Abstract
Nonreceptor protein tyrosine kinases phosphorylate proteins, thereby activating many intracellular signaling pathways and mediating protein-protein interactions. Protein phosphorylation is regulated in large part by the subcellular localization of these kinases and their respective substrates. Src is the most studied of these kinases, although other members of the Src family have been shown to be important in the differentiation of specific cell types. Src and Src family members are reported to be membrane-associated, but detergent-extraction studies have demonstrated a major difference in the solubility of Src compared with other members of the Src family (Fgr, Fyn, Lck, Lyn, and Yes), suggesting that their subcellular distributions may be different. By immunoelectron microscopy, we demonstrate that, unlike Src, the Src-related kinases are associated with electron-dense cytoplasmic domains and plasma membrane domains that correspond in size and frequency to endocytotic vesicles and coated pits. Clusters of labeling for these kinases also were seen adjacent to granule membranes. These kinases colocalize with the coated vesicle protein, clathrin, confirming their association with this class of endocytotic vesicle. We hypothesize that this vesicular association of Src-related kinases indicates a role for them in the endocytotic vesicle–mediated uptake and trafficking of plasma proteins into platelet granules.
ONE OR MORE MEMBERS of the Src family of nonreceptor tyrosine kinases are expressed in most cell types,1 but their roles in cell differentiation and function remain poorly defined. Knowledge of the subcellular distribution of these macromolecules can provide insights into their function in specific cells. The large number of Src family kinases expressed in platelets, including Fyn, Lyn, and Yes,2-5 together with our recent identification of Fgr and Lck,6 makes this cell an ideal model for such an investigation, but with the exception of Src, platelet ultrastructural localizations of Src family members have not been defined. Detergent-extraction studies indicate that in contrast to Src, the Src-related kinases are largely detergent-insoluble, suggesting a different subcellular localization of the Src-related kinases versus Src. Some subcellular fractionation studies of detergent-extracted cells have been used as evidence that the Src-related kinases are associated with caveolae,7-9 but this conclusion has been questioned because Src-related kinases in cell types lacking caveolae also are detergent-insoluble.10,11 Immunofluorescence studies of Lck in activated T cells suggested some endosomal localization, but Lck was on plasma membranes in unactivated T cells.10 Most of these studies have been on cell lines. Surprisingly, no one has applied immunogold electron microscopy to the question of Src-related kinase localization. Here, we used immunoelectron microscopy to define the subcellular localizations of the Src family of tyrosine kinases in platelets. We report that, unlike Src, the labeling for the Src-related kinases Fgr, Fyn, Lck, and Lyn is concentrated in clusters over electron-dense cytoplasmic domains and plasma membrane patches resembling endocytotic vesicles and coated pits, respectively. Clustered labeling also was present adjacent to granules. Labeling for these kinases colocalized with clathrin, indicating that they are associated with endocytotic vesicles.
MATERIALS AND METHODS
Briefly, 10 mL blood was collected from the abdominal aorta of metofane-anesthetized retired breeder Wistar rats obtained from Harlan Industries (Indianapolis, IN) or from the antecubital vein of human volunteers into acid-citrate-dextrose with 8 μmol/L PGE1 (Sigma Chemical Co, St Louis, MO) and immediately dripped into 10 vol 8% paraformaldehyde in 0.1 mol/L PIPES buffer (pH 7.2) and then fixed for 2 hours at room temperature. The red blood cells were then sedimented by centrifugation (750g for 20 minutes at room temperature), and the supernatant containing the platelets was removed. The platelets were pelleted by centrifugation (3,200g for 10 minutes at room temperature) and washed three times (2,300g for 10 minutes) with 0.1 mol/L PIPES buffer (pH 7.2).
Subsequently, they were prepared for cryosectioning and immunolabeling as previously described.12 Diluted antibodies were microfuged before use. Following incubation with primary antibody, the sections were rinsed and then incubated with either goat anti–rabbit IgG or goat anti–mouse IgG bridge antibodies that had been extensively cross-absorbed against the IgG of several other species, followed by rabbit anti–goat IgG conjugated to colloidal gold (10-nm probe). Similar results were obtained using protein A–gold in all experiments. Immunolabeling for Fgr was performed with a four-step procedure using goat anti–rabbit IgG and mouse anti–goat IgG as sequential bridge antibodies, followed by goat anti–mouse IgG conjugated to colloidal gold (10-nm probe). Double-labeling experiments were performed using the following sequence: polyclonal or monoclonal primary antibody, appropriate bridge antibody, immunogold probe (5 nm), blocking for 10 minutes in 1% glutaraldehyde in phosphate-buffered saline (PBS), polyclonal or monoclonal antibody, appropriate bridge antibody, immunogold probe (10 nm). Controls included reversing the order of the primary antibodies and substitution of primary antibodies with purified rabbit or mouse IgG.
Antibodies.Affinity-purified polyclonal antibodies to Fyn, Lck, Lyn, Src, Rap 1b, and caveolin were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Affinity-purified rabbit polyclonal antibodies to mouse Fgr were generously provided by Dr Clifford Lowell (University of California, San Francisco). Polyclonal antibodies to the following were generously supplied as indicated: Talin (Dr Mary Beckerle, University of Utah, Salt Lake City); P-selectin (Dr Michael Berndt, Baker Medical Research Institute, Monash University, Prahran, Victoria, Australia); glycoprotein IIb (GP IIb) and PECAM-1 (Dr Peter Newman, Blood Center of Southeastern Wisconsin, Milwaukee); and platelet factor-4 (PF-4) (Dr David Kuter, Massachusetts General Hospital, Boston). Rabbit polyclonal antisera to murine Lyn was a kind gift from Dr Tony DeMarco (University of California, San Francisco). Antifibrinogen antibodies were purchased from Accurate Chemical and Scientific Corp (Westbury, NY). Anti-Src monoclonal antibodies were generously provided by Dr Joan Brugge (ARIAD Pharmaceuticals, Cambridge, MA). Monoclonal antibodies to clathrin (monoclonal anti-clathrin heavy chain antibody, “X22”) were a kind gift from Dr Francis Brodsky (University of California, San Francisco). Monoclonal antibodies to tubulin were purchased from Amersham Life Science Inc (Arlington Heights, IL). Bridging antibodies (goat anti–mouse IgG, goat anti–rabbit IgG, and mouse anti–goat IgG) extensively cross-absorbed against IgGs of numerous species were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Immunogold probes were purchased from Jackson ImmunoResearch Laboratories, Sigma Chemical Co, and Amersham Life Sciences Inc.
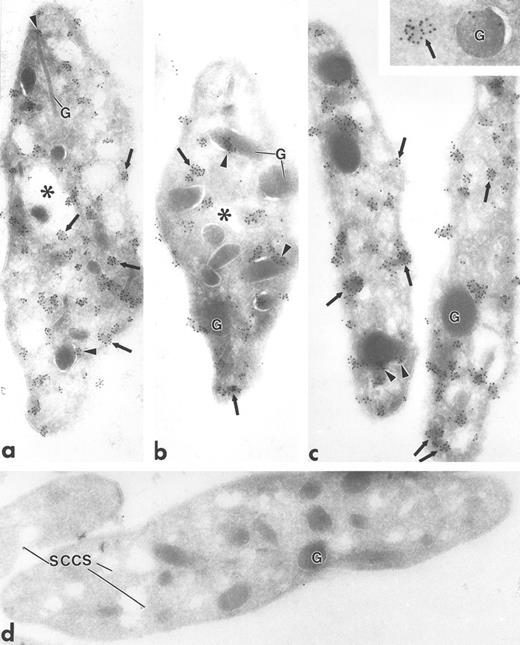
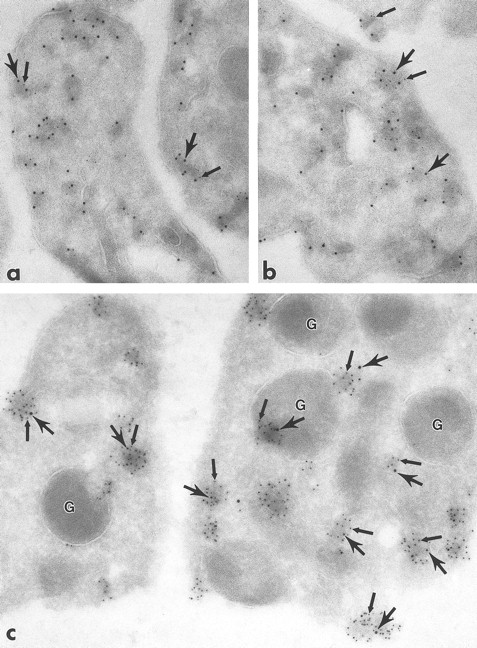
Ultrathin frozen sections of human (a and b), Wistar rat (c and d), and mouse platelets (inset) immunolabeled with polyclonal antibodies to Fyn (a), Lck (b and c), and Fgr (inset). (d) Section of a Wistar rat platelet incubated with Lck peptide–absorbed anti-Lck antibodies. Arrows indicate label for these Src-related kinases on platelet plasma membranes and electron-dense cytoplasmic patches. Arrowheads indicate clusters of label closely associated with alpha granule (G) membranes. Asterisks indicate lumena of the SCCS system, a channel system in continuity with the outside space. (a) Original magnification × 43,000; (b) × 43,000; (c) × 50,000; (d) × 46,000; (inset) × 70,000.
Ultrathin frozen sections of human (a and b), Wistar rat (c and d), and mouse platelets (inset) immunolabeled with polyclonal antibodies to Fyn (a), Lck (b and c), and Fgr (inset). (d) Section of a Wistar rat platelet incubated with Lck peptide–absorbed anti-Lck antibodies. Arrows indicate label for these Src-related kinases on platelet plasma membranes and electron-dense cytoplasmic patches. Arrowheads indicate clusters of label closely associated with alpha granule (G) membranes. Asterisks indicate lumena of the SCCS system, a channel system in continuity with the outside space. (a) Original magnification × 43,000; (b) × 43,000; (c) × 50,000; (d) × 46,000; (inset) × 70,000.
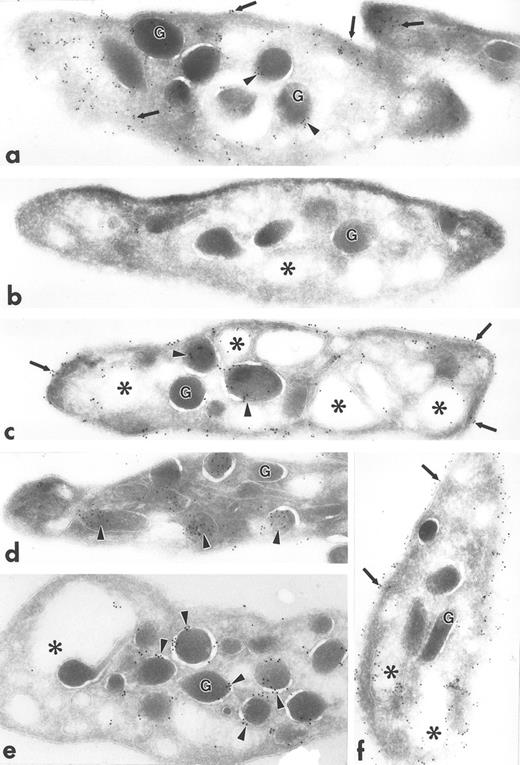
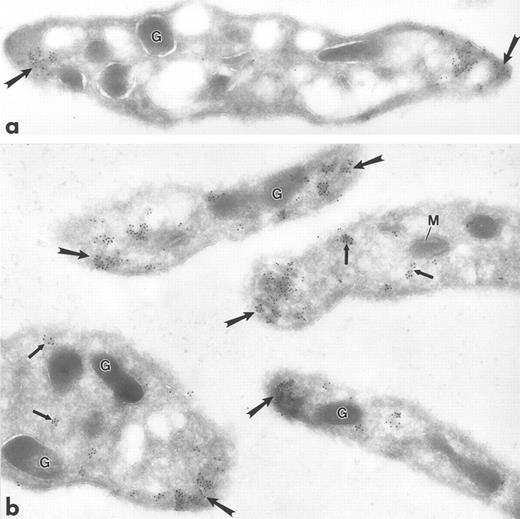
Ultrathin frozen sections of human (a) and Wistar rat (b) platelets isolated from fixed, whole blood and immunolabeled with monoclonal antibody to tubulin (a) or polyclonal antibody to Lyn (b). Tubulin is concentrated at the poles of the discoid platelet (arrows). Lyn is present on electron-dense cytoplasmic domains (small arrows) and is also concentrated at the poles of the discoid platelets (large arrows). G, granule; M, mitochondrion. Original magnification × 45,000.
Ultrathin frozen sections of human (a) and Wistar rat (b) platelets isolated from fixed, whole blood and immunolabeled with monoclonal antibody to tubulin (a) or polyclonal antibody to Lyn (b). Tubulin is concentrated at the poles of the discoid platelet (arrows). Lyn is present on electron-dense cytoplasmic domains (small arrows) and is also concentrated at the poles of the discoid platelets (large arrows). G, granule; M, mitochondrion. Original magnification × 45,000.
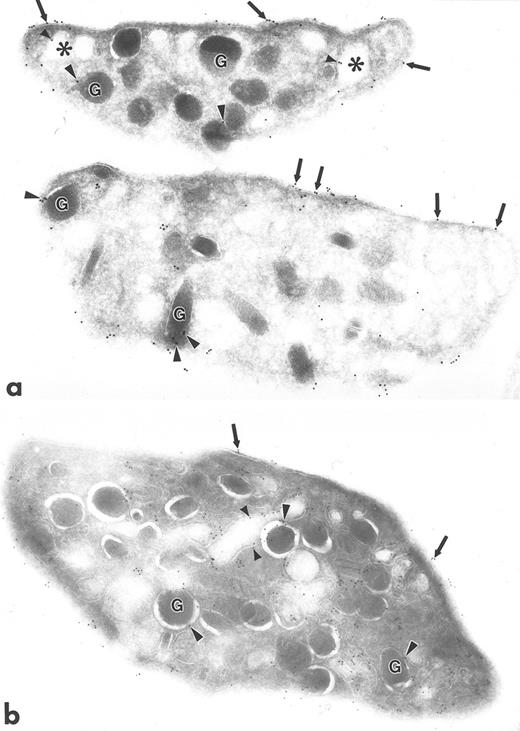
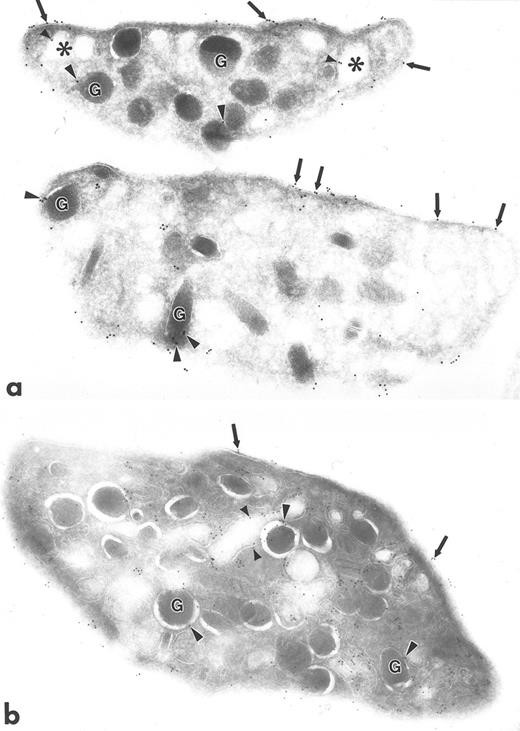
Ultrathin frozen sections of human platelets (a to f) immunolabeled with polyclonal antibodies against talin (a), caveolin (b), GP IIb (c), PF-4 (d), P-selectin (e), and PECAM-1 (f) using the 3-step labeling procedure. With the exception of caveolin, which is negative, each of these exhibits a unique labeling pattern that is unlike the clustered labeling pattern obtained with antibodies to the Src-related kinases seen in Fig 1a to d. Original magnification × 47,000.
Ultrathin frozen sections of human platelets (a to f) immunolabeled with polyclonal antibodies against talin (a), caveolin (b), GP IIb (c), PF-4 (d), P-selectin (e), and PECAM-1 (f) using the 3-step labeling procedure. With the exception of caveolin, which is negative, each of these exhibits a unique labeling pattern that is unlike the clustered labeling pattern obtained with antibodies to the Src-related kinases seen in Fig 1a to d. Original magnification × 47,000.
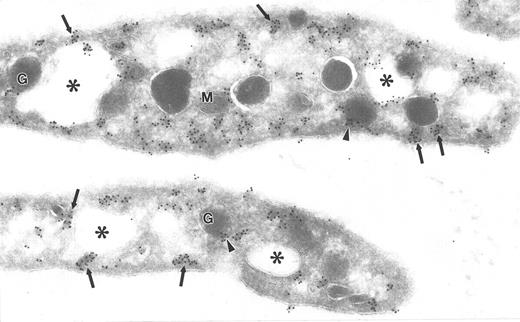
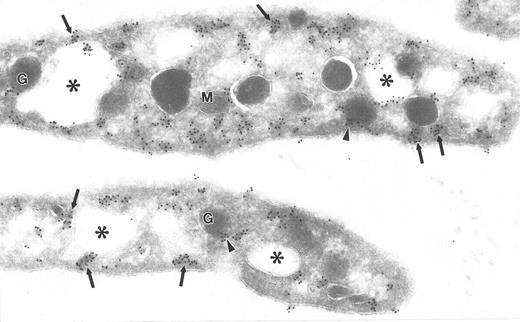
Ultrathin frozen sections of human platelets isolated from fixed, whole blood and immunolabeled with monoclonal antibody to Src (a) or with polyclonal antibody to Src (b). Arrows indicate label for Src on plasma membranes. Arrowheads indicate label on alpha granule (G) membranes and SCCS (⋅) membranes. Original magnification × 43,000.
Ultrathin frozen sections of human platelets isolated from fixed, whole blood and immunolabeled with monoclonal antibody to Src (a) or with polyclonal antibody to Src (b). Arrows indicate label for Src on plasma membranes. Arrowheads indicate label on alpha granule (G) membranes and SCCS (⋅) membranes. Original magnification × 43,000.
Ultrathin frozen section of human platelets immunolabeled with monoclonal antibodies to clathrin. Arrows indicate immunogold clusters overlying electron-dense cytoplasmic and subplasmalemmal patches. Arrowheads indicate label closely associated with alpha granules (G). Asterisks indicate lumena of the SCCS system. M, mitochondrion. Original magnification × 56,000.
Ultrathin frozen section of human platelets immunolabeled with monoclonal antibodies to clathrin. Arrows indicate immunogold clusters overlying electron-dense cytoplasmic and subplasmalemmal patches. Arrowheads indicate label closely associated with alpha granules (G). Asterisks indicate lumena of the SCCS system. M, mitochondrion. Original magnification × 56,000.
Ultrathin frozen sections of human platelets isolated as in Fig 1 and immunolabeled with monoclonal antibodies to clathrin followed by goat anti–mouse IgG bridge antibody and 5-nm immunogold particles. After blocking in 1% glutaraldehyde in PBS for 10 minutes, the sections were immunolabeled with polyclonal antibodies to Fyn, followed by goat anti–rabbit IgG bridge antibody and 10-nm immunogold particles. (a) and (b) Label for Fyn and clathrin are associated (small and large arrows, respectively). Similar results were obtained with protein A–gold or IgG-gold probes. In no double-label experiment was the amount of 10-nm gold probe the same as in a single label for the same primary antibody, probably due to steric hindrance of the gold particles. G, alpha granule. Original magnification × 70,000.
Ultrathin frozen sections of human platelets isolated as in Fig 1 and immunolabeled with monoclonal antibodies to clathrin followed by goat anti–mouse IgG bridge antibody and 5-nm immunogold particles. After blocking in 1% glutaraldehyde in PBS for 10 minutes, the sections were immunolabeled with polyclonal antibodies to Fyn, followed by goat anti–rabbit IgG bridge antibody and 10-nm immunogold particles. (a) and (b) Label for Fyn and clathrin are associated (small and large arrows, respectively). Similar results were obtained with protein A–gold or IgG-gold probes. In no double-label experiment was the amount of 10-nm gold probe the same as in a single label for the same primary antibody, probably due to steric hindrance of the gold particles. G, alpha granule. Original magnification × 70,000.
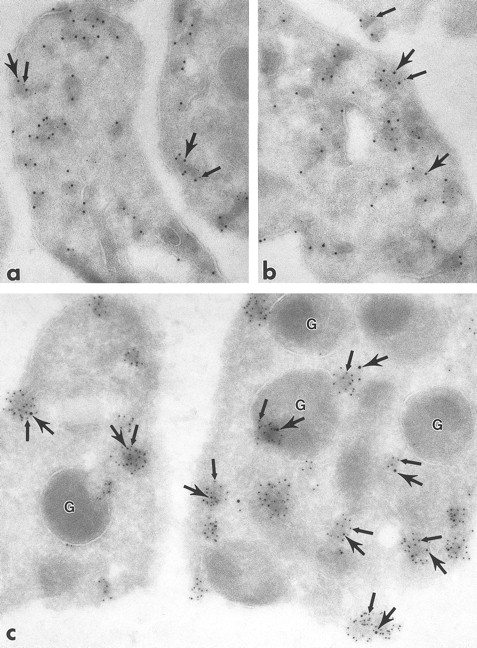
Ultrathin frozen sections of human platelets immunolabeled with monoclonal antibodies to clathrin followed by polyclonal antibodies to Lck (a and b) or with the reverse sequence of label (c). Sections were incubated with either monoclonal antibodies to clathrin (a and b) or polyclonal antibodies to Lck (c) followed by appropriate bridge antibody and 5-nm immunogold particles. After blocking in 1% glutaraldehyde in PBS for 10 minutes, the sections were immunolabeled with either polyclonal antibodies to Lck (a and b) or monoclonal antibodies to clathrin (c), followed by appropriate bridge antibody and 10-nm immunogold particles. In all figures, 5-nm gold particles are indicated by small arrows and 10-nm gold particles by large arrows. (a) to (c), Label for clathrin and Lck are associated. (c) Label for Lck is in clusters (small arrows) and clathrin is associated with these clusters (large arrows). Similar results were obtained with protein A–gold or IgG-gold probes. In no case was the level of 10-nm gold the same as in a single label for the same primary antibody, probably due to steric hindrance of the gold particles. G, granule. (a) Original magnification × 84,000; (b) × 84,000; (c) × 117,000.
Ultrathin frozen sections of human platelets immunolabeled with monoclonal antibodies to clathrin followed by polyclonal antibodies to Lck (a and b) or with the reverse sequence of label (c). Sections were incubated with either monoclonal antibodies to clathrin (a and b) or polyclonal antibodies to Lck (c) followed by appropriate bridge antibody and 5-nm immunogold particles. After blocking in 1% glutaraldehyde in PBS for 10 minutes, the sections were immunolabeled with either polyclonal antibodies to Lck (a and b) or monoclonal antibodies to clathrin (c), followed by appropriate bridge antibody and 10-nm immunogold particles. In all figures, 5-nm gold particles are indicated by small arrows and 10-nm gold particles by large arrows. (a) to (c), Label for clathrin and Lck are associated. (c) Label for Lck is in clusters (small arrows) and clathrin is associated with these clusters (large arrows). Similar results were obtained with protein A–gold or IgG-gold probes. In no case was the level of 10-nm gold the same as in a single label for the same primary antibody, probably due to steric hindrance of the gold particles. G, granule. (a) Original magnification × 84,000; (b) × 84,000; (c) × 117,000.
Polyclonal antibodies to Fyn and Lck were neutralized with the specific Fyn or Lck peptide, respectively, used to generate the antibodies. These were prepared according to the manufacturer's instructions (Santa Cruz Biotechnology).
RESULTS
Fgr, Fyn, Lck, and Lyn demonstrated a strikingly clustered pattern of immunogold labeling over electron-dense cytosolic domains of resting human and rodent platelets (Figs 1a to c and 2b). The electron-dense domains were smaller in size than platelet granules. The clusters of Fgr, Fyn, and Lck (Fig 1a to c) were evenly distributed throughout the platelets, and were not over recognizable organelles such as mitochondria, etc. In addition to the cytoplasmic clusters, all of these kinases were associated to variable degrees with the plasma membrane mainly as clusters, but also as single gold particles. Immunogold clusters for all of these kinases also were present immediately adjacent to alpha granule membranes. The labeling pattern for Lyn (Fig 2b) differed somewhat in that in addition to the clustered immunogold cytoplasmic pattern already described, gold particles were concentrated at the poles of discoid platelet profiles, similar to the labeling pattern obtained using monoclonal antibodies to tubulin (Fig 2a). Two different sources of anti-Lyn antibodies yielded similar results. Control sections incubated with purified rabbit or mouse IgG in place of primary antibody showed negligible label (not shown). Sections incubated with Fyn and Lck peptide–absorbed anti-Fyn or anti-Lck antibodies showed insignificant gold label, not above control levels (Fig 1d).
Polyclonal antibodies to other platelet proteins including talin (Fig 3a), membrane glycoproteins GP IIb (Fig 3c) and PECAM-1 (Fig 3f), alpha granule membrane protein P-selectin (Fig 3e), and alpha granule matrix proteins PF-4 (Fig 3d) and fibrinogen (not shown) yielded abundant immunogold labeling, but none showed a clustered pattern like that of the Src-related kinases. Talin was associated with platelet plasma, surface-connected canalicular system (SCCS), and alpha granule membranes and was present in the cytosol, not associated with identifiable organelles. GP IIb was localized to the plasma membrane and SCCS membranes, as well as to the inner leaflet of some alpha granule membranes. PECAM-1 was present extensively along plasma and SCCS membranes. PF-4 and fibrinogen were concentrated within alpha granules, but fibrinogen was present also to a lesser extent on plasma membranes, which was probably label for plasma fibrinogen bound to platelets fixed in whole blood. P-selectin was present almost exclusively on alpha granule membranes. Sections incubated with anti–Rap 1b antibodies showed label along plasma and SCCS membranes, and occasionally associated with alpha granule membranes (not shown). Sections incubated with anti-caveolin antibodies were almost completely devoid of gold label, consistent with our unpublished biochemical data (C.W.J., unpublished results, November 1995).
The subcellular localization of Src was distinctly different from that of the Src-related kinases. Src was not clustered; instead, it was associated with plasma membranes, with membranes of the SCCS, and to a lesser extent with alpha granule membranes, and demonstrated some diffuse, nonorganelle labeling (Fig 4), in agreement with previous reports.13 14 The labeling intensity for Src was greater using polyclonal antibodies (Fig 4b), although the pattern of labeling was similar to that obtained with monoclonal antibodies to Src (Fig 4a).
The electron-dense cytoplasmic domains containing immunolabel for the Src-related kinases resembled endocytotic vesicles in size and distribution. To ascertain if the cytoplasmic domains were indeed endocytotic vesicles, platelets were immunolabeled for the coated vesicle protein, clathrin. Clathrin label was present in clusters overlying electron-dense cytoplasmic domains, plasma membrane domains (coated pits), and immediately adjacent to alpha granule membranes, similar to the pattern for Src-related kinases (Fig 5). To determine if clathrin and specific Src-related kinases were colocalized, double-labeling experiments were performed. A significant portion of total clathrin and Fyn colocalized over these domains (Fig 6a and b), as did clathrin and Lck (Fig 7a to c), strongly suggesting that Src-related kinases are associated with coated pits and endocytotic vesicles. Control sections in which primary antibodies were replaced with purified rabbit or mouse IgG showed negligible gold label (not shown). This demonstration of Fyn and Lck with coated vesicles suggests that these proteins are involved in the uptake and incorporation of plasma proteins into platelet granules.
DISCUSSION
Six members (Src, Fgr, Fyn, Lck, Lyn, and Yes) of the Src family of tyrosine kinases are present in platelets.2-6 However, the roles of these kinases in platelet function have not been defined. To gain clues as to their possible functions, we have examined the subcellular localization of the Src family members Fgr, Fyn, Lck, Lyn, and Src in platelets using ultrastructural immunogold analysis. Fgr, Fyn, Lck, and Lyn all showed a striking clustered labeling pattern over electron-dense cytoplasmic domains, which was in sharp contrast to the association of Src with platelet plasma membranes, SCCS membranes, and alpha granule membranes. Some Fgr, Fyn, Lck, and Lyn label also was associated with the plasma membrane (coated pits), and some was clustered adjacent to alpha granule membranes. In addition, Lyn was concentrated at the platelet poles. The electron-dense domains labeled by the Fgr, Fyn, Lck, and Lyn antibodies resembled endocytotic vesicles. Double-labeling experiments performed with antibodies to clathrin and the Src family members Fyn and Lck demonstrated colocalization of these two Src family members with clathrin, indicating their association with endocytotic vesicles. The small size of the electron-dense domains does not allow us to distinguish between vesicle membrane and interior Fgr, Fyn, Lck, and Lyn label.
Platelets contain clathrin-coated vesicles,15-19 and actively endocytose a number of plasma proteins, including fibrinogen, by receptor-mediated endocytosis.20,21 These proteins are transported to and stored in alpha granules,20-24 and are released upon platelet activation at sites of vascular injury. In platelets, clathrin has been demonstrated immunocytochemically in the cytoplasm and on the cytoplasmic faces of alpha granule, SCCS, and plasma membranes, and has been shown to colocalize with the adhesive proteins von Willebrand factor, fibronectin, and fibrinogen.19 In contrast to other cells, platelet coated vesicles retain their coating during trafficking to and for a period after fusion with alpha granules.16 To our knowledge, this is the first reported association of Src-related kinases and clathrin-coated membranes in a nonactivated cell actively endocytosing plasma proteins. A previous immunofluorescence analysis of Lck localization in the Jurkat T-cell line revealed transposition of Lck from the plasma membrane to internal cytoplasmic vesicles upon activation by CD2 antibody, suggesting the possibility that Lck becomes associated with an endocytotic vesicle under these conditions.25 However, the type of vesicle was not characterized.
Clustered labeling for Fgr, Fyn, Lck, and Lyn was seen at the platelet surface and in the platelet interior, as was label for clathrin, suggesting that these kinases are associated with coated pits and are thus involved in the initial stages of endocytosis.
That the vesicles with which Fgr, Fyn, Lyn, and Lck are associated are involved in endocytotic transport to alpha granules is suggested by the location of some electron-dense domains bearing the Src family kinases, immediately adjacent to alpha granules. This immunogold labeling was also usually seen in clusters, and was never distributed in a pattern that would suggest alpha granule membrane association.
Detergent-extraction studies indicate that detergent-insoluble Fyn, Lyn, Lck, and Yes localize in the same sucrose density fractions as caveolae in studies of cell lines.7-9 It was concluded that these detergent-insoluble Src-related kinases were associated with caveolae.7 However, two observations suggest that this is not the case in platelets. First, our immunogold analyses demonstrated colocalization of Fyn and Lck with clathrin; caveolae do not have clathrin coats. Second, we did not detect the caveolar protein, caveolin, in platelets immunocytochemically or by Western blot analysis (Dr Carl Jackson, unpublished results). Caveolar association of the Triton-insoluble Src-related kinases also is questionable in other cell lineages, because these kinases are also Triton-insoluble in cells lacking caveolae or caveolin, such as lymphocytes and neuroblastoma cells.10,11 26
The different localization of Src versus the other Src family members studied here is consistent with differences in their detergent extractability.6,9,27-31 We found Fgr, Fyn, Lck, and Lyn in resting platelets to be mostly detergent-insoluble, whereas the majority of Src is detergent-soluble.6 This difference in detergent solubility is related to the presence of two cysteine residues in the N-terminus of Src-related kinases that are lacking in Src.32 At least one of these cysteine residues is palmitylated as a posttranslational modification, which facilitates the binding of these tyrosine kinases to detergent-insoluble membrane lipid domains. Src cannot be palmitylated because it lacks these cysteine residues.32 All of the Src family kinases can be myristylated near their N-terminus.32 It is unknown as to whether the detergent-insoluble portion of Src is made so by binding to a detergent-insoluble lipid domain via the myristate moiety.
Whether Fgr, Fyn, Lck, and Lyn play an active role in endocytosis or are merely transported with the vesicles cannot be determined from our analysis. However, one study provides evidence for involvement of Lck in the regulation of the rate of endocytosis. In that report, the rate of endocytosis of CD4 was decreased when CD4 and Lck were coexpressed in a cell line, but not when CD4 was expressed alone.33
NOTE ADDED IN PROOF
Polyclonal anti-Lck antibodies generously provided by Dr André Veillette (McGill University, Montreal, Quebec, Canada) showed a similar labeling pattern in platelets as that obtained with other anti-Lck antibodies used in this work.
Supported in part by Grant No. R01 HL51546 (C.W.J. and P.E.S.) from the National Heart, Lung, and Blood Institute, Cancer Center Support Grants No. P30 CA21765 and P01 CA20180 from the National Cancer Institute, Public Health Service, Department of Health and Human Services, and by American Lebanese Syrian Associated Charities.
Address reprint requests to Paula E. Stenberg, PhD, Department of Pathology L113, Oregon Health Sciences University, 3181 SW Sam Jackson Park Rd, Portland, OR 97201.