Abstract
Transformation of hematopoietic cells by the Bcr-abl oncoprotein leads to constitutive tyrosine phosphorylation of a number of cellular polypeptides that function in normal growth factor-dependent cell proliferation. Recent studies have shown that the CrkL adaptor protein and the Cbl protooncoprotein are constitutively tyrosine phosphorylated and form a preformed complex in cells expressing Bcr-abl. In the current study, we have examined cytokine-dependent tyrosine phosphorylation of Cbl and its association with Crk proteins. Erythropoietin (EPO) and interleukin-3 induced a dose and time-dependent tyrosine phosphorylation of Cbl in both EPO-dependent Ba/F3 and DA-3 transfectants, and the erythroid cell line HCD-57. Furthermore, once phosphorylated, Cbl associated with Crk adaptor proteins. Of the three Crk isoforms expressed in hematopoietic cells (CrkL, CrkII, and CrkI), tyrosine phosphorylated Cbl binds preferentially to CrkL and CrkII. The amount of Cbl associated with CrkL and CrkII exceeded the fraction of Cbl associated with Grb2 indicating that unlike other receptor systems, the Cbl-Crk association represents the dominant complex of Cbl in growth factor-stimulated hematopoietic cells. In factor-dependent hematopoietic cell lines, CrkL constitutively associated with the guanine nucleotide release factor, C3G, which is known to interact via Crk src-homology 3 (SH3) domains. Our data suggest that the inducible Cbl-Crk association is a proximal component of a signaling pathway downstream of multiple cytokine receptors.
ERYTHROPOIETIN (EPO), the primary regulator of erythropoiesis,1,2 provides both proliferative and differentiative signals to erythroid progenitors. Through binding of its cognate ligand, the 66 kD EPO receptor (EPO-R) elicits several signal transduction cascades. EPO stimulation of factor-dependent cell lines leads to a rapid activation of the cytoplasmic tyrosine kinase, JAK2,3,4 which is weakly associated with the juxtamembrane domain of the EPO-R. JAK2 then phosphorylates itself and individual tyrosine residues of the EPO-R cytoplasmic region. These phosphotyrosine residues subsequently serve as docking sites for proteins containing src-homology 2 (SH2) domains including STAT55-8 and Shp2.9,10 EPO and interleukin-3 (IL-3) activate the Ras/Raf1/MAP kinase pathway by recruitment of Grb2, either directly through binding to the receptor or indirectly, via adaptor molecules such as Shc11-15 or Shp2.9,15 The SH2 domain of Grb2 binds to a YXN motif16,17 and its SH3 domains are constitutively bound to the guanine nucleotide release factor (GNRF ), Son of Sevenless (Sos).18-24 This interaction then allows Sos to convert Ras to its GTP-bound active form.
Bcr-abl–mediated transformation of hematopoietic cells results in deregulated tyrosine kinase activity and constitutive assembly of tyrosine phosphorylated signaling complexes normally observed only after mitogenic growth factor stimulation. Examples of constitutively tyrosine phosphorylated substrates include STAT5,25 Shc,26 Shp2,27 Paxillin,28 and Vav.29 Analysis of additional tyrosine phosphorylated substrates in Bcr-abl transformed cells have recently identified p120Cbl30-32 and CrkL.30-36 Cbl was originally described as the transforming oncogene of the Cas NS-1 retrovirus resulting in pre-B–cell lymphomas and myelogenous leukemia in mice.37 However, p120cbl, the product of c-Cbl,38 is nontransforming. We and others have recently identified Cbl as a target of tyrosine phosphorylation in response to stimulation through a number of cell surface receptors including the T-cell receptor,39-42 B-cell receptor,43-47 epidermal growth factor (EGF ) receptor,48-53 colony stimulating factor-1 receptor,54,55 and FcγRII/RIII receptor.54,56 Interestingly, GM-CSF and EPO also affect tyrosine phosphorylation of Cbl in UT-7 cells.57
Crk proteins are the cellular homologues of v-crk, which was originally described as an oncogene from the avian retroviruses CT1058 and ASV-1.59 Three Crk protein variants are expressed in hematopoietic cells: CrkI (28 kD)60; an alternatively spliced CrkII (40 and 42 kD)60; and CrkL (36 kD).61 CrkII and CrkL contain an amino terminal SH2 domain followed by two SH3 domains, whereas the carboxy terminal SH3 domain of CrkII is not found in CrkI. Crk proteins are adaptors with no known catalytic activity. As Grb2 mediates SH3 dependent interaction with Sos, CrkL binds C3G, a unique GNRF. Recent studies performed in COS cells suggest that C3G can catalyze GTP exchange of a distinct Ras family member, Rap1.62
We and others have recently shown that a prominent complex is induced between tyrosine phosphorylated Cbl and the SH2 domain of Crk proteins on antigen receptor stimulation of T cells63-65 and EGF stimulation of mammary epithelial cells.53 Therefore, recent results that GM-CSF and EPO activated the tyrosine phosphorylation of Cbl57 and that tyrosine phosphorylated Cbl formed a complex with CrkL in Bcr-abl transformed cells30-32 prompted us to investigate if such a complex was induced by normal hematopoietic growth factor stimulation. Using EPO- and IL-3–dependent hematopoietic cell lines, we demonstrate that these cytokines also induce tyrosine phosphorylation of Cbl which promotes complex formation with CrkL and CrkII. CrkL was constitutively associated with the GNRF, C3G. In contrast to other receptor systems, growth factor stimulation did not induce a detectable tyrosine phosphorylation of Grb2-associated Cbl. These results suggest a distinct role for Crk-Cbl complexes in hematopoietic growth factor signaling pathways.
MATERIALS AND METHODS
Cells and cell culture.Ba/F3 and DA-3 cells (generously provided by J. Ihle, Memphis, TN) were maintained in RPMI 1640 medium supplemented with 10% (vol/vol) fetal calf serum (FCS) and 5% conditioned medium from WEHI-3 cells (IL-3 medium). Ba/F3-EPO-R and DA-3-EPO-R cells were maintained in RPMI 1640 medium supplemented with 10% (vol/vol) FCS and 0.5 U/mL of human erythropoietin (Kirin Brewery, Tokyo, Japan). HCD-57 cells were cultured in IMDM supplemented with 30% FCS and 0.1 U/mL of human recombinant EPO (Kirin Brewery, Tokyo, Japan).
Analysis of Cbl tyrosine phosphorylation and identification of associated proteins.For cytokine depletion, cell lines were incubated in RPMI 1640/1% bovine serum albumin (BSA) (no supplemental growth factor) for an 8-hour period and then stimulated for various periods with either no factor, IL-3 (Kirin Brewery), or EPO (Kirin Brewery) at 37°C. Cell lysates were prepared in 50 mmol/L Tris-HCl (pH 8.0) 150 mmol/L NaCl, 1.0% Triton X-100 plus phosphatase and protease inhibitors as previously described.66 Immunoprecipitations were performed with the following rabbit polyclonal antibodies: anti-Cbl,64 anti-CrkL, anti-CrkII, anti-Grb2, or anti-C3G (all from Santa Cruz Biotechnology, Santa Cruz, CA). The anti-Crk monoclonal antibody (Transduction Laboratories, Lexington, KY) recognizes CrkI and CrkII.64 Immune complexes were isolated with Protein A-Sepharose, washed three times with 50 mmol/L Tris HCl (pH 8.0), 150 mmol/L sodium chloride, 0.1% Triton X-100, 10 mmol/L sodium pyrophosphate, 10 mmol/L sodium fluoride, 5 mmol/L EDTA, and 1 mmol/L sodium orthovanadate, and prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously.66
For immunodepletion experiments, 2 mg of DA-3-EPO-R lysate was incubated in the presence of 1 μg anti-CrkII antibody and Protein A-Sepharose overnight. The supernatant was then incubated with 1 μg of anti-CrkI/II antibody for 1 hour, followed by a 1-hour incubation with Protein A-Sepharose. The immunoprecipitations were washed and analyzed by SDS-PAGE as described above.
Following electrophoretic transfer of proteins to nitrocellulose, the membranes were blocked and incubated with the anti-phosphotyrosine monoclonal antibody, 4G10, washed in 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L sodium chloride, 0.1% Triton X-100 (TBST), followed by horseradish peroxidase (HRP) conjugated sheep antimouse immunoglobulin G (Amersham, Arlington Heights, IL), followed by washing in TBST. After enhanced chemiluminescence detection, the membrane was stripped by incubation in 62.5 mmol/L Tris-HCl (pH 6.8), 2% (wt/vol) sodium dodecyl sulfate, 100 mmol/L β-mercaptoethanol for 1 hour at 55°C. Membranes were blocked and reprobed with the following antibodies: anti-Cbl (Santa Cruz Biotechnology), anti-CrkL, anti-CrkII, anti-Crk, anti-Grb2, or anti-C3G. Incubations were performed with the relevant secondary reagent, either HRP-Protein A (Amersham) or HRP-sheep antimouse IgG and the membrane was washed before enhanced chemiluminescence detection.
RESULTS
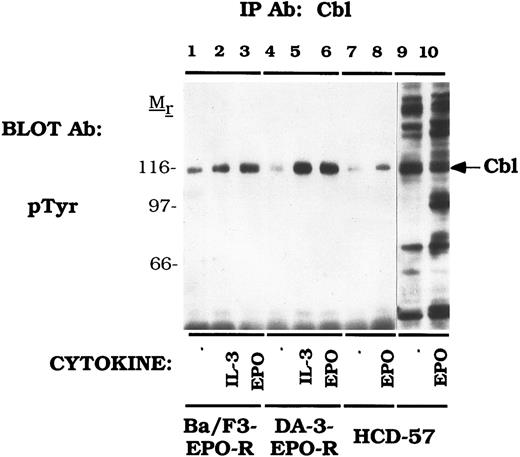
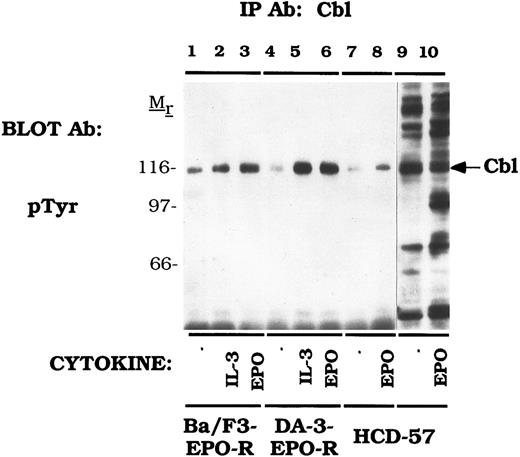
Cbl is tyrosine phosphorylated in response to IL-3 and EPO.Our initial experiments using two-dimensional electrophoresis demonstrated that EPO induces the tyrosine phosphorylation of several 120-kD proteins (data not shown). To investigate whether Cbl represented one of these phosphoproteins, we used anti-Cbl immunoprecipitations to assess the tyrosine phosphorylation of Cbl in cell lines expressing the endogenous EPO-R or transfected EPO-R transfectants (Fig 1). IL-3 (lanes 2 and 5) and EPO (lanes 3 and 6) activated tyrosine phosphorylation of Cbl in Ba/F3-EPO-R or DA-3-EPO-R cells. There was an elevated level of uninduced tyrosine phosphorylation in Ba/F3-EPO-R cells (lane 1). EPO also stimulated the tyrosine phosphorylation of Cbl in an erythroid cell line, HCD-57, expressing the endogenous EPO-R polypeptide (lane 8). DA-3-EPO-R cells were used throughout the remainder of this study due to a lower level of endogenous phosphorylation and more robust activation. These results confirm and extend the results of previous studies of EPO and GM-CSF induction of Cbl tyrosine phosphorylation in human UT-7 cells.57
EPO and IL-3 activate tyrosine phosphorylation of Cbl. Ba/F3 (lanes 1-3), DA-3-EPO-R (lanes 4-6), and HCD-57 (lanes 7-10) cells were depleted of cytokine for 4 hours and stimulated with no factor (lanes 1, 4, 7, and 9), 50 U of murine IL-3 (lanes 2 and 5) or 50 U/mL of human EPO (lanes 3, 6, 8, and 10) for 15 minutes. Following cell lysis, an immunoprecipitation was performed with an anti-Cbl polyclonal antibody. Lysate controls are shown in lanes 9 and 10. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with an anti-phosphotyrosine (pTyr) monoclonal antibody 4G10. Molecular mass standards are indicated.
EPO and IL-3 activate tyrosine phosphorylation of Cbl. Ba/F3 (lanes 1-3), DA-3-EPO-R (lanes 4-6), and HCD-57 (lanes 7-10) cells were depleted of cytokine for 4 hours and stimulated with no factor (lanes 1, 4, 7, and 9), 50 U of murine IL-3 (lanes 2 and 5) or 50 U/mL of human EPO (lanes 3, 6, 8, and 10) for 15 minutes. Following cell lysis, an immunoprecipitation was performed with an anti-Cbl polyclonal antibody. Lysate controls are shown in lanes 9 and 10. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with an anti-phosphotyrosine (pTyr) monoclonal antibody 4G10. Molecular mass standards are indicated.
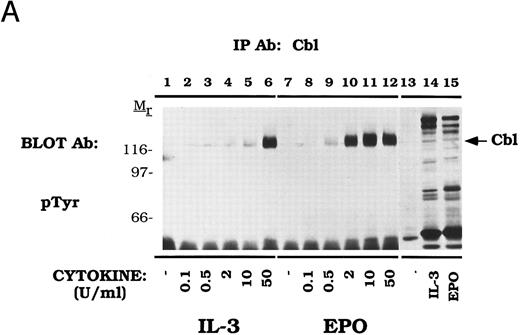
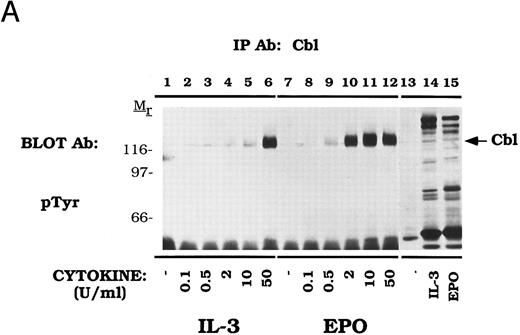
Cbl tyrosine phosphorylation displays dose and time-dependence in DA-3-EPO-R cells.The dose-dependence of Cbl tyrosine phosphorylation was next examined (Fig 2A). DA-3-EPO-R cells were depleted of cytokine and then stimulated with increasing concentrations of either IL-3 or EPO. Immunoprecipitations were performed with an anti-Cbl antibody, followed by detection with a monoclonal anti-phosphotyrosine antibody, 4G10. Increasing concentrations of IL-3 or EPO resulted in tyrosine phosphorylation of Cbl. Cbl tyrosine phosphorylation was observed at cytokine concentrations as low as 2 U/mL IL-3 or 0.5 U/mL EPO.
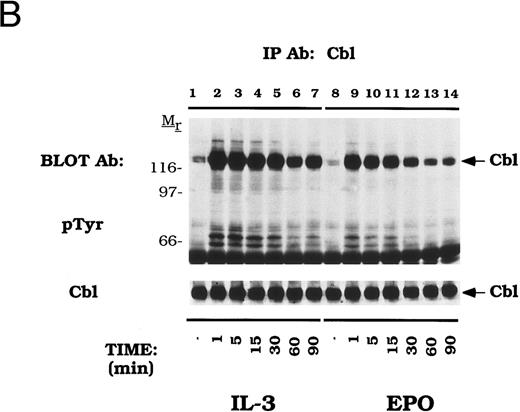
Dose-dependent activation and time-dependent activation of Cbl tyrosine phosphorylation in DA-3-EPO-R cells. (A) DA-3-EPO-R cells were depleted of cytokine for 8 hours and then stimulated with no added factor (lanes 1, 7, and 13), or various concentrations of IL-3 (lanes 2-6 and 14) or EPO (lanes 8-12 and 15) for 15 minutes as shown. Following cell lysis, an immunoprecipitation was conducted with anti-Cbl polyclonal antibody. Lysate controls corresponding to 50 U/mL stimulation are shown in lanes 13-15. Western blot analysis using the monoclonal anti-phosphotyrosine 4G10 antibody was performed (pTyr immunoblot). Molecular mass standards are indicated. (B) DA-3-EPO-R cells were depleted of cytokine for 8 hours and then stimulated with no added factor (lanes 1 and 8), 50 U/mL IL-3 (lanes 2-7), or 50 U/mL EPO (lanes 9-14) for various times as shown. Following cell lysis, an immunoprecipitation was conducted with anti-Cbl polyclonal antibody. Western blotting using the monoclonal anti-phosphotyrosine 4G10 was performed (pTyr immunoblot). The membrane was then stripped and reprobed with a Cbl polyclonal antibody. Molecular mass standards are indicated.
Dose-dependent activation and time-dependent activation of Cbl tyrosine phosphorylation in DA-3-EPO-R cells. (A) DA-3-EPO-R cells were depleted of cytokine for 8 hours and then stimulated with no added factor (lanes 1, 7, and 13), or various concentrations of IL-3 (lanes 2-6 and 14) or EPO (lanes 8-12 and 15) for 15 minutes as shown. Following cell lysis, an immunoprecipitation was conducted with anti-Cbl polyclonal antibody. Lysate controls corresponding to 50 U/mL stimulation are shown in lanes 13-15. Western blot analysis using the monoclonal anti-phosphotyrosine 4G10 antibody was performed (pTyr immunoblot). Molecular mass standards are indicated. (B) DA-3-EPO-R cells were depleted of cytokine for 8 hours and then stimulated with no added factor (lanes 1 and 8), 50 U/mL IL-3 (lanes 2-7), or 50 U/mL EPO (lanes 9-14) for various times as shown. Following cell lysis, an immunoprecipitation was conducted with anti-Cbl polyclonal antibody. Western blotting using the monoclonal anti-phosphotyrosine 4G10 was performed (pTyr immunoblot). The membrane was then stripped and reprobed with a Cbl polyclonal antibody. Molecular mass standards are indicated.
The time dependence of Cbl activation in DA-3-EPO-R cells was next tested (Fig 2B). Tyrosine phosphorylation of Cbl occurred as early as 1 minute after cytokine stimulation and was shown to dissipate by 90 minutes.
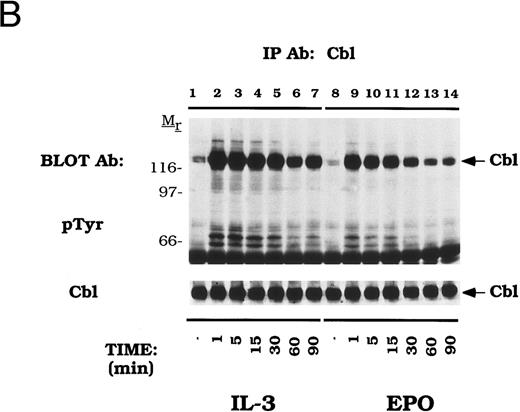
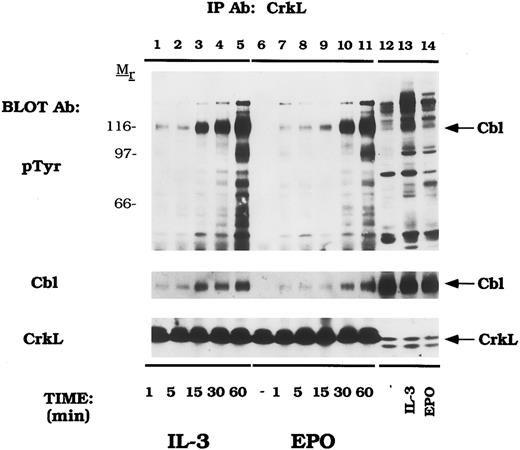
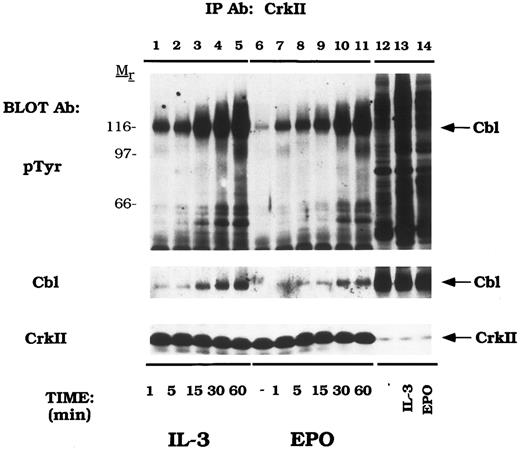
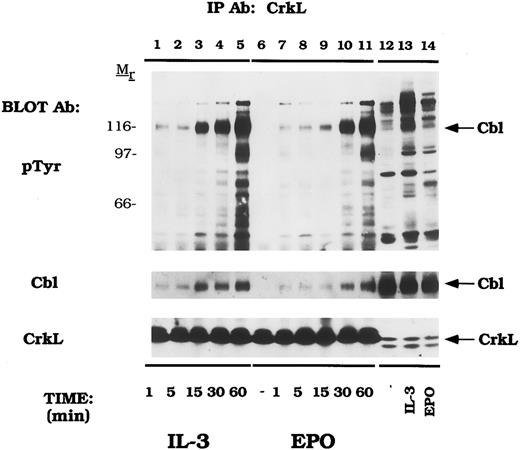
Cytokines induce tyrosine phosphorylation of Cbl and binding to CrkL and CrkII.We next examined EPO- and IL-3–dependent association of the Crk family of proteins with tyrosine phosphorylated Cbl. DA-3-EPO-R cells were depleted of cytokine and then stimulated with IL-3 or EPO for increasing time periods. Lysates were then immunoprecipitated with peptide-specific antibodies to CrkL (Fig 3) and CrkII (Fig 4). A 120-kD phosphoprotein displayed time-dependent association with CrkL in response to either IL-3 or EPO stimulation. Stripping and reprobing this blot with a peptide-specific anti-Cbl antibody identified this protein as Cbl (Cbl immunoblot). Equal amounts of CrkL were immunoprecipitated in this experiment as demonstrated by stripping and reprobing the blot with a peptide-specific CrkL antibody (CrkL immunoblot). The CrkL antibody was immune specific since no CrkII or CrkI signal was observed in the CrkL immunoprecipitations as detected by reprobing the blot with the relevant antibodies (data not shown).
EPO and IL-3 activate the formation of Cbl/CrkL complexes. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 6 and 12), 50 U/mL of murine IL-3 (lanes 1-5 and 13) or 50 U/mL of human EPO (lanes 7-11 and 14) for various periods of time as shown. Following cell lysis, an immunoprecipitation was performed with a CrkL polyclonal antibody. Lysate controls from 15 minute stimulations are shown in lanes 12-14. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody followed by HRP-Sheep antimouse IgG. The blot was then stripped and reprobed with either Cbl or CrkL polyclonal antibodies. Molecular mass standards are indicated.
EPO and IL-3 activate the formation of Cbl/CrkL complexes. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 6 and 12), 50 U/mL of murine IL-3 (lanes 1-5 and 13) or 50 U/mL of human EPO (lanes 7-11 and 14) for various periods of time as shown. Following cell lysis, an immunoprecipitation was performed with a CrkL polyclonal antibody. Lysate controls from 15 minute stimulations are shown in lanes 12-14. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody followed by HRP-Sheep antimouse IgG. The blot was then stripped and reprobed with either Cbl or CrkL polyclonal antibodies. Molecular mass standards are indicated.
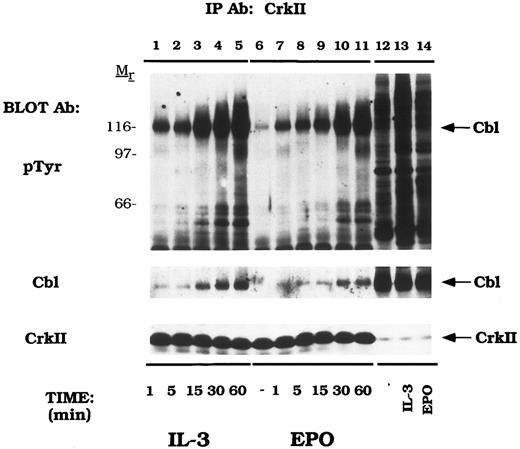
EPO and IL-3 activate the formation of Cbl/CrkII complexes. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 6 and 12), 50 U/mL of murine IL-3 (lanes 1-5 and 13) or 50 U/mL of human EPO (lanes 7-11 and 14) for various periods of time as shown. Following cell lysis, an immunoprecipitation was performed with a CrkII polyclonal antibody. Lysate controls from 15 minute stimulations are shown in lanes 12-14. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody followed by HRP-Sheep antimouse IgG. The blot was then stripped and reprobed with either Cbl or CrkII polyclonal antibodies. Molecular mass standards are indicated.
EPO and IL-3 activate the formation of Cbl/CrkII complexes. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 6 and 12), 50 U/mL of murine IL-3 (lanes 1-5 and 13) or 50 U/mL of human EPO (lanes 7-11 and 14) for various periods of time as shown. Following cell lysis, an immunoprecipitation was performed with a CrkII polyclonal antibody. Lysate controls from 15 minute stimulations are shown in lanes 12-14. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody followed by HRP-Sheep antimouse IgG. The blot was then stripped and reprobed with either Cbl or CrkII polyclonal antibodies. Molecular mass standards are indicated.
Similarly, Cbl displayed a time-dependent association with CrkII (Fig 4). The amount of a 120-kD phosphoprotein, Cbl, associated with CrkII increased over time on IL-3 and EPO stimulation (Cbl immunoblot). Equal amounts of CrkII were immunoprecipitated in this experiment as shown by reprobing with a peptide-specific anti-CrkII antibody (CrkII immunoblot). The CrkII antibody was specific as no CrkL or CrkI were immunoprecipitated in this experiment (data not shown).
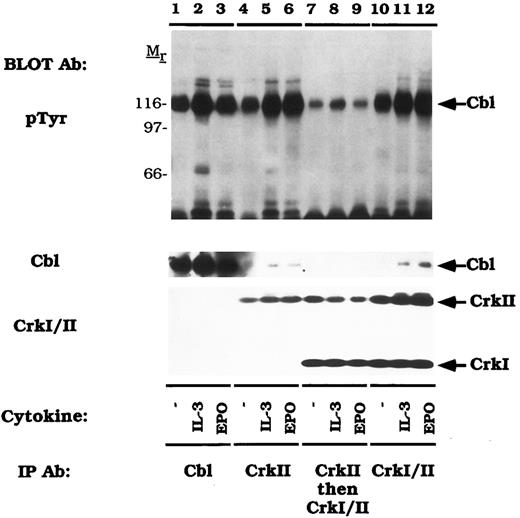
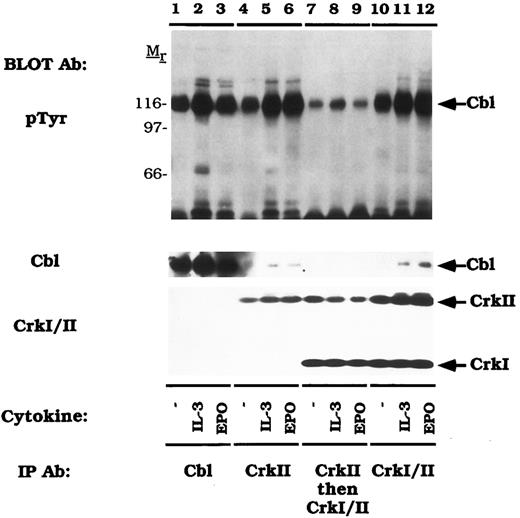
Variable binding of activated Cbl to CrkI and CrkII.We next assessed the relative binding of tyrosine phosphorylated Cbl to CrkI and Crk II (Fig 5). For these studies we used a monoclonal antibody that recognizes both CrkI and CrkII (anti-CrkI/II). Again, IL-3 or EPO stimulated the tyrosine phosphorylation of Cbl (lanes 1-3). An anti-CrkII specific antibody (lanes 4-6) or the anti-CrkI/II antibody (lanes 10-12) immunoprecipitated the tyrosine phosphorylated Cbl polypeptide. Supernatants that had been immunodepleted with the CrkII antibody (lanes 4-6) were next reimmunoprecipitated with the anti-CrkI/II antibody (lanes 7-9). The amount of co-immunoprecipitated tyrosine phosphorylated Cbl in this second immunoprecipitation was markedly reduced when the lysates were precleared with the CrkII-specific antibody (compare lanes 7-9 to lanes 4-6). The pTyr blot was purposefully overexposed to reveal the signal in lanes 7-9. While approximately half of the CrkII was immunoprecipitated with the CrkII specific antibody (CrkI/II immunoblot, lanes 4-6), most of the tyrosine phosphorylated Cbl was found in this immune complex (pTyr immunoblot, lanes 4-6). Taken together, these data demonstrate that activated Cbl binds selectively to CrkII, not CrkI, following cytokine induction.
Tyrosine phosphorylated Cbl preferentially associates with CrkII. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1, 4, 7, and 10), 50 U/mL of murine IL-3 (lanes 1, 5, 8, and 11) or 50 U/mL of human EPO (lanes 3, 6, 9, and 12) for 15 minutes. Following cell lysis, an immunoprecipitation was performed with either an anti-Cbl antibody (lanes 1-3), an anti-CrkII antibody (lanes 4-6), and anti-CrkII antibody followed by an anti-Crk antibody (lanes 7-9), or an anti-Crk antibody (lanes 10-12). The anti-Crk antibody recognizes both CrkI and CrkII. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody and then stripped and reprobed with either a Cbl polyclonal antibody or a Crk monoclonal antibody. Molecular mass standards are indicated.
Tyrosine phosphorylated Cbl preferentially associates with CrkII. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1, 4, 7, and 10), 50 U/mL of murine IL-3 (lanes 1, 5, 8, and 11) or 50 U/mL of human EPO (lanes 3, 6, 9, and 12) for 15 minutes. Following cell lysis, an immunoprecipitation was performed with either an anti-Cbl antibody (lanes 1-3), an anti-CrkII antibody (lanes 4-6), and anti-CrkII antibody followed by an anti-Crk antibody (lanes 7-9), or an anti-Crk antibody (lanes 10-12). The anti-Crk antibody recognizes both CrkI and CrkII. Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody and then stripped and reprobed with either a Cbl polyclonal antibody or a Crk monoclonal antibody. Molecular mass standards are indicated.
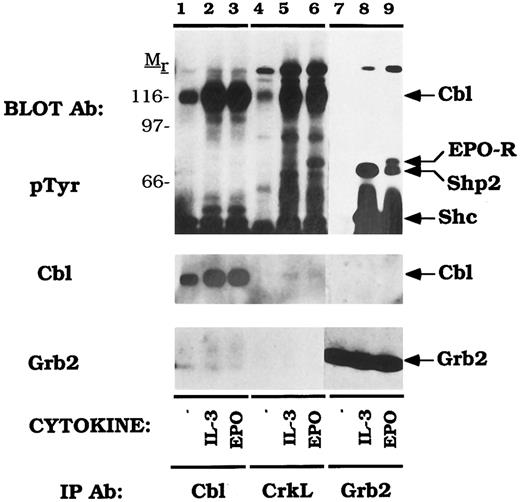
Cbl-Crk association exceeds Cbl-Grb2 association in vivo.Cbl is constitutively associated with the SH3 domains of Grb2 and this fraction of Cbl is a prominent target of tyrosine phosphorylation after stimulation of T and B lymphocyte antigen receptors.41,45 Cbl was also shown to associate with Grb2 in vitro using GST fusions and in Cbl immunoprecipitations in cytokine-dependent UT-7 cells but tyrosine phosphorylation of this fraction was not assessed.57 In order to compare the amount of Cbl associated with either Grb2 or CrkL in DA-3-EPO-R cells, immunoprecipitations were performed with a peptide-specific Grb2 antibody and a CrkL antibody (Fig 6). Immunoprecipitation with a peptide-specific Cbl antibody confirmed that IL-3 or EPO stimulation of DA-3-EPO-R cells increased Cbl tyrosine phosphorylation (lanes 2 and 3) and enhanced association of Cbl with CrkL (lanes 5 and 6) and CrkII (data not shown). Grb2 immunoprecipitated Shc (52 kD) and Shp2 (68 kD) after IL-3 or EPO stimulation (lanes 8 and 9) and the EPO-R (72 kD) after EPO stimulation (lane 9), as previously demonstrated.9,11,15 An additional unknown 145-kD phosphoprotein, most likely SHIP,67-69 a recently identified inositol 5′-phosphatase was observed to co-immunoprecipitate in both the CrkL and Grb2 immunoprecipitates (lanes 3-9). However, no 120 kD phosphoproteins were observed in anti-Grb2 immunoprecipitates (lanes 7-9). The 72-kD EPO-R was shown to co-immunoprecipitate with CrkL (lane 6) and Grb2 (lane 9) EPO stimulation of DA-3-EPO-R cells. Stripping and reprobing the nitrocellulose with an anti-Grb2 antibody demonstrated that a low, but detectable amount of Grb2 co-immunoprecipitated with Cbl (Grb2 immunoblot, lanes 1-3). IL-3 or EPO-dependent association of Cbl with CrkL or CrkII was also observed in Ba/F3-EPO-R cells (data not shown). Thus, while a number of Grb2-associated polypeptides were prominently tyrosine phosphorylated, Cbl was not among these. Moreover, the fraction of Cbl associated with Crk proteins far exceeded the amount of Cbl constitutively bound to Grb2.
Cytokine induced Cbl/Crk complexes exceed Cbl/Grb2 complexes in DA-3-EPO-R cells. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1, 4, and 7), 50 U/mL of murine IL-3 (lanes 2, 5, and 8) or 50 U/mL of human EPO (lanes 3, 6, and 9) for 15 minutes. Following cell lysis, an immunoprecipitation was performed with either an anti-Cbl polyclonal antibody (lanes 1-3), an anti-CrkL polyclonal antibody (lanes 4-6), or an anti-Grb2 polyclonal antibody (lanes 7-9). Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody and then stripped and reprobed with either Cbl or Grb2 polyclonal antibodies. The exposure periods of the pTyr immunoblot are 3 minutes (lanes 1-6) and 1 minute (lanes 7-9). The migration of EPO-R, Shp2, and Shc were determined by stripping and reprobing the membrane with specific antibodies (data not shown). Molecular mass standards are indicated.
Cytokine induced Cbl/Crk complexes exceed Cbl/Grb2 complexes in DA-3-EPO-R cells. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1, 4, and 7), 50 U/mL of murine IL-3 (lanes 2, 5, and 8) or 50 U/mL of human EPO (lanes 3, 6, and 9) for 15 minutes. Following cell lysis, an immunoprecipitation was performed with either an anti-Cbl polyclonal antibody (lanes 1-3), an anti-CrkL polyclonal antibody (lanes 4-6), or an anti-Grb2 polyclonal antibody (lanes 7-9). Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody and then stripped and reprobed with either Cbl or Grb2 polyclonal antibodies. The exposure periods of the pTyr immunoblot are 3 minutes (lanes 1-6) and 1 minute (lanes 7-9). The migration of EPO-R, Shp2, and Shc were determined by stripping and reprobing the membrane with specific antibodies (data not shown). Molecular mass standards are indicated.
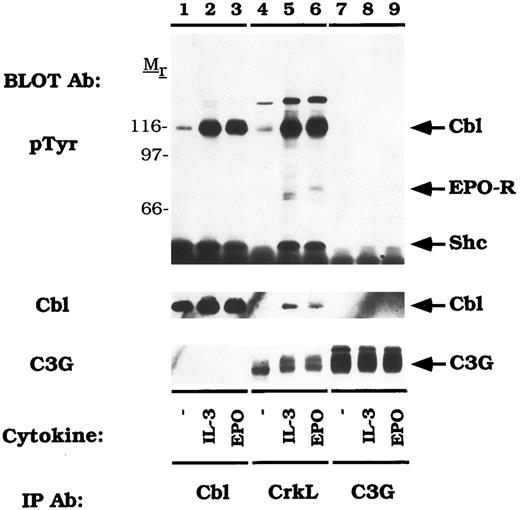
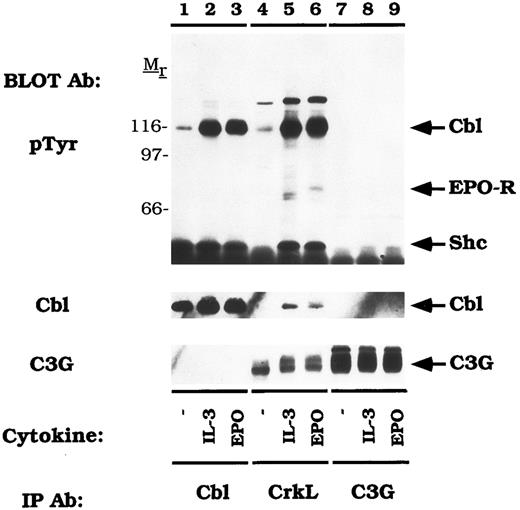
CrkL constitutively associates with the guanine nucleotide release factor, C3G.Previous studies have shown that the Crk SH3 domain binds to a unique guanine nucleotide release factor, C3G.70 Therefore, we tested the association of CrkL and C3G in DA-3-EPO-R cells (Fig 7). When the blot was probed with an anti-C3G antibody, a 130-140 kD protein was observed in the CrkL immunoprecipitation which co-migrated with C3G (C3G immunoblot, lanes 7-9). However, C3G was not detected to co-immunoprecipitate with Cbl (C3G immunoblot, lanes 1-3). Stripping and reprobing the blot with an anti-Cbl antibody confirmed that Cbl associates with CrkL (Fig 7, lanes 5 and 6) but not with C3G (lanes 7-9). CrkL failed to associate with either Cbl or C3G when the membrane was reprobed with a peptide-specific CrkL antibody (data not shown). Interestingly, C3G appeared to migrate with a slower mobility after IL-3 or EPO stimulation (lanes 4-6). This probably corresponds to serine or threonine phosphorylation as previously described for the related GNRF, Sos.71 72
CrkL constitutively associates with C3G. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1, 4, and 7), 50 U/mL of murine IL-3 (lanes 2, 5, and 8) or 50 U/mL of human EPO (lanes 3, 6, and 9) for 15 minutes. Following cell lysis, an immunoprecipitation was performed with either an anti-Cbl antibody (lanes 1-3), an anti-CrkL antibody (lanes 4-6), or an anti-C3G antibody (lanes 7-9). Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody and then reprobed with either a Cbl or C3G polyclonal antibodies. The assignment of EPO-R and Shc was determined by stripping and reprobing the membrane with peptide-specific antibodies (data not shown). Molecular mass standards are indicated.
CrkL constitutively associates with C3G. DA-3-EPO-R cells were depleted of cytokine for 8 hours and stimulated with no factor (lanes 1, 4, and 7), 50 U/mL of murine IL-3 (lanes 2, 5, and 8) or 50 U/mL of human EPO (lanes 3, 6, and 9) for 15 minutes. Following cell lysis, an immunoprecipitation was performed with either an anti-Cbl antibody (lanes 1-3), an anti-CrkL antibody (lanes 4-6), or an anti-C3G antibody (lanes 7-9). Immune complexes were resolved by SDS-PAGE and blotted to nitrocellulose. The immunoblot was probed with 4G10 monoclonal anti-phosphotyrosine antibody and then reprobed with either a Cbl or C3G polyclonal antibodies. The assignment of EPO-R and Shc was determined by stripping and reprobing the membrane with peptide-specific antibodies (data not shown). Molecular mass standards are indicated.
There was an EPO-dependent association of the 72 kD phosphoprotein with CrkL (Fig 6; pTyr immunoblot, lane 6; Fig 7, pTyr immunoblot, lane 6) and with C3G on long exposures of the anti-phosphotyrosine immunoblot (data not shown). Stripping and reprobing these blots with an anti-EPO-R antibody confirmed the identity of this phosphoprotein (data not shown). The EPO-R contains a sequence motif Y484SHP, which represents a potential binding site for the Crk SH2 domain.73 However, the EPO-R was only shown to associate with CrkL (Figs 6 and 7) and not with CrkII (Figs 4 and 5). Whether this presents differences in antibody affinity or individual Crk SH2 domain selectivity remains to be investigated.
DISCUSSION
Cbl has emerged as an important signal transduction protein downstream of a number of tyrosine kinase associated cell surface receptors. Recent analyses have focused on delineating the association of Cbl with other signaling proteins, since Cbl possesses a large proline-rich region that mediates binding to SH3 domains and a number of potential tyrosine phosphorylation sites. One phosphorylation-dependent interaction of significant interest is the complex of Cbl with Crk proteins, which was observed constitutively in Bcr-abl transformed cells 30-32 and on receptor stimulation of B47 and T lymphocytes63,64,74 and EGF-dependent mammary cells.53 Given that Bcr-abl–induced phosphorylation commonly involves substrates normally involved in growth factor signaling we have examined the association of Cbl and Crk in IL-3 and EPO-dependent hematopoietic cells.
In the current work, we show that IL-3 and EPO stimulation of cytokine-dependent hematopoietic cells activates tyrosine phosphorylation of Cbl. In other experiments, Cbl was phosphorylated in response to IL-2, IL-15, and EPO but not IL-4 stimulation of CTLL-EPO-R cells (data not shown). Tyrosine phosphorylation of Cbl resulted in its association with CrkL and CrkII. IL-3 and EPO dependent Cbl tyrosine phosphorylation displays rapid induction similar to other substrates of cytokine-dependent tyrosine phosphorylation. These data complement those describing constitutive association of Cbl and CrkL in cell lines transformed with Bcr-abl30-32 and the T-cell receptor dependent binding of Cbl and Crk adaptor proteins.63,64 74
Ras activation is mediated by the interaction of the adaptor protein Grb2 with the GNRF, Sos, resulting in the conversion of Ras to its GTP-bound form.18-24 A parallel signaling pathway has been suggested which couples the Crk adaptor proteins to the GNRF, C3G, which acts to convert Rap1 to an activated state.62 Here we show a stable association of C3G with CrkL. Although a ternary Cbl-CrkL-C3G complex could not be detected in IL-3 or EPO-dependent cells (Fig 7), such a complex was observed in T-cell receptor stimulated lymphocytes engineered to overexpress Cbl.64 Therefore, the ability to detect a ternary Cbl-CrkL-C3G complex may be dependent on the expression level of these proteins, antibody specificity, or stability of this complex under the lysis and/or immunoprecipitation conditions used in this study. The cytokine-dependent stimulation of Rap1 remains to be tested.
Cbl binds to SH3 domains of Grb239,41,45,49,53,55,63 and thereby forms a constitutive complex distinct from the Grb2-Sos complex. Notably, this pool of Cbl is a target of rapid tyrosine phosphorylation on stimulation of T-39,41 and B-lymphocyte antigen receptors45 and the EGF receptor49,53 Surprisingly, here we show that Grb2-associated Cbl is a substantially smaller pool compared with the amount of Cbl that is associated with Crk proteins. More importantly, Grb2 associated Cbl was not detectably tyrosine phosphorylated on IL-3 or EPO stimulation. Thus, Cbl-Crk is the predominant in vivo association in factor-dependent hematopoietic cells as compared with the Cbl-Grb2 complex. Earlier published in vitro studies completed with bacterially expressed fusion proteins must be interpreted with caution. Given that Grb2 and Crk proteins preferentially associate with Sos and C3G, respectively, it appears that selective Crk-C3G complex formation may regulate the balance of nucleotide exchange factors on cytokine stimulation. In this regard it is noteworthy that the C3G target, Rap1, has been shown to down-regulate Ras activity.62
It is unclear how the EPO-R signals to Cbl. We have not obtained any evidence that Cbl associates with the EPO-R. However, tyrosine phosphorylated Cbl binds CrkL and/or CrkII. Both the EPO-R (Y484SHP) and Cbl (Y360LFP, Y681MTP, and Y774DVP) contain putative binding sites for the Crk SH2 domain.73 In vitro mixing experiments using the isolated SH2 domain of Crk expressed as a GST fusion protein demonstrate that in vitro Crk can bind both to Cbl and the EPO-R (data not shown). Cbl Y774 is thought to mediate Crk binding based on phosphopeptide competition studies completed in the analysis of T-cell receptor activation.64 65 Crk proteins bind to both EPO-R and Cbl in vitro; however, in vivo the predominant complex appears to be Cbl-Crk. Mutagenesis of the corresponding tyrosines of Cbl and the EPO-R will be necessary to better address these questions. Alternatively, the EPO-R could signal to Cbl by novel adaptor proteins.
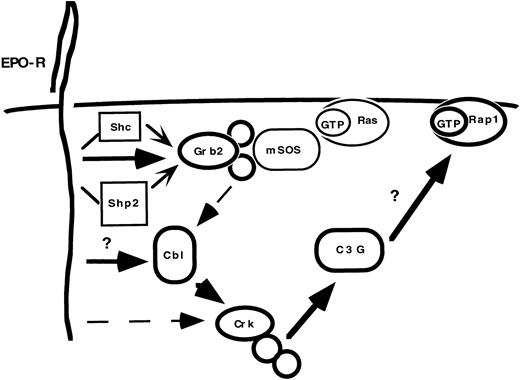
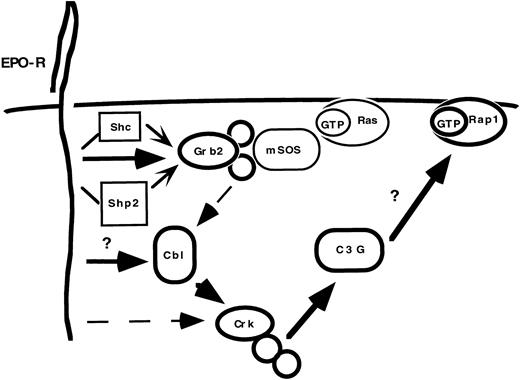
Taken together, our data support two parallel and interrelated signal transduction pathways downstream of the EPO-R (Fig 8). EPO-R activation results in the dose- and time-dependent tyrosine phosphorylation of Cbl. Once phosphorylated, Cbl can interact with complexes of Crk adaptor proteins, which associate with a specific GNRF, C3G. It is unclear how Cbl couples to the EPO-R. However, immunoprecipitation experiments demonstrate that only a small fraction of Cbl associates with Grb2 in DA-3 Cells. Crk proteins can potentially interact with the tyrosine phosphorylated EPO-R through an SH2-dependent association. However, immunoprecipitation experiments reveal that CrkL, CrkII, and CrkI associate with Cbl. Future experiments will address whether EPO activates Rap1.
EPO activates the Cbl-Crk-C3G cascade. EPO activates multiple signaling cascades within hematopoietic cells. A critical adaptor protein necessary for Ras activation, Grb2, can bind constitutively to Cbl. However, the predominant complex following cytokine activation is the Cbl-Crk complex. The preferred complexes are indicated by bold lines and larger arrowheads. Dashed lines indicate associations that occur in vitro, based on immunoprecipitation experiments (ie, Grb2-Cbl and EPO-R-Crk), but may have lesser significance in vivo. It remains unclear how EPO-R couples to Cbl and whether EPO-R activates Rap1.
EPO activates the Cbl-Crk-C3G cascade. EPO activates multiple signaling cascades within hematopoietic cells. A critical adaptor protein necessary for Ras activation, Grb2, can bind constitutively to Cbl. However, the predominant complex following cytokine activation is the Cbl-Crk complex. The preferred complexes are indicated by bold lines and larger arrowheads. Dashed lines indicate associations that occur in vitro, based on immunoprecipitation experiments (ie, Grb2-Cbl and EPO-R-Crk), but may have lesser significance in vivo. It remains unclear how EPO-R couples to Cbl and whether EPO-R activates Rap1.
Cbl tyrosine phosphorylation has been demonstrated in several systems suggesting that it plays a vital role in mitogenesis and/or other cellular responses to receptor stimulation. The present findings suggest an approach to assess the role of Cbl-Crk association in mitogenesis through overexpression of various mutant Cbl and Crk proteins in factor-dependent hematopoietic cell lines.
ACKNOWLEDGMENT
We acknowledge the kind gift of IL-15 from David Cosman and Judy Giri, Immunex Corp, Seattle, WA. We thank Bernard Mathey-Prevot, Martin Carroll, Cheryl Miller, and Sonya Penfold for helpful comments on the manuscript.
Supported by the National Institutes of Health Grant No. RO1 DK 43889-01 to A.D.D. and AR6308 to H.B. and American Cancer Society Grant No. IM-770 to H.B. D.L.B. is a Special Fellow of the Leukemia Society of America and is also supported by the Medical Research Council of Canada. K.A.R. is a predoctoral fellow of the Howard Hughes Medical Institute and Ryan Foundation. A.D.D. is a Scholar of the Leukemia Society of America.
Address reprint requests to Alan D. D'Andrea, MD, Dana-Farber Cancer Institute, Pediatric Oncology, 44 Binney St, Boston, MA 02115.