To the Editor:
Pericentric inversion of chromosome 16, translocation (16; 16), and del(16q), resulting in a chimeric fusion of the CBFβ and MYH11 genes, are typically seen in the M4Eo French-American-British (FAB) subset of acute myelogenous leukemias (AML). Karyotypic detection of these abnormalities can be difficult, particularly in the minimal residual disease (MRD) setting. To date, only 15 patients, in four publications,1-4 have been studied for MRD by reverse transcription-polymerase chain reaction (RT-PCR) detection of CBFβ/MYH11 transcripts. These studies showed the feasibility of the technique in MRD follow-up.
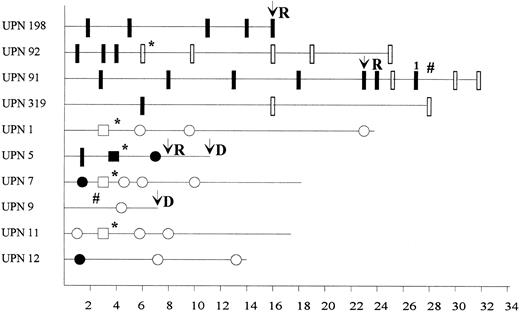
We have studied MRD by RT-PCR in peripheral blood, bone marrow, or cytapheresis samples from 10 patients (Fig 1). Nine were positive at diagnosis for the common A-type transcript and one (UPN1) for a variant of the E-type5 transcript. Patients UPN 198, 92, 91, and 319 were studied at Necker-Enfants Malades (Paris, France) using C1/M1/C2 primers and seminested PCR as previously described,1 whereas patients UPN 1, 5, 7, 9, 11, and 12 were tested at Institut Paoli-Calmettes (Marseille, France) with C4 (5′-AGGCCTTTGAAGAGGCTGGG-3′ ) and M0 (5′-AGCGGGCCCTGGAGACCCAG-3′ ) primers in a single-round PCR followed by hybridization with an internal P3b CBFβ probe (5′-AGACAGGTCTCTCATCGGGAGG-3′ ) and autoradiography.6 The two laboratories achieved the same level of sensitivity (10−4 cellular equivalent ± 1 log) as controlled by dilution experiments of a positive control RNA (ME-1 cell line or patient diagnostic material) performed during each testing.
Detection of residual disease by RT-PCR in blood, cytapheresis product, or bone marrow. (*) Autologous transplantation; (#) allogeneic bone marrow transplantation; D, death; R, relapse; UPN, unique patient number; (•) RT-PCR–positive result in peripheral blood; (○) RT-PCR–negative result in peripheral blood; (▪) RT-PCR–positive result in cytapheresis product; (□) RT-PCR–negative result in cytapheresis product; (▮) RT-PCR–positive result in bone marrow; () RT-PCR–negative result in bone marrow; (1) weak positivity.
Detection of residual disease by RT-PCR in blood, cytapheresis product, or bone marrow. (*) Autologous transplantation; (#) allogeneic bone marrow transplantation; D, death; R, relapse; UPN, unique patient number; (•) RT-PCR–positive result in peripheral blood; (○) RT-PCR–negative result in peripheral blood; (▪) RT-PCR–positive result in cytapheresis product; (□) RT-PCR–negative result in cytapheresis product; (▮) RT-PCR–positive result in bone marrow; () RT-PCR–negative result in bone marrow; (1) weak positivity.
All patients achieved clinical and cytological complete remission (CR) after induction therapy (cytarabine and anthracycline-containing regimens). Residual positivity was observed in 52% (13 of 25) of samples taken within 8 months after diagnosis. Of the 6 patients analyzed before the second month, 5 showed strong PCR positivity, whereas only 1 patient was negative. This early RT-PCR positivity did not predict for early relapse. Molecular conversion to RT-PCR negativity within 8 months was observed in 6 patients, none of whom have relapsed, with a median follow-up of 18 months (range, 7 to 24 months), although 1 patient (UPN 9) died at 7 months after diagnosis from graft-versus-host disease. In contrast, 3 of the 4 patients remaining positive by 8 months have relapsed at 16, 22, and 8 months. We conclude that, in contrast to early (<2 months) PCR positivity, absence of PCR conversion within the first 8 months seems to be predictive for hematologic relapse and could represent an indication for more intensive therapy, if these data are confirmed in a larger series. The superior predictive value of CBFβ/MYH11 detection in AML in comparison with BCR/ABL in CML could be explained by its lower sensitivity, in keeping with results obtained for PML/RARα detection in acute promyelocytic leukemia7 and ALL-1/AF-4 in acute lymphoblastic leukemia.8
We also report the detection of CBFβ/MYH11 fusion transcripts in cytapheresis products in four patients who underwent peripheral blood stem cell (PBSC) mobilization using granulocyte colony-stimulating factor (G-CSF ) after the third course of chemotherapy. Three patients were RT-PCR negative in the cytapheresis products and are still in hematologic and molecular complete remission at 18 to 24 months from diagnosis, whereas the patient who showed clear RT-PCR positivity in the cytapheresis products experienced early relapse. These data correlate with PCR results obtained in peripheral blood independently from G-CSF PBSC mobilization, showing that growth factor use does not seem to recruit leukemic cell progenitors despite the in vitro proliferative effect on AML blasts. Moreover, these data suggest that RT-PCR analysis of CBFβ/MYH11 transcripts could be used to objectively assess blastic contamination and the efficacy of purging treatment in cytapheresis products.
Large prospective series and longer follow-up are required to more accurately determine the value of MRD detection by RT-PCR, using CBFβ-MYH11 fusion transcripts as the molecular marker, to refine prognosis and modulate postremission treatment modalities in accordance with the risk of relapse.
ACKNOWLEDGMENT
Supported in part by the “Association pour la Recherche contre le Cancer,” the “Ligues contre le Cancer des bouches-du-Rhône et du Var,” and the “Fédération Nationale des Centres de Lutte Contre le Cancer.”