Abstract
The stress-activated protein/c-Jun N-terminal kinases (SAPK/JNK) have been shown to be activated by pro-inflammatory cytokines, as well as physical and chemical stresses. We now show that a variety of hematopoietic growth factors, including Steel locus factor (SLF ), granulocyte-macrophage colony-stimulating factor (GM-CSF ), and interleukin-3 (IL-3), all of which promote the growth and survival of various lineages of hematopoietic cells, activate the stress-activated protein kinases in the factor-dependent cell line MC/9. These hematopoietic growth factors activated both 46- and 55-kD isoforms of both SAPKγ and SAPKα. Furthermore, we demonstrate that SAPK activation correlated with the phosphorylation of SAPK/ERK kinase-1 (SEK1) after treatment with SLF or GM-CSF. Interestingly, IL-4, a cytokine with distinctive and important effects on the immune system, was the exception among the hematopoietic growth factors we examined in failing to induce activation of SAPKγ, SAPKα, or SEK1. These findings show that activation of SAPK is involved, not only in responses to stresses, but also in signaling by growth factors that regulate the normal development and function of cells of the immune system.
THE STRESS-ACTIVATED protein kinases (SAPK) or the c-jun N-terminal kinases (JNK) are mammalian homologues of the MAPK family.1-3 Ten isoforms of SAPK/JNK have been identified that are generated by alternate splicing of three genes in both the rat (SAPKα,β,γ) and the human (JNK2,3,1).4,5 Most of these isoforms are widely expressed, with the exception of SAPKβ/JNK3, which is expressed predominantly in neural tissue. SAPK/JNK are activated by growth factors, pro-inflammatory cytokines, UV irradiation, heat shock, protein synthesis inhibitors, bacterial lipopolysaccharide, T-cell activators, and ligation of CD40.1-3,6-9 Like other kinases in the MAPK family, SAPK/JNK are activated by phosphorylation on threonine and tyrosine residues of a TXY motif2 by a dual-specificity MAPK kinase (MKK) known as SEK1 or MKK4.10-13 Experiments with a series of dominant-inhibitory and constitutively activated mutant proteins have indicated that SAPK/JNK lies downstream of Ras or of Cdc42 and Rac, depending on the activating stimuli.14-16
Hematopoietic growth factors including Steel locus factor (SLF ), interleukin-3 (IL-3), IL-4, and granulocyte-macrophage colony-stimulating factor (GM-CSF ) are members of a large structural family of polypeptide growth factors that regulate the growth, survival, and differentiation of cells in many tissues, including those of the immune system. The receptor for SLF is a typical homodimeric protein-tyrosine kinase receptor, resembling the platelet-derived growth factor receptor.17 SLF acts on pluripotent hematopoietic stem cells, the progenitors of a variety of hematopoietic lineages, and mature mast cells,18 but also has an important developmental role on cells of nonhematopoietic origin, including germ cells and cells derived from the neural crest.18 SLF is produced constitutively by stromal cells and is essential for the development of the hematopoietic system. In contrast, the receptors for IL-3, GM-CSF, and IL-4 are distinctive heterodimers, the subunits of which lack enzymatic activity and belong to the hematopoietin receptor superfamily. Ligand-binding to these receptors activates associated kinases, including members of the JAK and src protein kinase families.19,20 These three cytokines are released by activated cells of the immune system and link the immune system with the regulation of the growth and function of cells of hematopoietic origin. IL-3 stimulates the growth, survival, and differentiation of pluripotent hematopoietic stem cells, progenitors of all erythroid and myeloid cells, mature macrophages, eosinophils, megakaryocytes, mast cells, or basophils.19 GM-CSF is more restricted in its activity, targeting the progenitors and mature cells of the macrophage, neutrophil, and eosinophil lineages.19 IL-4 is active on cells of both hematopoietic and nonhematopoietic origin but is particularly important for the differentiation of specific classes of B and T lymphocytes and the downregulation of macrophage activity.20
Here we show that IL-3, GM-CSF, and SLF activate both 46- and 55-kD isoforms of SAPKγ and SAPKα in hematopoietic cells. Furthermore, these growth factors activate SEK1, the only kinase identified as upstream of SAPK/JNK. Interestingly, IL-4 failed to activate SAPKγ, SAPKα, or SEK1. These findings suggest a role for SAPK/JNK in the normal function of these hematopoietic growth factors that regulate the growth and development of the lymphohematopoietic system.
MATERIALS AND METHODS
Antibodies and reagents.The N-terminus of c-Jun (1-169) fused to glutathione S-transferase was expressed in bacteria, and purified by affinity chromatography on glutathione Sepharose 4B beads (Pharmacia Biotech Inc, Quebec, Canada). The antibodies specific for isoforms of JNK1 or JNK2 were purchased from Pharmingen (Mississauga, Ontario, Canada) or Santa Cruz Biotechnology (Santa Cruz, CA), respectively. The anti-phospho-MKK4 specific antibody was purchased from New England Biolabs (Beverly, MA). RPMI 1640 was purchased from Canadian Life Technologies (Burlington, Ontario, Canada) and fetal calf serum (FCS) from Intergen (Purchase, NY). Recombinant SLF was provided by James Wieler, and synthetic cytokines were provided by I. Clark-Lewis, both of the Biomedical Research Centre (Vancouver, BC, Canada).
Cell culture and stimulation conditions.The factor-dependent hematopoietic cell line MC/9 was passaged in RPMI 1640 supplemented with 10% FCS, 10 μmol/L 2-mercaptoethanol, and 3% IL-3 conditioned medium as described.21 Before stimulation, cells were cultured overnight in 0.3% IL-3–conditioned medium, washed three times with phosphate-buffered saline (PBS), and then incubated at 107 cells/mL in serum-free medium buffered with 10 mmol/L HEPES (N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]) pH 7.2, for 1 hour. Cells were stimulated with the following doses of synthetic growth factors: IL-4, 20 μg/mL; GM-CSF, 10 μg/mL; IL-3, 20 μg/mL. All stimulations had been shown in preliminary experiments to give maximal levels of tyrosine phosphorylation of the respective receptors.
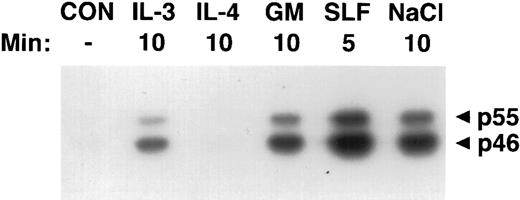
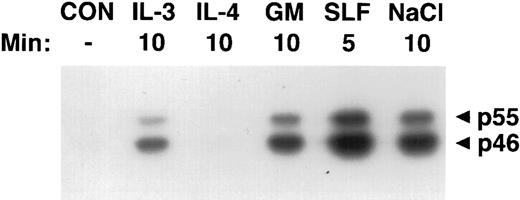
Activation of SAPKγ by hematopoietic growth factors. MC/9 cells were incubated at 37°C in RPMI 1640 for 1 hour and then left untreated (CON), or treated with saturating doses of IL-3 (IL-3), IL-4 (IL-4), GM-CSF (GM), SLF (SLF ), or 0.2 mol/L NaCl (NaCl) for the indicated times. Immunoprecipitated SAPKγ was analyzed using an in-gel kinase assay after SDS-PAGE with a separating gel containing GST-c-jun. The phosphorylation of c-Jun was assessed by autoradiography and the presence of 46- and 55-kD splice variants of SAPKγ are indicated.
Activation of SAPKγ by hematopoietic growth factors. MC/9 cells were incubated at 37°C in RPMI 1640 for 1 hour and then left untreated (CON), or treated with saturating doses of IL-3 (IL-3), IL-4 (IL-4), GM-CSF (GM), SLF (SLF ), or 0.2 mol/L NaCl (NaCl) for the indicated times. Immunoprecipitated SAPKγ was analyzed using an in-gel kinase assay after SDS-PAGE with a separating gel containing GST-c-jun. The phosphorylation of c-Jun was assessed by autoradiography and the presence of 46- and 55-kD splice variants of SAPKγ are indicated.
Immunoprecipitation conditions.After stimulation, cells were lysed in solubilization buffer (50 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% [vol/vol] Nonidet P-40 [NP-40], 1 mmol/L sodium molybdate, 200 mmol/L sodium orthovanadate, 1 mmol/L sodium fluoride, 50 mmol/L β-glycerol phosphate, 10 μg/mL aprotinin, 10 μg/mL soybean trypsin inhibitor, 0.7 μg/mL pepstatin, 2 μg/mL leupeptin, and 40 μg/mL phenylmethylsulfonyl fluoride). To monitor the levels of tyrosine phosphorylation in each sample, a portion of the cell lysate was routinely resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with the antiphosphotyrosine antibody 4G10. The remaining lysate was subjected to immunoprecipitation using antibodies, or affinity precipitation using GST-c-Jun bound to glutathione Sepharose.
Protein-kinase assays.The kinase activity of SAPK was measured using an in vitro kinase assay with the N-terminus of c-Jun as a substrate. The cell lysate was mixed with GST-c-Jun and 20 μL packed volume of glutathione Sepharose beads. After 2 hours the beads were washed extensively with solubilization buffer and once with kinase assay buffer (25 mmol/L HEPES pH 7.2, 25 mmol/L magnesium chloride, 2 mmol/L dithiothreitol, 50 mmol/L β-glycerol phosphate, and 0.5 mmol/L sodium vanadate). The kinase reaction was initiated by the addition of 20 μL of kinase assay buffer containing 10 μCi of [γ-32P]ATP, and stopped after 20 minutes at 30°C by the addition of SDS-sample buffer.
For in-gel kinase assays, the cell lysate was mixed with an antibody specific for JNK1 or JNK2 and protein A-Sepharose beads for 2 hours. The beads were washed extensively in solubilization buffer and immunoprecipitated proteins were eluted by boiling in SDS-sample buffer. The samples were analyzed by SDS-PAGE using a separating gel containing 1 mg of bacterially expressed GST-c-Jun. The gel was then sequentially washed twice in buffer A (20% isopropanol and 50 mmol/L Tris, pH 8) for 30 minutes, twice in buffer B (50 mmol/L Tris, pH 8 and 5 mmol/L 2-mercaptoethanol [2-Me]) for 30 minutes, once in buffer C (6 mmol/L guanidine HCl, 50 mmol/L Tris, pH 8, and 5 mmol/L 2-Me) for 1 hour, several times in cold buffer D (0.05% [vol/vol] Tween-20, 50 mmol/L Tris, pH 8, and 5 mmol/L 2-Me) over 15 to 18 hours, and once in kinase buffer (40 mmol/L HEPES, pH 7.2, 2 mmol/L dithiothreitol, 15 mmol/L magnesium chloride, 1 mmol/L manganese chloride, 0.3 mmol/L vanadate, and 0.1 mmol/L EGTA) for 30 minutes. The kinase assay was initiated by the addition of 20 mL of kinase buffer containing 100 μCi of [γ-32P]ATP and incubated for 1 hour. The gel was then rinsed with buffer E (5% trichloroacetic acid and 1% sodium pyrophosphate) repeatedly until gel appears cold. The gel was then dried using a gel dryer and phosphorylated c-Jun was detected using autoradiography.
RESULTS
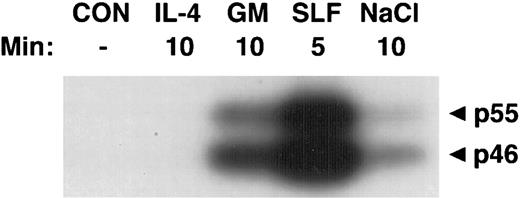
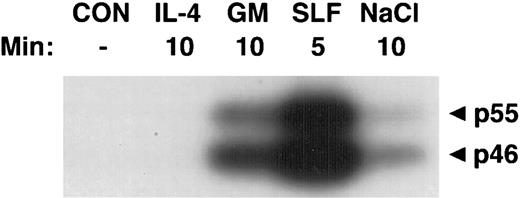
Activation of SAPKγ and SAPKα in response to hematopoietic growth factors.To determine whether SAPK/JNK was involved in responses to IL-3, GM-CSF, or SLF, we stimulated MC/9 mast cells with these factors and immunoprecipitated SAPKγ or SAPKα, using antibodies that precipitated both the 46- and 55-kD isoforms of JNK1 or JNK2, respectively. The precipitate was subjected to SDS-PAGE and renaturation, and SAPK activity was assessed using an “in-gel” kinase assay. Stimulation with either GM-CSF, IL-3, or SLF induced activation of both 46- and 55-kD isoforms of SAPKγ (Fig 1). Similarly, GM-CSF or SLF induced the activation of both 46- and 55-kD isoforms of SAPKα (Fig 2). It was of particular interest to investigate the effect of IL-4 on SAPK activity, because we had previously shown that IL-4 failed to activate Ras,21,22 Erk-1/2 MAP kinases,22,23 or p38 MAP kinase.24 Unlike the other hematopoietins, IL-4 failed to activate either SAPKγ (Fig 1) or SAPKα (Fig 2).
Activation of SAPKα by hematopoietic growth factors. MC/9 cells were incubated at 37°C in RPMI 1640 for 1 hour and then left untreated (CON), or treated with saturating doses of IL-4 (IL-4), GM-CSF (GM), SLF (SLF ), or 0.2 mol/L NaCl (NaCl) for the indicated times. Immunoprecipitated SAPKα was analyzed after SDS-PAGE with a separating gel containing GST-c-Jun, using an in-gel kinase assay. The phosphorylation of c-Jun was assessed by autoradiography and the presence of 46- and 55-kD splice variants of SAPKα are indicated.
Activation of SAPKα by hematopoietic growth factors. MC/9 cells were incubated at 37°C in RPMI 1640 for 1 hour and then left untreated (CON), or treated with saturating doses of IL-4 (IL-4), GM-CSF (GM), SLF (SLF ), or 0.2 mol/L NaCl (NaCl) for the indicated times. Immunoprecipitated SAPKα was analyzed after SDS-PAGE with a separating gel containing GST-c-Jun, using an in-gel kinase assay. The phosphorylation of c-Jun was assessed by autoradiography and the presence of 46- and 55-kD splice variants of SAPKα are indicated.
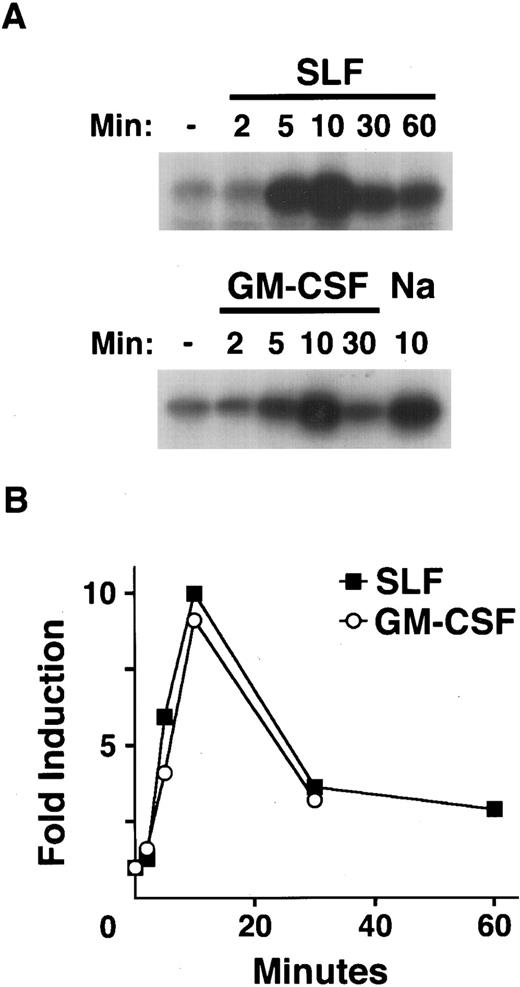
Kinetics of SAPK activation by hematopoietic growth factors.To examine the kinetics of the activation of SAPK/JNK induced by treatment of MC/9 cells with SLF, IL-3, and GM-CSF, we used GST-c-Jun to preferentially immunoprecipitate activated SAPK.4,25 26 Treatment with SLF or GM-CSF resulted in a rapid increase in the activation of SAPK (Fig 3). Cells treated with SLF exhibited detectable activation of SAPK after 2 minutes, with maximal activation of SAPK occurring around 10 minutes (Fig 3A). In cells treated with GM-CSF the activation of SAPK again peaked at around 10 minutes (Fig 3A). In both instances the activation of SAPK was transient and was returning to basal levels by 30 minutes (Fig 3B). IL-4 failed to activate SAPK over similar time courses (data not shown).
Kinetics of SAPK activation by hematopoietic growth factors. After 1 hour in RPMI 1640 at 37°C, MC/9 cells were left untreated, or treated with saturating doses of GM-CSF (GM-CSF ), SLF (SLF ), or 0.2 mol/L NaCl (Na) as a positive control for the indicated times. Cell extracts were prepared and GST-c-Jun was used as both affinity reagent and substrate for an in vitro kinase assay. (A) The phosphorylation of c-Jun was assessed by autoradiography. (B) The fold activation of SAPK in cells treated with SLF (▪) or GM-CSF (○) compared to untreated cells was quantitated using densitometry.
Kinetics of SAPK activation by hematopoietic growth factors. After 1 hour in RPMI 1640 at 37°C, MC/9 cells were left untreated, or treated with saturating doses of GM-CSF (GM-CSF ), SLF (SLF ), or 0.2 mol/L NaCl (Na) as a positive control for the indicated times. Cell extracts were prepared and GST-c-Jun was used as both affinity reagent and substrate for an in vitro kinase assay. (A) The phosphorylation of c-Jun was assessed by autoradiography. (B) The fold activation of SAPK in cells treated with SLF (▪) or GM-CSF (○) compared to untreated cells was quantitated using densitometry.
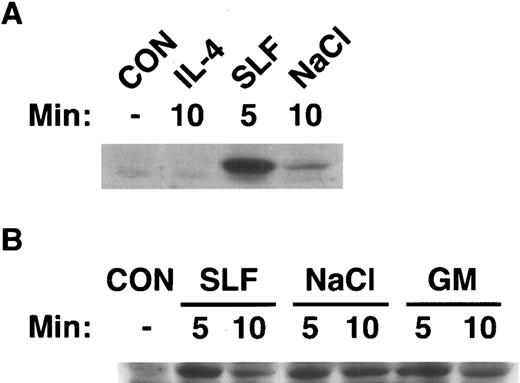
Activation of SAPK by hematopoietic growth factors correlates with activation of SEK1.Currently, SEK1/MKK4 is the only established upstream activator of SAPK/JNK.10-13 Therefore, we determined whether there was a correlation between activation of SAPK by hematopoietic growth factors and activation of SEK1. We immunoblotted cell extracts from MC/9 cells that had been stimulated with IL-4 or SLF (Fig 4A), or GM-CSF, SLF, or 0.2 mol/L NaCl (Fig 4B) with an antibody that specifically recognized the activated form of human MKK4 and cross-reacted with SEK1. It was evident (Fig 4) that stimulation of cells with GM-CSF, SLF, or NaCl induced rapid phosphorylation of SEK1. This phosphorylation was transient and was returning to basal levels by 10 minutes. These data indicate the activation of SEK1 preceded the activation of SAPK, consistent with a role in activation of SAPK by these stimuli. In keeping with our finding that IL-4 failed to stimulate the enzymatic activity of SAPK, IL-4 failed to induce phosphorylation of SEK1 (Fig 4). The activation of SAPK in all cases correlated with activation of SEK1.
Activation of SEK1/MKK4 by hematopoietic growth factors. MC/9 cells were incubated at 37°C in RPMI 1640 for 1 hour before simulation. Cells were then left untreated (CON), or (A) treated with maximal doses of IL-4 (IL-4) or SLF (SLF ) or (B) treated with maximal doses of GM-CSF (GM), SLF (SLF ), or 0.2 mol/L NaCl (NaCl) for the indicated times. After lysis, cell extracts were analyzed by SDS-PAGE, and were immunoblotted with an antibody that specifically recognizes the activated form of SEK1/MKK4.
Activation of SEK1/MKK4 by hematopoietic growth factors. MC/9 cells were incubated at 37°C in RPMI 1640 for 1 hour before simulation. Cells were then left untreated (CON), or (A) treated with maximal doses of IL-4 (IL-4) or SLF (SLF ) or (B) treated with maximal doses of GM-CSF (GM), SLF (SLF ), or 0.2 mol/L NaCl (NaCl) for the indicated times. After lysis, cell extracts were analyzed by SDS-PAGE, and were immunoblotted with an antibody that specifically recognizes the activated form of SEK1/MKK4.
DISCUSSION
These experiments using MC/9 mast cells show that a series of hematopoietic growth factors which regulate the normal development and function of cells of the immune system activate both SAPKγ (Fig 1) and SAPKα (Fig 2). One of these hematopoietic growth factors, SLF, signals through a tyrosine kinase receptor, while the others, IL-3 and GM-CSF, signal through receptors of the hematopoietin receptor superfamily.17 20 Cells treated with GM-CSF, IL-3, or SLF exhibited increased activity of 46- and 55-kD proteins corresponding to different splice variants of SAPKγ (Fig 1). Similarly, cells treated with GM-CSF or SLF activated both 46- and 55-kD splice variants of SAPKα (Fig 2). Together our results indicate that IL-3 (Fig 1), GM-CSF (Figs 1 and 2), and SLF (Figs 1 and 2) stimulate the activation of 46- and 55-kD isoforms of both SAPKγ and SAPKα in MC/9 cells.
The kinetics of SAPK activation in MC/9 cells treated with GM-CSF or SLF resembled that observed for activation of Erk22,23 and p38 MAPK24 by these factors. The activation of SAPK by SLF showed similar kinetics to that induced by GM-CSF (Fig 3A). In all cases the activation of SAPK following stimulation with these factors was rapid and transient (Fig 3B). We also used an antibody specific for the activated form of MKK4 to analyze the ability of these growth stimuli to activate SEK1/MKK4, the only known upstream activator of SAPK/JNK.10-13 The phosphorylation of SEK1 induced by treatment of cells with SLF, GM-CSF, or 0.2 mol/L NaCl was evident at 5 minutes and was declining by 10 minutes when levels of SAPK/JNK activity were peaking. The earlier kinetics of phosphorylation of SEK1 compared with activation of SAPK are consistent with SEK1 being upstream of SAPK in the cellular response to these stimuli. These results suggest that SEK1 is involved in the activation of SAPK in response to SLF and GM-CSF. There have been reports of other activators of SAPK/JNK,27 but these proteins have not been characterized yet and their role, if any, in the responses we have seen remains to be determined.
IL-4 was unique among the hematopoietins that we examined in this study in failing to increase the activation of SAPKγ (Fig 1) or SAPKα (Fig 2). We also examined the ability of IL-4 to activate SAPK over more extended time courses up to 1 hour, but failed to see any increased SAPK activity (data not shown). As noted, the inability of IL-4 to activate SAPK correlated with its inability to induce phosphorylation of SEK1 (Fig 4).
This failure of IL-4 to activate SAPK/JNK correlates with its inability to activate either the ERK22,23 or the p38 MAP kinase pathway.24 IL-4 is also notable for its inability to activate Ras.21,28 We hypothesize that hematopoietic growth factors activate p38 MAPK and SAPK/JNK in a Ras-dependent fashion. Indeed, other growth factors activate SAPK/JNK through Ras-dependent mechanisms as dominant inhibitory mutants of Ras have been shown to prevent the activation of SAPK/JNK in response to EGF and NGF.29 Furthermore, SAPK/JNK activity was increased by the expression of constitutively active mutants of Ras.15 However, dominant inhibitory mutants of Cdc42 or Rac1 prevented SAPK/JNK activation by Ras,15 and constitutively active mutants of Cdc42 or Rac1 increased SAPK/JNK activity.14-16 These findings suggest that Ras was mediating SAPK/JNK activation through either Cdc42 or Rac1. Observations that the activation of SAPK/JNK by tumor necrosis factor (TNF-α) is not inhibited by over-expression of dominant-negative Ras, however, suggest that there may also be other Ras-independent pathways of SAPK/JNK activation.15 Further studies using dominant inhibitory mutants of Ras and the Rho family of small GTPases will be required to elucidate the hierarchy of proteins leading to SAPK/JNK activation by hematopoietic growth factors.
Hematopoietic growth factors promote cell-cycle progression in cells of the immune system. The functional significance of the activation of SAPKγ/JNK1 and SAPKα/JNK2 by hematopoietic growth factors, for example in the promotion of cell-cycle progression, is unknown. Clearly the best argument for a role of SAPK/JNK in cell-cycle progression is their ability to phosphorylate and activate the transcription factors: c-Jun, ATF-2, and Elk-1.1,2,30-32 SAPK/JNK may have an obligate role in cell-cycle regulation because SAPK/JNK are the only known kinases capable of phosphorylating c-Jun on serines 63 and 73 and thereby increasing its transcriptional activity.1,2,30 The observation that c-Jun is required for the transformation of fibroblasts by activated Ras implies a role for SAPK/JNK in growth downstream of Ras.33
Hematopoietic growth factors not only provide growth signals that promote growth of hematopoietic cells, but also signals that suppress apoptosis. In some cells activation of SAPK correlates not with suppression of apoptosis but with its induction.34-36 It is possible that activation of the SAPK/JNK pathway in the absence of other signals such as ERK activity results in a pro-apoptotic signal, whereas the integrated activation of ERK, SAPK/JNK, and p38 MAPK pathways, stimulated by factors such as GM-CSF, IL-3, and SLF, delivers a signal for the suppression of apoptosis. However, our data indicate that the activation of ERK, SAPK/JNK, or p38 MAPK pathways is not essential for the suppression of apoptosis as IL-4, which we have shown fails to activate any of these kinases, nevertheless suppresses the apoptosis of many hematopoietic cells, including the factor-dependent cell line MC/9 used in these experiments.
In conclusion, we have shown that SAPKγ and SAPKα are activated in response to IL-3, GM-CSF, and SLF but not IL-4. Furthermore, our results suggest that SAPK activation is mediated by SEK1, as both SLF and GM-CSF, but not IL-4, activated SEK1 before activation of SAPK. Importantly, our results show that SAPK/JNK activity is involved not only in response to stress, but also in signaling by hematopoietic growth factors that, in some cases such as SLF, regulate the normal growth and development of the hematopoietic system.
ACKNOWLEDGMENT
We thank M. Gold for assistance in setting up the in-gel kinase assay, I. Clark-Lewis and J. Wieler for cytokines, and P. Orchansky for critical reading of the manuscript.
Supported by The Medical Research Council of Canada and The National Cancer Institute of Canada. I.N.F. was supported by an MRC Studentship.
Address reprint requests to John W. Schrader, MBBS, PhD, 2222 Health Sciences Mall, The Biomedical Research Centre, The University of British Columbia, Vancouver, British Columbia, V6T 1Z3 Canada.