Abstract
HTK is a receptor tyrosine kinase of the Eph family. To characterize the involvement of HTK in hematopoiesis, we generated monoclonal antibodies against HTK and investigated its expression on human bone marrow cells. About 5% of the bone marrow cells were HTK+, which were also c-Kit+, CD34low, and glycophorin A−/low. Assays of progenitors showed that HTK+c-Kit+ cells consisted exclusively of erythroid progenitors, whereas HTK−c-Kit+ cells contained progenitors of granulocytes and macrophages as well as those of erythroid cells. Most of the HTK+ erythroid progenitors were stem cell factor-dependent for proliferation, indicating that they represent mainly erythroid burst-forming units (BFU-E). During the erythroid differentiation of cultured peripheral CD34+ cells, HTK expression was upregulated on immature erythroid cells that corresponded to BFU-E and erythroid colony-forming units and downregulated on erythroblasts with high levels of glycophorin expression. These findings suggest that HTK is selectively expressed on the restricted stage of erythroid progenitors, particularly BFU-E, and that HTK is the first marker antigen that allows the purification of erythroid progenitors. Furthermore, HTKL, the ligand for HTK, was expressed in the bone marrow stromal cells. Our findings provide a novel regulatory system of erythropoiesis mediated by the HTKL-HTK signaling pathway.
RECEPTOR TYROSINE kinases (RTKs) play an important role in pleiotropic cell functions such as cell proliferation and differentiation.1 RTKs transduce signals for diverse cell functions by phosphorylating tyrosines on themselves and other proteins in response to their ligands. RTKs form a large family of growth factor receptors, and some of them are involved in hematopoiesis. Colony-stimulating factor-1 (CSF-1) receptor/c-Fms and its ligand, macrophage colony-stimulating factor, participate in the proliferation and differentiation of monocytes, macrophages, and osteoclasts.2 The c-Kit receptor and its ligand, stem cell factor (SCF ), and Flt3/Flk2 and its ligand, FL, play important roles in the early stage of hematopoiesis.3,4 Recently, several novel RTKs have been isolated by means of the polymerase chain reaction (PCR) technique.5 Some of these receptors, eg, TIE or TEK, are expressed on hematopoietic stem cells and are assumed to be involved in early hematopoiesis.6 7
RTKs are classified into many subfamilies according to their structural characteristics. The Eph family composes the largest subfamily of RTKs, and it is further categorized into Eck-related (Eck, Ehk1, Ehk2, Ehk3/MDK-1, Sek1, Hek, and Eph) and Elk-related kinases (Elk, Nuk, Hek2, and HTK).8,9 Ligands for the Eph family receptors are also categorized into glycosylphosphatidylinositol-anchored (B61, ELF-1, AL-1/RAGS, Ehk1-L/LERK3, and LERK4) and transmembrane proteins (Elk-L/LERK2 and HTKL). These groupings correlate well with the binding specificities of the ligands to their receptors. The glycosylphosphatidylinositol-anchored ligands bind to the Eck-related RTKs, whereas the transmembrane ligands bind to the Elk-related RTKs. Some biologic functions of the Eph family receptors and ligands have been elucidated. AL-1/RAGS and ELF-1, the ligands for Ehk1/Rek7 and Hek/Mek4, respectively, contribute to the formation of neuronal pathways from the retina to the tectum during chick brain development10,11 and to axon bundle formation in vitro.12 B61, the ligand for Eck, plays a role in tumor necrosis factor-α–induced angiogenesis and the migration of endothelial cells.13 These findings suggest that the Eph family receptors and their ligands play important roles in the formation of spatial boundaries that may be involved in organizing the developing body plan.
HTK is an Elk-related kinase that was isolated from a human hepatoma cell line, Hep3B, and from human CD34+ bone marrow mononuclear cells.14 We also isolated HTK from a human immature hematopoietic cell line, UT-7.15 HTK has a cysteine-rich region and two fibronectin type III repeats in the extracellular domain and a single uninterrupted catalytic domain in the intracellular domain.14 Unlike other members of the Eph family that are predominantly expressed in the nervous system, HTK is expressed broadly in other tissues.14 In the hematopoietic system, HTK is expressed on a subset of monocytic cells of human cord blood.16 However, the precise meaning of HTK on these cells has not been determined. To understand the functional role of HTK in the hematopoietic system further, we established monoclonal antibodies (MoAbs) against human HTK and characterized its expression on bone marrow mononuclear cells. We found that HTK was selectively expressed on bone marrow erythroid progenitors, especially on erythroid burst-forming units (BFU-E). In addition, HTKL, the ligand for HTK, was expressed in stromal cells of human bone marrow. Our findings suggested that HTK and its ligand function as a novel regulatory system of human erythropoiesis.
MATERIALS AND METHODS
Cell lines.The human megakaryoblastic cell line, UT-7,17 was maintained in Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Inc, Grand Island, NY) containing 10% fetal calf serum (FCS; CSL Ltd, Parkville, Australia) and 2 U/mL recombinant human erythropoietin (Epo; provided by Snow Brand Milk Products Co, Tochigi, Japan).
Preparation of human bone marrow and peripheral blood cells.Human bone marrow and peripheral blood cells were obtained from normal healthy volunteers who had given informed consent. Cells diluted with phosphate-buffered saline (PBS) were centrifuged over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) for 30 minutes at 400g. Mononuclear cells recovered from the interface were resuspended in staining medium consisting of 5% FCS and 0.01% sodium azide in PBS.
Northern blotting.Total RNA was extracted from cell lines using acid guanidinium thiocyanate-phenol-chloroform, and poly(A)+ RNA was selected using Oligotex-dT30 (Takara Shuzo, Shiga, Japan). Two micrograms of poly(A)+ RNA was loaded onto a formaldehyde gel, which was then Northern-blotted using a Zeta-Probe membrane (Bio-Rad, Richmond, CA). Human lymphohematopoietic tissues were Northern-blotted using a Human Multiple Tissue Northern Blot (Clontech, Palo Alto, CA) containing 2 μg of poly(A)+ RNA per lane. Hybridization and washes were performed according to the manufacturer's instructions. The 746-bp Sma I-Sma I fragment derived from human HTK cDNA and the 200-bp EcoRI-EcoRI fragment derived from human HTKL cDNA were [α-32P]dCTP–labeled using a Megalabel kit (Amersham International plc, Buckinghamshire, UK) and hybridized to the blot.
Detection of HTKL mRNA by reverse transcription (RT)-PCR.Total RNA was extracted as above. The first-strand cDNA was synthesized from 2 μg of total RNA using Superscript II reverse transcriptase and random hexamer oligonucleotides (Life Technologies, Inc). Amplification by PCR was performed using 10% of the cDNA as a template. The cycling parameters were 1 minute at 94°C, 2 minutes at 58°C, and 3 minutes at 72°C for 40 cycles. The primer sequences of HTKL were as follows: sense, 5′-AGACCAAGCAGACAGATGCAC-3′; and antisense, 5′-GTTGATCCAGCAGAACTTGCA-3′. Human β-actin was used as a reference gene. The primer sequences were as follows: sense, 5′-CTGGACTTCGAGCAAGAGAT-3′; and antisense, 5′-TCGTCATACTCCTGCTTGCT-3′. Five percent of these reaction mixtures was resolved by electrophoresis on a 2% agarose gel and stained with ethidium bromide.
Expression of HTK protein.A partial cDNA encoding the entire extracellular domain of HTK was fused in-frame to human IgG1Fc (HTKex-Fc). This HTKex-Fc cDNA was inserted into pMKITNeo (provided by Dr K. Maruyama, Tokyo Medical and Dental University, Tokyo, Japan) and then transfected into COS7 cells by electroporation for transient production. HTKex-Fc protein was purified from the conditioned medium of transfected COS7 cells using a protein G-Sepharose column (Pharmacia Biotech). A partial cDNA encoding the entire extracellular domain of HTK was tagged with a FLAG octapeptide (DYKDDDDK; HTKex-FLAG) and inserted into pMKITNeo. HTKex-FLAG protein was purified from the conditioned medium of transfected COS7 cells using the anti-FLAG MoAb M2 affinity gel (Eastman Kodak, New Haven, CT). A full-length HTK cDNA was tagged with a FLAG octapeptide (HTK-FLAG) and inserted into pMKITNeo, which was introduced into Ba/F3 cells by electroporation. Cells were selected in the presence of G418 (Wako Pure Chemical Industries Ltd, Osaka, Japan). Among several resistant clones, that with the highest level of HTK expression (BaF3/HTK) was selected by Western blotting with the anti-FLAG MoAb M2. Ba/F3 cells transfected with vector alone (BaF3/vector) served as negative controls.
Generation of MoAb and polyclonal antibody (PoAb) against HTK.Hybridomas were produced by fusing mouse myeloma cells with spleen cells from Balb/c mice immunized with purified HTKex-FLAG protein. Three positive hybridomas were selected by cells by fluorescence-activated cell sorter (FACS; Becton Dickinson Immunocytometry Systems, San Jose, CA), using the BaF3/HTK cells as indicators. The antibody produced by one of the hybridomas, designated 38-1E (IgG2a, κ), was used in this study. Mouse anti-HTK PoAb was raised in mice immunized with purified HTKex-FLAG protein.
Preparation of Antibody-Sepharose.Fifteen microliters of protein G-Sepharose was incubated with 3 μg of anti-HTK MoAb or isotype-matched mouse control IgG (antitrinitrophenol, IgG2a, κ; Pharmingen, San Diego, CA) in lysis buffer (10 mmol/L Tris [pH 7.8], 1% Triton X-100, 150 mmol/L NaCl, 1 mmol/L EDTA, 50 μg/mL aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride, 100 μmol/L leupeptin, and 25 μmol/L pepstatin A) for 1 hour at 4°C with gentle rotation.
Immunoprecipitaion and Western blotting.Cells were lysed on ice in lysis buffer. Cell lysates were cleared by centrifugation for 15 minutes at 15,000g at 4°C and then gently rotated with anti-HTK MoAb-Sepharose for 2 hours at 4°C. Immunoprecipitates were washed with cold lysis buffer and boiled for 3 minutes in sample buffer (50 mmol/L Tris [pH 6.8], 1% sodium dodecyl sulfate [SDS], 10% glycerol, 1 mmol/L dithiothreitol, and 0.008% bromophenol blue) with 5% 2-mercaptoethanol (2-ME). Proteins were separated on a 6.5% polyacrylamide gel and transferred onto polyvinylidene fluoride membranes (Nihon Millipore Ltd, Tokyo, Japan). Membranes were incubated for 2 hours at room temperature in 5% bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO) dissolved in TBS-T (20 mmol/L Tris [pH7.4], 150 mmol/L NaCl, 0.1% Tween-20). Membranes were washed and then incubated for 1 hour at room temperature with anti-HTK PoAb, followed by 1 hour at room temperature with horseradish peroxidase (HRP)-conjugated sheep antimouse Ig antibody (Amersham International plc). Proteins were visualized using the ECL detection system (Amersham International plc).
Cell surface biotinylation.BaF3/HTK and BaF3/vector cells were biotinylated using the ECL protein biotinylation system (Amersham International plc). Briefly, 1 × 107 cells in PBS were labeled with biotinylation reagent on ice for 30 minutes. The cells were washed twice with RPMI medium 1640 (Life Technologies, Inc) and incubated for 10 minutes in TBS (20 mmol/L Tris [pH 7.4], 150 mmol/L NaCl). The cells were lysed and then precleared with the control IgG-Sepharose. Proteins were immunoprecipitated with anti-HTK MoAb-Sepharose in RIPA buffer (1% sodium deoxycholate and 0.1% SDS in lysis buffer) overnight. Immune complexes were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) with 10% polyacrylamide. Proteins were transferred onto a polyvinylidene fluoride membrane, probed with HRP-conjugated streptavidin (Amersham International plc), and visualized with the ECL detection system.
FACS analysis and cell sorting.Before staining, Fc receptors on cells were blocked with 5 μg/106cells of mouse IgG2a, κ (antitrinitrophenol) for 30 minutes on ice. Mononuclear cells were stained with biotinylated anti-HTK MoAb, followed by allophycocyanin-conjugated streptavidin (Caltag Laboratories, San Francisco, CA) with combinations of the following antibodies: phycoerythrin (PE)-conjugated anti-CD14 MoAb (Dakopatts, Glostrup, Denmark), PE-conjugated anti-CD33 MoAb (Pharmingen), fluorescein isothiocyanate (FITC)-conjugated NU-4A1 (anti-CD34 MoAb; Nichirei Corp, Tokyo, Japan), FITC-conjugated NU-c-Kit (anti-c-Kit MoAb, Nichirei Corp), or FITC-conjugated anti-glycophorin A MoAb (Immunotech, Marseille, France). The cells were suspended in the staining medium containing propidium iodide. FACS analysis and cell sorting were performed on a FACS Vantage (Becton Dickinson Immunocytometry Systems) equipped with 488 nm argon and 632 nm He/Ne lasers. Data from more than 10,000 mononuclear cells were collected and analyzed using LYSYS II software on a CONSORT 32 system (Becton Dickinson Immunocytometry Systems). The fluorescence intensity of individual cells was measured as relative fluorescence units. Residual erythrocytes and dead cells were gated out using forward and side scatter channels and by propidium iodide staining at the time of data analysis and sorting. Hematopoietic cell lines were stained with biotinylated anti-HTK MoAb followed by FITC-conjugated streptavidin (Life Technologies, Inc), as described above.
Blocking specific binding of anti-HTK antibodies.In the FACS and Western blotting analyses, specific binding of anti-HTK MoAb and PoAb to HTK protein was blocked by incubating the antibodies with a 50-fold excess molar of HTKex-Fc for 1 hour at room temperature before staining.
Colony assay.Sorted cells were plated in methylcellulose medium.18 Briefly, cells were embedded in 1 mL of 1.3% methylcellulose (1,500 centipoise; Aldrich Chemical Co, Milwaukee, WI), 30% FCS, 1% deionized BSA, 50 μmol/L 2-ME, 2 U/mL human Epo, 100 ng/mL human SCF, and 20 ng/mL human interleukin-3 (IL-3) in IMDM. SCF and IL-3 were provided by Amgen Inc (Thousand Oaks, CA) and Sandoz Pharma Ltd (Basel, Switzerland), respectively. The culture dishes were incubated in a humidified atmosphere at 37°C with 5% CO2 .
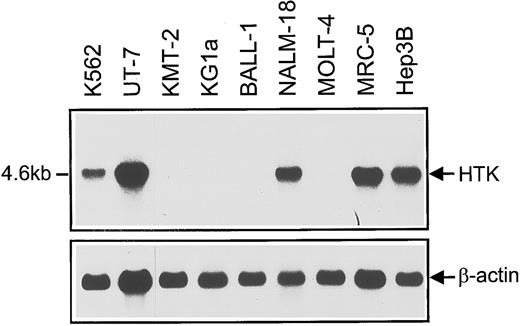
Northern blots of HTK expression in hematopoietic and nonhematopoietic control cell lines (MRC-5 and Hep3B). Poly(A)+ RNA (2 μg) was loaded on each lane. The Sma I-Sma I fragment of HTK cDNA (746 bp) was used as a probe. Loading was normalized using a β-actin probe.
Northern blots of HTK expression in hematopoietic and nonhematopoietic control cell lines (MRC-5 and Hep3B). Poly(A)+ RNA (2 μg) was loaded on each lane. The Sma I-Sma I fragment of HTK cDNA (746 bp) was used as a probe. Loading was normalized using a β-actin probe.
Culture of bone marrow stromal cells.Briefly, 5 × 106 bone marrow cells were suspended in 5 mL of IMDM containing 12.5% FCS, 12.5% horse serum (Summit Biotechnology, Greeley, CO), 50 μmol/L 2-ME, and 1 μmol/L hydrocortisone (Japan Upjohn, Tokyo, Japan) and were cultured for 3 to 5 weeks.
The in vitro proliferation and differentiation of erythroid progenitors purified from peripheral blood.The method is described elsewhere,19 but we applied a minor modification. In brief, human peripheral CD34+ blood cells were obtained by a multistep purification including Ficoll-Paque density centrifugation, platelet depletion by density centrifugation using 10% BSA, adherent cell depletion by nylon-fiber, and positive selection with anti-CD34 (My10) MoAb using immunomagnetic microspheres and chymopapain treatment. To expand and generate differentiated erythroid progenitors, the purified CD34+ cells were cultured in the liquid phase as described.20 In brief, the purified cells were suspended at various concentrations ranging from 1,000 to 5,000 cells/mL in 5 mL IMDM containing 20% FCS, 10% pooled human AB serum, 1% deionized BSA, 50 μmol/L 2-ME, 10 μg/mL insulin (porcine, activity 26.3 US Pharmacopoeia U/mg; Calbiochem, Behring Diagnostics, La Jolla, CA), 2 U/mL Epo, 50 U/mL IL-3, and 50 ng/mL SCF in a 25-cm2 culture flask. After incubation for the indicated periods at 37°C in a 5% CO2 atmosphere, the cells were collected, washed twice, and stored at 4°C.
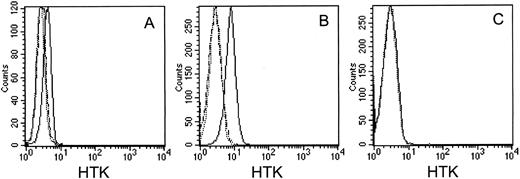
FACS analysis of HTK expression on cell lines with MoAb, 38-1E, against the extracellular domain of HTK. (A) UT-7 cells, (B) BaF3/HTK cells, and (C) BaF3/vector cells. The cells were stained with biotinylated 38-1E (solid lines) or biotinylated 38-1E that had been incubated with a 50-fold excess molar of HTKex-Fc (broken lines), followed by FITC-conjugated streptavidin. The dotted line indicates negative controls stained with FITC-conjugated streptavidin only.
FACS analysis of HTK expression on cell lines with MoAb, 38-1E, against the extracellular domain of HTK. (A) UT-7 cells, (B) BaF3/HTK cells, and (C) BaF3/vector cells. The cells were stained with biotinylated 38-1E (solid lines) or biotinylated 38-1E that had been incubated with a 50-fold excess molar of HTKex-Fc (broken lines), followed by FITC-conjugated streptavidin. The dotted line indicates negative controls stained with FITC-conjugated streptavidin only.
RESULTS
Expression of HTK in hematopoietic cell lines.Northern blots showed that a 4.6-kb HTK mRNA was expressed in the hematopoietic cell lines K562, UT-7 (erythromegakaryoblastic), and NALM-18 (pre-B), but not in KMT-2, KG1a (myeloid), BALL-1 (B), and MOLT-4 (T) (Fig 1). We previously showed that the HTK expression in erythromegakaryoblastic cell lines is markedly downregulated when megakaryocytic differentiation is induced by phorbol myristate acetate.21 On the other hand, Bennett et al14 have reported that HTK is expressed in human monocytic cell lines. These findings indicated that the expression of HTK is restricted to some hematopoietic cell lineages.
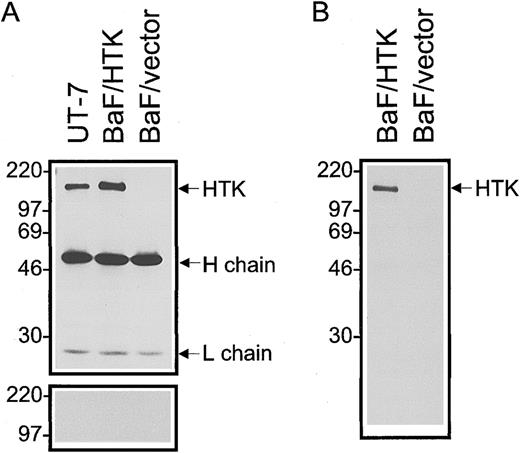
Characterization of anti-HTK MoAb.FACS analysis showed that the anti-HTK MoAb, 38-1E, reacted with UT-7 and BaF3/HTK cells that express HTK, but not with BaF3/vector control cells (Fig 2A through C). To confirm the specific reactivity of 38-1E with the HTK protein, we stained cells with 38-1E that had been incubated with a 50-fold excess molar of HTKex-Fc protein. This soluble HTK protein completely blocked the binding of 38-1E to UT-7 and BaF3/HTK cells (Fig 2A and B). The MoAb, 38-1E, immunoprecipitated a protein with a molecular weight of 120 kD from lysates of UT-7 and BaF3/HTK cells, but not of BaF3/vector cells (Fig 3A). An excess amount of HTKex-Fc protein completely blocked the reactivity of mouse anti-HTK PoAb with the 120-kD protein in Western blotting (Fig 3A), indicating that the 120-kD protein is HTK protein. No other major protein was detected by cell surface biotinylation followed by immunoprecipitation with 38-1E (Fig 3B). These data confirmed that 38-1E specifically reacts with the HTK protein on the cell surface.
Detection of HTK protein in cell lines by Western blotting. (A) Proteins were immunoprecipitated from lysates of UT-7, BaF3/HTK, and BaF3/vector cells with anti-HTK MoAb, 38-1E. The immunoprecipitates were separated by SDS-PAGE (10% polyacrylamide) under reducing conditions, transferred, and probed with anti-HTK PoAb (upper column). The membrane was reprobed with anti-HTK PoAb that had been incubated with a 50-fold excess molar of HTKex-Fc (lower column). (B) Proteins in the cell lysates of surface-biotinylated cell lines were immunoprecipitated with 38-1E. Immune complexes were separated by SDS-PAGE (10% polyacrylamide) under reducing conditions, transferred, and probed with HRP-conjugated streptavidin. The positions of the molecular mass markers (in kilodaltons) are indicated on the left.
Detection of HTK protein in cell lines by Western blotting. (A) Proteins were immunoprecipitated from lysates of UT-7, BaF3/HTK, and BaF3/vector cells with anti-HTK MoAb, 38-1E. The immunoprecipitates were separated by SDS-PAGE (10% polyacrylamide) under reducing conditions, transferred, and probed with anti-HTK PoAb (upper column). The membrane was reprobed with anti-HTK PoAb that had been incubated with a 50-fold excess molar of HTKex-Fc (lower column). (B) Proteins in the cell lysates of surface-biotinylated cell lines were immunoprecipitated with 38-1E. Immune complexes were separated by SDS-PAGE (10% polyacrylamide) under reducing conditions, transferred, and probed with HRP-conjugated streptavidin. The positions of the molecular mass markers (in kilodaltons) are indicated on the left.
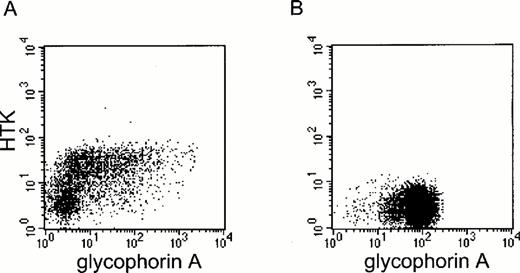
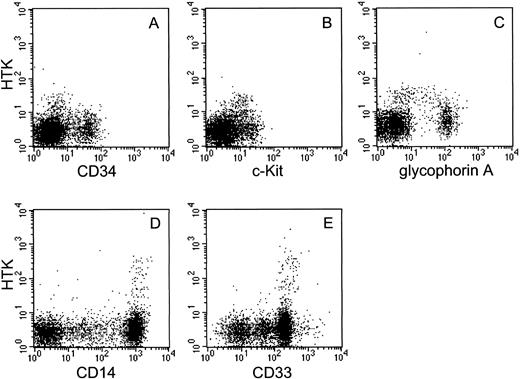
Expression of HTK on human bone marrow and peripheral blood cells.We investigated HTK expression on primary hematopoietic cells by FACS analysis using the anti-HTK MoAb, 38-1E, in combination with MoAbs against hematopoietic marker antigens. About 5% of the bone marrow mononuclear cells expressed HTK. The two-color analysis showed that HTK+ cells also expressed c-Kit and a low level of CD34 (Fig 4A and B). These expression profiles of HTK+ cells were confirmed by a three-color analysis of HTK, c-Kit, and CD34, which showed that HTK+c-Kit+ cells also expressed a low level of CD34 (data not shown). Most HTK+ cells did not express the lineage-specific marker antigens CD3, CD14, CD19, CD20, and CD33 (data not shown), but approximately 43% of them expressed a low level of glycophorin A (Fig 4C). A two-color analysis of peripheral blood cells showed that HTK was expressed on a subset of CD14+ (3.4%) and CD33+ cells (3.9%) (Fig 4D and E).
Two-color analysis of human bone marrow and human peripheral blood cells. Human bone marrow mononuclear cells were stained with biotinylated anti-HTK MoAb, 38-1E, and (A) anti-CD34, (B) anti-c-Kit, or (C) anti-glycophorin A. Peripheral blood cells were also stained with 38-1E and (D) anti-CD14 or (E) anti-CD33.
Two-color analysis of human bone marrow and human peripheral blood cells. Human bone marrow mononuclear cells were stained with biotinylated anti-HTK MoAb, 38-1E, and (A) anti-CD34, (B) anti-c-Kit, or (C) anti-glycophorin A. Peripheral blood cells were also stained with 38-1E and (D) anti-CD14 or (E) anti-CD33.
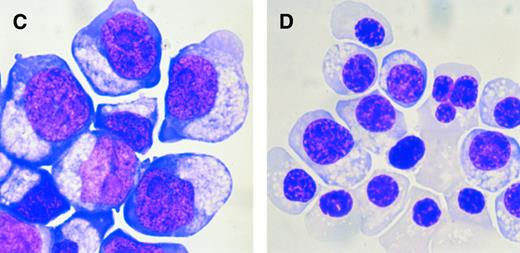
Cytochemical characterization of HTK+ bone marrow cells.We sorted HTK+ bone marrow cells using a FACS Vantage and examined their morphology. HTK+ cells showed a blastic appearance, some of which tended to differentiate towards the erythroid lineage (Fig 5). Cytochemical analysis showed that HTK+ cells were negative for myeloperoxidase, periodic acid-Schiff's, naphthol AS-D chloroacetate, and α-naphthyl butyrate staining (data not shown). These results indicated that the HTK+ cells are immature. On the other hand, HTK+ cells in the peripheral blood were typically monocytic in appearance (data not shown).
The morphology of sorted HTK+ cells. HTK+c-Kit+ cells in human bone marrow mononuclear cells were sorted by FACS Vantage (May-Grünwald-Giemsa staining, original magnification × 400).
The morphology of sorted HTK+ cells. HTK+c-Kit+ cells in human bone marrow mononuclear cells were sorted by FACS Vantage (May-Grünwald-Giemsa staining, original magnification × 400).
Progenitor assay of sorted bone marrow HTK+ cells.To assess colony-forming ability, sorted HTK+ cells were cultured in the presence of hematopoietic growth factors. As shown in Fig 4A, HTK+ cells also expressed c-Kit. Thus, we divided the c-Kit+ cell fraction into HTK+ and HTK− fractions and compared their colony-forming ability (Fig 6A). The HTK−c-Kit+ fraction formed various kinds of colonies derived from granulocyte-macrophage colony-forming units (CFU-GM), erythroid colony-forming units (CFU-E), BFU-E, and mixed colony-forming units (CFU-MIX). On the other hand, HTK+c-Kit+ cells formed predominantly erythroid colonies and bursts in the presence of IL-3, SCF, and Epo. About 2.8% and 3.7% of the HTK+c-Kit+ cells showed colony formation on days 7 and 14 of culture, respectively. Generally, HTK+c-Kit+ cells formed smaller erythroid bursts than HTK−c-Kit+ cells (data not shown). Sequential observation showed that 85% of the erythroid colonies on day 7 proliferated up to day 14 and only 15% of them disappeared. Notably, HTK+c-Kit+ cells barely formed erythroid colonies in the absence of SCF or Epo (Fig 6B). These findings suggested that HTK+c-Kit+ cells consist exclusively of BFU-E. We divided HTK+ cells into glycophorin A− and glycophorin Alow fractions and compared their colony-forming ability. The HTK+glycophorin Alow cells did not form colonies, whereas the HTK+glycophorin A− cells formed more than HTK+c-Kit+ cells. About 4.4% and 4.9% of the HTK+glycophorin A− cells formed colonies on days 7 and 14 of culture, respectively.
Colony formation of sorted bone marrow cells. Fractionated bone marrow cells were cultured in methylcellulose medium containing the following factors: (A) IL-3 + SCF + Epo; (B) IL-3 + SCF + Epo (FULL), IL-3 + Epo [SCF(−)], or IL-3 + SCF [Epo(−)]. Five thousand cells of each fraction (HTK−c-Kit+ and HTK+c-Kit+) were plated. The numbers of colonies were counted on days 7 and 14. Bars show the mean of duplicate culture dishes. E, GM, or MIX indicate colonies derived from CFU-E/BFU-E, CFU-GM, or CFU-MIX, respectively. Results are representative of at least seven independent experiments.
Colony formation of sorted bone marrow cells. Fractionated bone marrow cells were cultured in methylcellulose medium containing the following factors: (A) IL-3 + SCF + Epo; (B) IL-3 + SCF + Epo (FULL), IL-3 + Epo [SCF(−)], or IL-3 + SCF [Epo(−)]. Five thousand cells of each fraction (HTK−c-Kit+ and HTK+c-Kit+) were plated. The numbers of colonies were counted on days 7 and 14. Bars show the mean of duplicate culture dishes. E, GM, or MIX indicate colonies derived from CFU-E/BFU-E, CFU-GM, or CFU-MIX, respectively. Results are representative of at least seven independent experiments.
Expression of HTK during erythroid differentiation.We purified immature BFU-E from peripheral blood using a multistep procedure including a positive selection with anti-CD34 MoAb19 20 and analyzed HTK expression during their differentiation in vitro. Peripheral blood contains mostly immature BFU-E and few mature BFU-E or CFU-E. Therefore, this procedure selectively enriches immature BFU-E. On day 6 of culture, the cells consisted of mainly immature erythroid cells. The progenitor assay showed that they contained mostly BFU-E or CFU-E (data not shown). About 50% of these immature erythroid cells were HTK−glycophorin A−. On the other hand, the remaining glycophorin A− as well as glycophorin Alow/+ cells expressed HTK (Fig 7A and C). On day 14 of culture, when the cells differentiated into mature erythroblasts with a high level of glycophorin A expression, none of these cells expressed HTK (Fig 7B and D). These studies indicated that HTK expression is precisely regulated during erythroid differentiation.
Expression of HTK on cultured erythroid cells. Purified immature BFU-E from human peripheral blood were cultured. The expression of HTK and glycophorin A on cultured cells was analyzed by FACS on (A) day 6 and (B) day 14. The morphology of cultured cells was also examined on (C) day 6 and (D) day 14 (May-Grünwald-Giemsa staining, original magnification × 400).
Expression of HTK on cultured erythroid cells. Purified immature BFU-E from human peripheral blood were cultured. The expression of HTK and glycophorin A on cultured cells was analyzed by FACS on (A) day 6 and (B) day 14. The morphology of cultured cells was also examined on (C) day 6 and (D) day 14 (May-Grünwald-Giemsa staining, original magnification × 400).
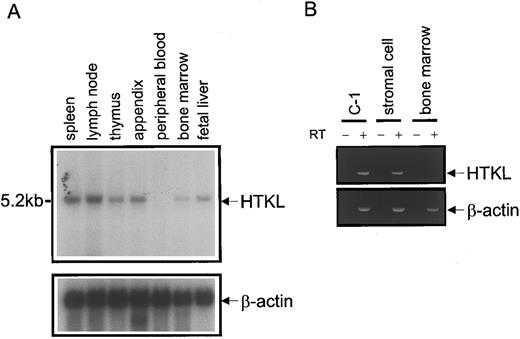
Expression of HTKL in hematopoietic system.Northern blots showed that HTKL mRNA was expressed in several human lymphohematopoietic tissues including bone marrow, but not in peripheral blood (Fig 8A). To determine which component of bone marrow expresses HTKL mRNA, we further analyzed the expression of HTKL mRNA by RT-PCR. The colon cancer cell line, C-1, that expresses HTKL was similarly processed as a positive control. HTKL mRNA was detected in bone marrow stromal cells, but not in bone marrow mononuclear cells (Fig 8B).
Expression of HTKL in lymphohematopoietic tissues. (A) Northern blots of HTKL expression in lymphohematopoietic tissues. The EcoRI-EcoRI fragment of HTKL cDNA (200 bp) was used as a probe. Loading was normalized using a β-actin probe. (B) RT-PCR analysis of HTKL mRNA. RT-PCR was performed on C-1 (positive control), bone marrow stromal, and bone marrow mononuclear cells with or without reverse transcriptase (RT + or RT −, respectively). Human β-actin was used as a reference gene. The PCR products were resolved by electrophoresis on a 2% agarose gel and stained with ethidium bromide.
Expression of HTKL in lymphohematopoietic tissues. (A) Northern blots of HTKL expression in lymphohematopoietic tissues. The EcoRI-EcoRI fragment of HTKL cDNA (200 bp) was used as a probe. Loading was normalized using a β-actin probe. (B) RT-PCR analysis of HTKL mRNA. RT-PCR was performed on C-1 (positive control), bone marrow stromal, and bone marrow mononuclear cells with or without reverse transcriptase (RT + or RT −, respectively). Human β-actin was used as a reference gene. The PCR products were resolved by electrophoresis on a 2% agarose gel and stained with ethidium bromide.
DISCUSSION
In this study, we analyzed HTK expression in human bone marrow mononuclear cells and showed that it is selectively expressed on committed erythroid progenitors, particularly BFU-E.
At a stage before hemoglobin synthesis, erythroid cells can be detected by their capacity to form colonies in semisolid culture medium. The nature of the colony formation enables very primitive progenitors (referred to as BFU-E) and relatively mature precursors (referred to as CFU-E) to be selectively determined. BFU-E are further divided into immature (or early) and mature (or late) BFU-E according to their proliferative capacity and responsiveness to Epo.19,22,23 Human immature BFU-E have few Epo receptor (EpoR) and are not responsive to Epo, whereas mature BFU-E express a low level of EpoR and are weakly responsive to Epo. These cells give rise to a number of CFU-E that are highly responsive to Epo.19 Development from the earliest BFU-E to the latest CFU-E is a continuous process. BFU-E require the growth factors such as IL-3, granulocyte-macrophage colony-stimulating factor, and SCF to develop into CFU-E.22,23 Among them, SCF is crucial, because mice lacking SCF (Sl mutants) or its receptor, c-Kit (W mutants), exhibit a significant reduction of CFU-E in their fetal liver.24 On the other hand, Epo and EpoR are indispensable for the proliferation and survival of CFU-E and their terminal differentiation.25
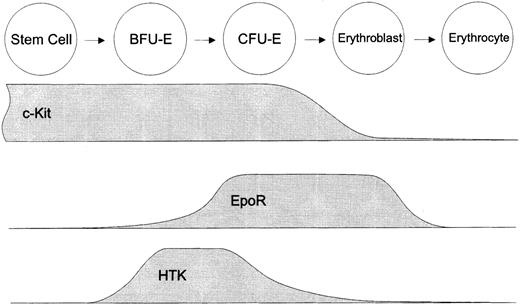
We found here that HTK+c-Kit+ cells gave rise to predominantly large erythroid colonies (bursts) by 14 days in culture and that most of them required SCF as well as Epo for proliferation. These findings suggested that HTK+c-Kit+ cells contain mainly BFU-E and fewer CFU-E. However, HTK was also expressed on some erythroblasts with low levels of glycophorin A, a marker antigen of mature erythroid cells. These HTK+glycophorin Alow cells showed no colony formation, indicating that these cells are erythroblasts at the early stage. HTK is thus mainly expressed at the BFU-E stage, and it is rapidly downregulated during further erythroid differentiation (Fig 9). This characteristic expression of HTK was confirmed by erythroid differentiation in vitro (Fig 7).
Schematic representation of hematopoietic factor receptors involved in the regulation of human erythropoiesis. HTK is a novel hematopoietic factor receptor expressed at the restricted stage of erythroid differentiation around BFU-E.
Schematic representation of hematopoietic factor receptors involved in the regulation of human erythropoiesis. HTK is a novel hematopoietic factor receptor expressed at the restricted stage of erythroid differentiation around BFU-E.
During the development of the earliest BFU-E to CFU-E, BFU-E undergo various stages of maturation. The majority of BFU-E (64%) in the HTK+c-Kit+ fraction gave rise to early appearing bursts that showed apparent colony formation by 7 days in culture and the remaining BFU-E gave rise to late appearing bursts. In addition, HTK was not detected on the CD34+ immature BFU-E isolated from fresh peripheral blood, but its expression was upregulated after an incubation in the presence of SCF and Epo (Fig 7). These findings suggested that HTK is initially expressed at the immature BFU-E stage and then reaches a peak at the mature BFU-E stage. Low levels of CD34 expression on HTK+ cells also indicated that they are mainly at the mature BFU-E stage, because CD34 expression has been shown to gradually decline during the differentiation of BFU-E into CFU-E.26
As shown in Fig 6, HTK−c-Kit+ cells contained a significant number of erythroid progenitors. However, they also contained CFU-MIX, which indicated that some of HTK− progenitors still had multipotentiality. In contrast, HTK+ erythroid progenitors were completely erythroid-committed progenitors. Moreover, HTK−c-Kit+ cells formed larger erythroid bursts than HTK+c-Kit+ cells in the presence of IL-3, SCF, and Epo. From these data, it is suggested that HTK− erythroid progenitors are more immature than that of HTK+ and that HTK+ cells are differentiated from HTK− multipotent or primitive erythroid progenitors (Fig 9).
To analyze the function of the hematopoietic progenitors, purification of them is necessary and crucial. There are several means of purifying and concentrating normal erythroid progenitors, including differential density centrifugation, immune selection using rosetting, FACS, and negative or positive selection by immunomagnetic separation.27,28 As shown in Fig 7, Sawada et al19 have purified BFU-E (purity of 56.6%, 1,400-fold enrichment) from human peripheral blood using a multistep procedure including a positive selection with anti-CD34 MoAb. From human bone marrow cells, Kannourakis and Johnson29 have purified BFU-E by a multistep procedure including positive selection with an MoAb. Although this procedure resulted in a 150-fold enrichment of BFU-E with 18% purity, there was considerable contamination with nonerythroid cells (36% colony-forming cells). MoAbs against several erythroid surface antigens, such as c-Kit, EpoR, glycophorin, and E-cadherin, have been generated. Among them, the c-Kit+ fraction contains erythroid progenitors (BFU-E and CFU-E) at a high frequency, but it is also contaminated with the progenitors of other lineages. EpoR is predominantly expressed in the erythroid lineage but is distributed broadly from progenitors to mature erythroblasts.30 Thus, HTK is the first surface antigen to be identified that is exclusively expressed on erythroid progenitors, especially on BFU-E, in human bone marrow cells. Positive selection using anti-HTK MoAb would be very useful for purifying erythroid progenitors and introducing this step into the described multistep procedures should increase the purity and yield of erythroid progenitors.
The plating efficiency of sorted HTK+c-Kit+ cells was low (Fig 6A), because HTK+ cells contain mature erythroid cells that have lost colony-forming ability. As described above, HTK+glycophorin Alow cells showed no colony formation, and HTK+glycophorin A− cells formed 1.3 to 1.6 times more colonies than HTK−c-Kit+ cells. Enrichment of erythroid progenitors using HTK MoAb remains to be an interesting project.
The biologic function of HTK has not yet been elucidated. To address this question, we cloned HTKL, the ligand for HTK, from the colon cancer cell line, C-1, using a BIAcore system.21 HTKL is a transmembrane protein of about 42 kD that is widely expressed in fetal and adult tissues.16 HTKL induces the phosphorylation of HTK only in the transmembrane or polymerized soluble form, indicating that cell-to-cell contact is required in transducing signals for HTKL. In this study, we showed that HTKL is expressed in bone marrow stromal cells. This finding suggested that the HTKL-HTK signaling pathway acts in the direct cell-to-cell interaction of HTK+ erythroid progenitors with stromal cells and that it plays a role in erythropoiesis. We previously showed that polymerized soluble HTKL stimulates the DNA synthesis of the immature hematopoietic cell line, UT-7.21 However, its mitogenicity is too subtle to be regarded as the main effect. The Eph family of RTKs and their ligands act as positional labels during neuronal development, particularly in the guidance of these cells.10-12 Positional information is also important within the bone marrow microenvironment, which comprises resident stromal cells, extracellular matrix, and various secreted cytokines. Within this microenvironment, BFU-E as well as multilineage progenitors form multicentric colonies (bursts), suggesting that these progenitors reside at the proper site. Close interactions of the hematopoietic progenitors with this microenvironment are supposed to control the homing site. Thus, it is possible that HTKL gives positional information to BFU-E via HTK. The HTKL-HTK signaling pathway is different from known hematopoietic growth factors in that this is functional only in the cell-to-cell contact. In view of this, we are performing functional assays of the HTKL-HTK system. Activated c-Kit associates with and activates EpoR.25 These findings indicate that the interaction of c-Kit and EpoR at or around the CFU-E stage may be involved in triggering subsequent cell proliferation and differentiation. HTK is coexpressed with c-Kit and/or EpoR on erythroid progenitors (Fig 9). It may be important to clarify the interactions among these erythroid factor receptors to further understand the precise regulation of erythropoiesis.
In summary, we showed that the Eph family RTK, HTK, is selectively expressed on bone marrow erythroid progenitors, particularly on BFU-E, and that its ligand, HTKL, is expressed in bone marrow stromal cells. Our findings suggested that HTK is the first marker antigen for erythroid progenitors and that the HTKL-HTK signaling pathway functions as a novel regulatory system of erythropoiesis in the direct cell-to-cell contact of HTK+ erythroid progenitors with stromal cells.
ACKNOWLEDGMENT
We thank Drs N. Komatsu and K. Maruyama for providing UT-7 cells and an expression vector, pMKITNeo, respectively.
Supported by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Toshio Suda, MD, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, Honjo 2-2-1, Kumamoto City 860, Japan.

![Fig. 6. Colony formation of sorted bone marrow cells. Fractionated bone marrow cells were cultured in methylcellulose medium containing the following factors: (A) IL-3 + SCF + Epo; (B) IL-3 + SCF + Epo (FULL), IL-3 + Epo [SCF(−)], or IL-3 + SCF [Epo(−)]. Five thousand cells of each fraction (HTK−c-Kit+ and HTK+c-Kit+) were plated. The numbers of colonies were counted on days 7 and 14. Bars show the mean of duplicate culture dishes. E, GM, or MIX indicate colonies derived from CFU-E/BFU-E, CFU-GM, or CFU-MIX, respectively. Results are representative of at least seven independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2757/4/m_bl_0030f6.jpeg?Expires=1769711474&Signature=e4eGOf7dv4Uw3LomgHK4aY5AUKuQkKJfL2aQD~-AhailOu5yI1GvRDPPew1BybTVmdgeaEAdxkbKz3RYvLt27F9RrelXoUmgUGBKpaj7VwF3nb5qxvjM~~MJh0XPvJb~aD2UR2Ej1iLbSQTJ9GGfujvw0eitFsjjQJhZs6aR6GAHF~~caLHAsUpwWz1c4pM1s3mkjYAe46jl~wjcDqY0JY2bfbSb0kvjInNXjEtqwZUdKA~V15mT-ovjE3CuNZnFKzaGw1agWl2GlnT~k5msaEeQQ480TckZHp0H4aylRXnjfy8GGEqhVmd60RuqFtoUyrQo7mHWiKBQ7UGW7mUexA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)