) or spleen (B) in the GVL system by donor cells (□), CD4+ T cells (▪), CD8+ T cells (▵), and SER-4+ macrophages (▴). Immunohistochemically stained frozen tissue sections were analyzed under the microscope. The data indicate the means and standard deviations from two experiments with 2 to 3 animals per time point.
Kinetics of infiltration of liver (A) or spleen (B) in the GVL system by donor cells (□), CD4+ T cells (▪), CD8+ T cells (▵), and SER-4+ macrophages (▴). Immunohistochemically stained frozen tissue sections were analyzed under the microscope. The data indicate the means and standard deviations from two experiments with 2 to 3 animals per time point.
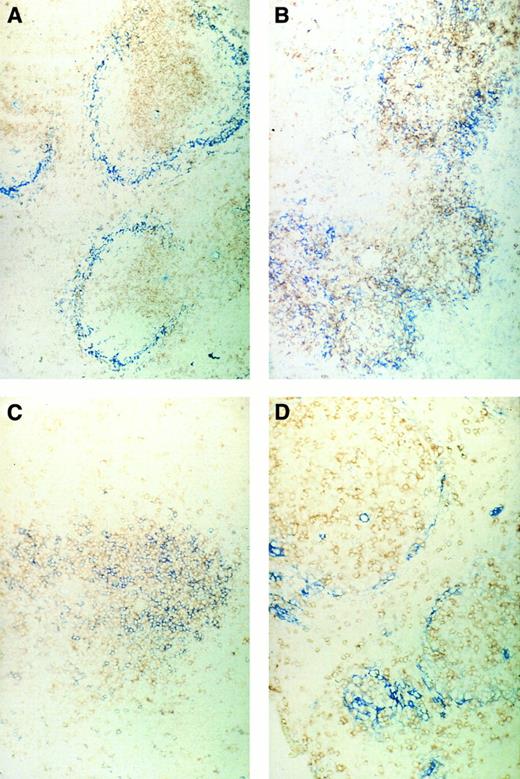
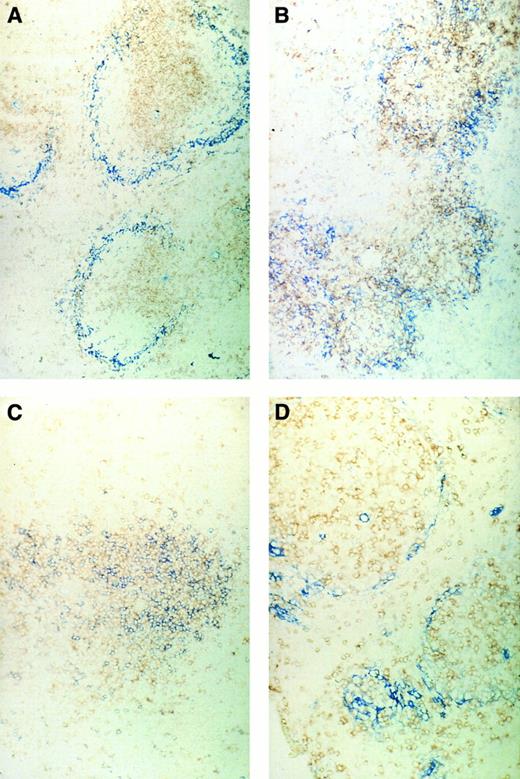
Immunohistochemical pictures of frozen tissue sections, double-stained for CD4 (brown) and sialoadhesin (blue) (A and B) and for CD4 (brown) and CD8 (blue) (C and D) in spleens of normal control mice (A and C) or ESb-MP tumor-bearing mice (d28) 5 days after ADI treatment (B and D) (original magnification × 160).
Immunohistochemical pictures of frozen tissue sections, double-stained for CD4 (brown) and sialoadhesin (blue) (A and B) and for CD4 (brown) and CD8 (blue) (C and D) in spleens of normal control mice (A and C) or ESb-MP tumor-bearing mice (d28) 5 days after ADI treatment (B and D) (original magnification × 160).
As can be seen from the results in Fig 2, donor cells were detectable much earlier in the spleen than in the liver. In the spleen, they were detectable 1 day after transfer, reached a maximum after 5 days, and then gradually disappeared until day 20. There was a higher content in the spleens of tumor bearing than in non–tumor-bearing mice, but the kinetics were similar. In contrast, in the nonlymphoid organ liver, the first time point of donor cell detection was day 5. No donor cells could be detected in the liver between days 1 and 4 after transfer, neither by immunohistology nor by using dye-labeled cells. In non–tumor-bearing mice, the level of donor cell infiltration increased to day 12 and then decreased again until day 20. In livers of tumor-bearing mice, there was a steady increase of donor cells up to day 20 that decreased only until day 30. Thus, the kinetics of donor cell infiltration of the liver and the content of donor cells after 3 weeks was different between tumor-bearing and non–tumor-bearing mice. These differences show aspects of GVL as distinct from GVH reactivity.
Figure 3 shows frozen sections from liver and spleen of either tumor-bearing or non–tumor-bearing recipients undergoing GVL or GVH reactions after adoptive transfer of allogeneic immune spleen cells. Five days after cell transfer (Fig 3C and D), many donor cells can be seen in the white pulp of recipient spleens, often associated with areas of necrosis. Figure 3A and B show that there were significantly more infiltrating donor cells in livers from tumor-bearing mice than in those from non–tumor-bearing mice.
Distinction Between CD4 and CD8 T Lymphocytes
In addition to the total number of infiltrating donor cells in spleen and liver, we determined the amounts of CD4 and CD8 staining T lymphocytes. As can be seen from Fig 4B, 1 day after cell transfer into the 5 Gy irradiated recipients there were about 50 CD4 T lymphocytes per white pulp, whereas there were less than 5 CD8 T cells detectable. Five days after transfer, the CD4 T cells had increased to 75 and the CD8 T cells to 30 cells per unit. From days 8 through 20, a steady state was reached that leveled off at about 55 CD4 and 30 CD8 T cells. Because donor cell infiltration in the spleen steadily decreased after day 8 (Fig 2), it appears that a large proportion of the CD4 and CD8 T cells in the spleen after day 8 were of host origin.
Figure 4A shows the results obtained with the liver. In contrast to the spleen, detectability of T cells in the liver started only around day 5. Thereafter, there was a steady increase of liver-infiltrating donor cells and of T cells with CD4 and CD8 markers up to day 20. At each time point, there was a higher number of CD4 T cells than of CD8 T cells. It can be concluded that, during the first 3 weeks after cell transfer, the livers of tumor-bearing mice become increasingly infiltrated with T lymphocytes of both CD4 and CD8 lineage.
Sialoadhesin-Positive Macrophages
With the help of the MoAb SER-4,24 we determined the localization and quantity of sialoadhesin-positive macrophages in the spleens and livers of tumor-bearing mice undergoing ADI. The number of these cells in the spleen (Fig 4A) remained rather constant around 50 per unit throughout the detection period. In contrast, in the liver (Fig 4B), we saw a dramatic increase from day 1 to 12 by a factor greater than 30. Thereafter, they slowly decreased to baseline level until day 30. The area under the curve of SER+ cells in the liver (days 1 through 30; 1,211 units) was 1.32-fold larger than the area of donor cells (days 5 through 30; 916 units).
Localization of SER+ Macrophages and T Lymphocytes in the Spleen
We first investigated the localization of CD4 or CD8 T lymphocytes in the spleens in relation to the position of SER+ macrophages. Figure 5A shows the typical structure in normal control mice of the white pulp with the T lymphocytes in the center and the stained blue SER+ macrophages surrounding. A double staining of CD4 and CD8 T lymphocytes (Fig 5C) showed a pattern of random mixture within the center of the white pulp. In contrast, tumor-bearing mice that were irradiated with 5 Gy on day 16 and then inoculated with immune spleen cells showed 5 days later a different structural organization in the spleen (Fig 5B and D). CD4 T lymphocytes and SER+ macrophages (Fig 5B) were not as nicely separated as in the white pulp from normal mice, and CD4 and CD8 T lymphocytes (Fig 5D) were more separated than in normal spleens. It seems as if the white pulp is in a process of reorganization after irradiation and cell transfer. Spleens of irradiated tumor-bearing control animals not treated by cell transfer showed SER staining such as in normal mice but with decreased intensity.30
Increase in SER+ Liver Macrophages Representing a Host Response
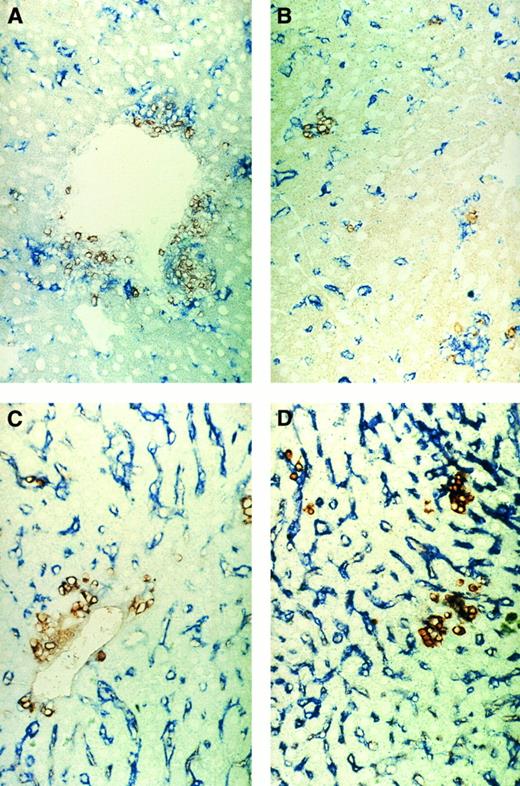
Figure 6 shows immunohistochemical double stainings from the liver. Figure 6A and B show the positioning between CD4 T lymphocytes and SER+ macrophages in areas of the periportal vein (A) or deep in the parenchyma (B). A close association between the CD4 T lymphocytes and the macrophages can be seen. Figure 6C and D show the positioning of total donor cells (brown) and SER+ macrophages. Whereas clusters of donor cells can be seen around portal veins (Fig 6C) and in the parenchyma (Fig 6D), there were also single donor cells with or without direct contact to SER+ macrophages.
Immunohistochemical pictures of frozen tissue sections, double-stained for CD4 (brown) and sialoadhesin (blue) (A and B) and for β2Mb (brown) and sialoadhesin (blue) (C and D) in livers from tumor-bearing mice (d43) 20 days after ADI treatment. (A and C) Areas of portal vein (original magnification × 160); (B and D) areas of parenchyma (original magnification × 160).
Immunohistochemical pictures of frozen tissue sections, double-stained for CD4 (brown) and sialoadhesin (blue) (A and B) and for β2Mb (brown) and sialoadhesin (blue) (C and D) in livers from tumor-bearing mice (d43) 20 days after ADI treatment. (A and C) Areas of portal vein (original magnification × 160); (B and D) areas of parenchyma (original magnification × 160).
These observations show two important and new findings. (1) The increase in SER+ liver macrophages represents a host response to ADI, because the cells were negative for the donor marker β2Mb (Fig 6C). (2) A close physical association exists between SER+ host macrophages and CD4 T lymphocytes (mostly donor T cells).
Quantitative Aspects and Statistical Analysis
For quantitative comparison of donor cell infiltration in the GVL versus the GVH system, we determined the areas under the curves shown in Fig 2. The data were obtained on the basis of the mean response curves. Further analysis via bootstrapping (1,000 samples) showed the 95% confidence interval (CI; 0.95) that is indicated in parentheses. The following values were obtained: GVL/GVH area (spleen) = 1.54 (1.50; 1.60); GVL/GVH area (liver, days 5 through 30) = 3.00 (2.75; 3.25).
Thus, for the spleen, the area of the GVL system was 1.54-fold larger than the GVH area. This means that 54% more cells infiltrated the spleens of tumor-bearing than those of non–tumor-bearing mice. For the liver, in the time interval of days 5 through 30, there were 200% more cells in the GVL than in the GVH system.
We next compared the number of donor cells in the spleen and liver to the sum of CD4 and CD8 T cells. The mean response curves with pointwise 95% confidence intervals (CI0.95; vertical bars) of the number of donor cells and the sum of CD4 and CD8 T cells are shown in Fig 7. Whereas for the spleen (Fig 7B) the difference between donor cell numbers and the value of the sum of CD4 and CD8 T cells seems to be constant over time, one can see that, for the liver (Fig 7A), the difference is growing, especially after day 8. To analyze the relations between donor cells and host cells on the one side and CD4 and CD8 T cells on the other side, a linear regression analysis was performed according to the following formula: (CD4 + CD8) = Host Cells + β × Donor Cells + Error, ignoring time effects or other confounding factors. Using this model, it was calculated that, for the spleen, the host cells contributed by a value of 61.3%, with a CI0.95 of 39.6% to 83%. The factor β was estimated as 0.64, with a CI0.95 of 0.13 to 1.16. The confidence interval includes 1, so that the difference between CD4 + CD8 T cells and donor cells seems to result solely from the contribution of the host cells.
Mean GVL response curves in liver (A) and spleen (B) with corresponding 95% confidence intervals. ( — ) Donor cells; (- - -) CD4 + CD8 cells.
Mean GVL response curves in liver (A) and spleen (B) with corresponding 95% confidence intervals. ( — ) Donor cells; (- - -) CD4 + CD8 cells.
With regard to the infiltration of the liver by T cells, the regression analysis showed (1) that the value for host T cells in the liver before cell transfer was not significantly different from 0 and (2) that the estimate of the factor β was 1.25, with a CI0.95 of 1.07 to 1.44. The value of factor β indicates the increasing difference between donor cells and the sum of CD4 and CD8 T cells.
In other words, in donor-cell–infiltrated livers, there were more CD4 + CD8 T cells than donor cells, with a growing difference, indicating a contribution of host T cells to the ADI-mediated liver inflammation.
SER Macrophages From GVL Livers Stimulate CD8 Antitumor Memory T Cells
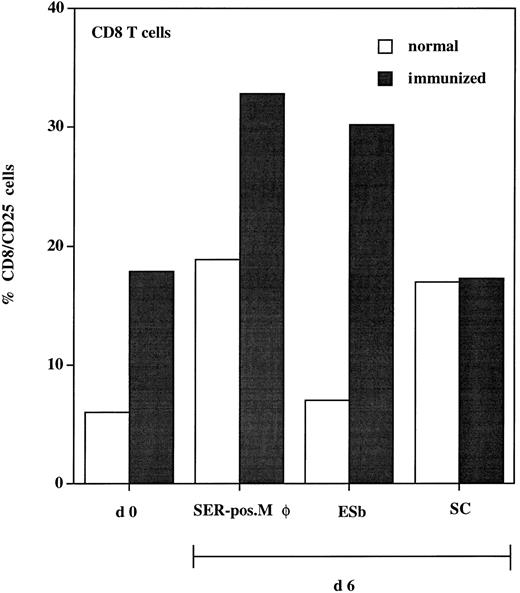
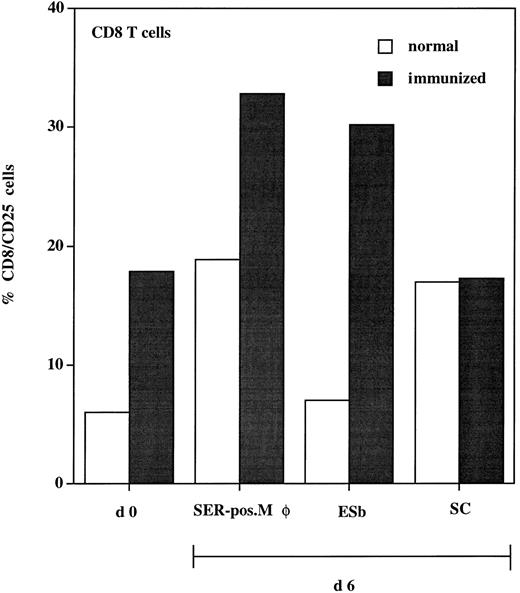
To test the functional significance of the close physical association of SER+ macrophages and T cells after ADI, we studied the antigen-presenting function of these SER-postive macrophages. These cells were isolated from livers after ADI and cocultured with spleen lymphocytes (responders) from immunized and normal (nonprimed) animals. To enhance the chances of testing for a tumor-specific response, we used immune cells from syngeneic DBA/2 mice. B10.D2 anti–ESb-MP ISPL contain, in addition to tumor-specific T cells, T-cell clones with reactivity to DBA/2 minor histocompatibility antigens.31 Activation of responders (CD8 T cells) was tested at days 0 and 6 by staining with antibodies against CD25 (IL-2 receptor α chain). Results show that, in every experimental situation, expression of CD25 was higher in immune spleen lymphocytes as compared with nonimmune lymphocytes (Fig 8). Within the immune lymphocytes, the highest expression of CD25 was seen after coculture with SER+ macrophages (30% of the CD8 population). This level was even higher than that expressed after coculture with ESb lymphoma cells (which were used as a positive control, because immune lymphocytes were generated after priming with these tumor cells). The syngeneic CD8 cytolytic T-cell response to ESb was previously characterized as being highly specific and directed against class I MHC (Kb)-restricted immunodominant peptide(s) as tumor antigen(s).32 When cocultured with normal liver sinusoidal cells, CD25 reached only 17% of the CD8 population. In conclusion, the association of SER+ macrophages with T cells has a functional significance, because coincubation of both populations enhances the expression of activation marker CD25 in the CD8 T cells.
Expression of activation marker (CD25) on CD8 T cells isolated from spleen of normal (□) and immunized (▪) DBA/2 mice. Animals were immunized three times with irradiated ESb 289 lymphoma cells. One week after the last challenge, spleen lymphocytes were isolated from immunized and normal (nonprimed) animals and double-stained for flow cytometry using antimouse MoAbs: FITC-conjugated anti-CD25 and PE-conjugated anti-CD8 (d0). Another part of lymphocytes (responders) was incubated for 6 days with the following stimulator cells (responder:stimulator cell ratio = 10:1): (1) SER+ liver macrophages (SER-pos. Mø), isolated from tumor-bearing DBA-2 mice 12 days after ADI using rosetting with unopsonized sheep erythrocytes; (2) 200 Gy irradiated ESb 289 cells (ESb); and (3) liver sinusoidal cells (SC) isolated from normal (non–tumor-bearing) animals. Lymphocytes were then stained using the same MoAbs as at day 0 followed by FACS analysis. Results were expressed as the percentage of CD25+ cells from the CD8 T-cell population.
Expression of activation marker (CD25) on CD8 T cells isolated from spleen of normal (□) and immunized (▪) DBA/2 mice. Animals were immunized three times with irradiated ESb 289 lymphoma cells. One week after the last challenge, spleen lymphocytes were isolated from immunized and normal (nonprimed) animals and double-stained for flow cytometry using antimouse MoAbs: FITC-conjugated anti-CD25 and PE-conjugated anti-CD8 (d0). Another part of lymphocytes (responders) was incubated for 6 days with the following stimulator cells (responder:stimulator cell ratio = 10:1): (1) SER+ liver macrophages (SER-pos. Mø), isolated from tumor-bearing DBA-2 mice 12 days after ADI using rosetting with unopsonized sheep erythrocytes; (2) 200 Gy irradiated ESb 289 cells (ESb); and (3) liver sinusoidal cells (SC) isolated from normal (non–tumor-bearing) animals. Lymphocytes were then stained using the same MoAbs as at day 0 followed by FACS analysis. Results were expressed as the percentage of CD25+ cells from the CD8 T-cell population.
DISCUSSION
We previously described an animal model for GVL in which the transfer of allogeneic immune T lymphocytes led to strong GVL effects associated with only mild GVH reactivity.9 This situation may be related to the clinical setting of allogeneic BMT in leukemia patients in whom both GVL and GVH reactivity can be seen and in whom a distinction between both activities has been difficult so far.33 34 In this study, we performed a systematic immuno-histochemical analysis of immune donor cell infiltration of lymphoid organs (spleen) and nonlymphoid organs (liver) and compared experimental situations of a pure GVH reaction (in non–tumor-bearing hosts) with reactivity in a GVL situation (in tumor-bearing hosts). A distinction between donor and host cells was made possible by making use of the fact that donor and host cells express a different allelic form of β2M, which noncovalently attaches to MHC class I molecules (α chains). By using a newly established method of staining with anti-β2Mb + antimouse Ig immune complexes and having excluded by quantitative FACScan analysis a possible exchange with host β2Ma chains, we could clearly detect and quantitate in frozen sections donor cells in host tissues.
The following new observations were made, which will be discussed. (1) There was a clear difference between the kinetics of infiltration of the spleen as compared with the liver. Both organs were infiltrated only transiently. (2) There was a clear difference in the extent of organ infiltration between the GVL and the GVH systems. (3) There was a strong and very conspicious increase of SER+ macrophages in the liver of tumor-bearing hosts after adoptive cell transfer. (4) These cells were shown to have T-cell–stimulatory capacity in a syngeneic antitumor CD8 T-cell response.
(1) Upon immune cell transfer into tumor-bearing mice, both the spleen and the liver became infiltrated, but there was a distinct difference in kinetics. Whereas infiltration of the spleen started immediately, reached a peak after about 5 days, and dissappeared within 3 weeks, the liver became infiltrated only after 5 days and reached its peak after 20 days to then disappear by day 30. Considering these differences in kinetics, one could postulate a link at day 5 after transfer between the decrease of cells in the spleen and the increase of cells in the liver. It cannot be excluded that there may be an active emigration of donor cells from the spleen to the liver, a route that is commonly used by metastasizing tumor cells when injected into the spleen.35
One month after adoptive cell transfer no donor cells could be seen anymore in either the spleen or the liver. The reason for their dissappearance remains unclear at present, but various possibilities could be discussed.
One possibility is dissappearance due to apoptosis perhaps induced by persistant stimulation via superantigens.36-38 This possibility exists because the host expresses viral superantigens of MMTV, in particular v-SAG-7 (Mlsa), which stimulates strongly v-β 6 T-cell receptor-positive T lymphocytes among the donor lymphocytes. To what extent the liver-infiltrating cells represent v-β 6-positive cells remains to be investigated.
Another possibility would be a host versus graft (HVG) response. This could have a delayed occurance because of the sublethal host irradiation and the time that is required for reconstitution of the radiation-induced defect. Whichever mechanism may be at work, the dissappearance of the donor cells may be important for the prevention of GVH disease and lethality. When comparing our GVL/GVH model with other systems,39-42 a major difference consists in the dose of radiaton used. Although we used only a sublethal dose of 5 Gy, many other systems used a lethal dose. This difference supresses completely HVG reactivity and thereby may favor GVH reactivity. The dose of radiation may thus have important consequences for host conditioning.34
(2) In this study, we were able to compare the GVL activity in the ADI model of ESb-MP lymphoma with a pure GVH system in which the same donor immune cells were used and injected into normal 5 Gy irradiated DBA/2 recipients. This allowed a comparative study of kinetics of organ infiltration and of quantities of infiltrated cells. In both organs, spleen and liver, there was a significantly higher organ infiltration by donor lymphocytes in the GVL than in the GVH situation. Whereas in the spleen there were about 1.5 times as many donor cells over the whole time course, in the liver the factor was 3.0. What was also remarkable in the liver was the difference in kinetics between the GVL and the GVH reactivity. GVL reactivity started earlier and lasted much longer (days 5 through 30). This means that at a time point, such as day 20, when the GVH reactivity was already terminated, there was still peak reactivity in the GVL situation. The most likely explanation for this difference would be the assumption that there was still tumor-associated antigen in the liver that attracted remaining immune T lymphocytes. Because at this time after therapy no liver metastases can be seen anymore, it has to be assumed that tumor-associated antigens may still persist in a processed form. This assumption is supported by the detection of potential antigen-presenting cells, namely SER+ macrophages in the liver. The appearance of these cells preceeded the appearance of the donor T cells. Many SER+ macrophages could be seen in tight clusters in association with CD4 T cells. Furthermore, the kinetics of dissappearence of SER+ macrophages was paralleled by a dissappearance of donor lymphocytes.
(3) The early appearance, the enormous increase, and then the disappearence of SER+ liver macrophages in the tumor-bearing host as a consequence of immune cell transfer is perhaps the most remarkable observation of this study. In normal liver tissue, these cells were hardly detectable. In a previous study, we had also stained livers from tumor-bearing mice after ADI treatment with the MoAb F4/80 that stains all liver macrophages. There was rather homogenous staining throughout the organ and no relationship to metastases or ADI therapy. Double-staining with SER-4 antibodies and antibodies to donor-type β2M showed that this macrophage subpopulation was of host origin. Donor T cells and CD4 T cells could be shown to form tight clusters with this particular subset of macrophages. In a previous study, we have isolated SER+ macrophages from spleens of normal animals and shown their ability to process and present antigen from human C-reactive protein to a respective responsive CD4 T-helper clone.30
(4) This study for the first time demonstrates an important new function of freshly ex vivo isolated SER macrophages from GVL livers in which ADI therapy led to their recruitment and activation and to destruction of liver metastases. Their ability to activate tumor primed CD8 memory T cells (Fig 8). Although no exogenous tumor antigen was added, these cells, which were isolated via a painstaking and long isolation procedure (enzyme perfusion, metrizamide gradient separation, plastic adhesion, and erythrocyte rosetting), were able to stimulate CD8 memory T cells just as tumor-stimulator cells. Because we showed before that ESb immune CD8 memory T cells recognize class I MHC-restricted tumor antigen peptide(s), these results suggest that the SER macrophages not only present exogenous antigens via class II MHC30 but also present exogenous antigens via class I MHC. This would mean that they could present antigens to CD4 as well as to CD8 immune T cells and thereby colocalize these immune cells at their cell surface. From recent further studies we know that SER macrophages from GVL livers also form clusters with CD8 T cells (S. Muerköster, unpublished observations). They could thus be the sites for synergistic CD4-CD8 immune T-cell interactions. Such synergism was previously shown to be a prerequisite for ADI therapy leading to complete tumor regression and eradication of macrometastases.9
We may speculate upon the question as to where the SER+ liver macrophages came from and what function they have. They could have been recruited from the blood and may perhaps be derived from blood monocytes. In the liver environment and perhaps with the help of donor-cell–derived cytokines, they could have differentiated into either a specialized subpopulation of SER+ macrophages or perhaps into a form of immature dendritic cells with macropinocytic activity.43 It is possible that the SER+ macrophages function as scavengers of the destroyed metastases. In addition, they appear to be capable of processing and presenting tumor-associated antigen via both MHC class I and II pathways.
We also compared the localization of SER+ macrophages and CD4 or CD8 T lymphocytes in the spleen of normal mice to those of tumor-bearing mice 5 days after ADI treatment. Whereas in normal spleens the SER+ macrophages were surrounding the white pulp in which CD4 and CD8 T cells appeared to be randomly distributed in the T cell area, in the tumor-bearing mouse, the white pulp structures were less well organized. It is possible that in the tumor-bearing, sublethally irradiated and reconstituted mouse, the normal spleen architecture had to be reconstituted first and that this process was guided and accelerated by the transfered immune cells. It has been shown before that, without immune cell transfer, the normal reconstitution of the lymphoid organs after 5 Gy irradiation is delayed, starting after about 12 days and reaching an overshooting reaction after about 3 weeks before hemostatic control mechanisms lead to normalization of the cellular contents.44 It is also possible that the sublethal irradiation that leads to depletion of lymphoid organs contributed to the early and preferential infiltration of lymphoid organs by the donor immune cells and that reconstitution of the lymphoid organs was a prerequisite for colonization of extralymphatic organs.
Differences between GVL and GVH reactivity have also been described in some45-47 but not all42 experimental model systems and recently also in the clinical situation. Transfer of escalating doses of donor leukocytes for treatment of relapse of CML after BMT showed that GVL effects could be obtained with lower cell numbers than those required for GVH.48 Allogeneic immunocompetent lymphocytes with or without stimulation via antigens and cytokines6,8 are receiving increasing attention for clinical application49 and promising results have already been reported from several groups.6-8,48 50
The findings of this study, together with our previous findings,9 have shown several types of donor-host interactions that may be of importance for the overall GVL therapy effect: (1) accelerated reconstitution of the spleen, (2) induction of a host response in form of an increase of SER+ macrophages in the liver, (3) physical contact of donor T cells with host SER+ macrophages in the liver, and (4) synergistic T-T–cell interactions (CD4 and CD8, donor and host derived) during targeting of metastases and mediation of antitumor reactivity. These new findings may lead to a better understanding of mechanisms of importance for effective cellular tumor immunotherapy.
M.R., V.U., and K.-H.L. contributed equally to this report.
Address reprint requests to Volker Schirrmacher, PhD, DKFZ, FSP7, Im Neuenheimer Feld 280, 69120 Heidelberg, Germany.