Abstract
We have previously shown that FLT-3 ligand (FL) mobilizes murine hematopoietic primitive and committed progenitor cells into blood dose-dependently. Whether FL also acts synergistically with granulocyte colony-stimulating factor (G-CSF ) to induce such mobilization has now been investigated. Five- to 6-week-old C57BL/6J mice were injected subcutaneously with recombinant human G-CSF (250 μg/kg), Chinese hamster ovarian cell-derived FL (20 μg/kg), or both cytokines daily for 5 days. The number of colony-forming cells (CFCs) in peripheral blood increased approximately 2-, 21-, or 480-fold after administration of FL, G-CSF, or the two cytokines together, respectively, for 5 days. The number of CFCs in bone marrow decreased after 3 days but was increased approximately twofold after 5 days of treatment with G-CSF. The number of CFCs in the bone marrow of mice treated with both FL and G-CSF showed a 3.4-fold increase after 3 days and subsequently decreased to below control values. The number of CFCs in spleen was increased 24.2- and 93.7-fold after 5 days of treatment with G-CSF alone or in combination with FL, respectively. The number of colony-forming unit-spleen (CFU-S) (day 12) in peripheral blood was increased 13.2-fold by G-CSF alone and 182-fold by G-CSF and FL used together after 5 days of treatment. Finally, the number of preCFU-S mobilized into peripheral blood was also increased by the administration of FL and G-CSF. These observations show that FL synergistically enhances the G-CSF–induced mobilization of hematopoietic stem cells and progenitor cells into blood in mice, and that this combination of growth factors may prove useful for obtaining such cells in humans for transplantation.
SMALL NUMBERS of hematopoietic stem cells and progenitor cells are present in the peripheral blood (PB) of mice, dogs, and humans,1-4 and large numbers of these cells are mobilized into blood by cytotoxic agents,5 various growth factors,6-8 or combinations of these.9,10 The FLT-3 ligand (FL) is a ligand for the FLT3/FLK2 tyrosine kinase receptor expressed on hematopoietic stem cells11,12 and stimulates the proliferation of hematopoietic stem cells and primitive progenitor cells in combination with other growth factors.13-15 We have previously shown that FL can mobilize murine hematopoietic primitive and committed progenitor cells into the blood.16 Given the potential clinical application of this molecule in the collection of allogeneic PB stem cells (PBSCs) from healthy donors for transplantation, it is important to establish a potent, nontoxic dosage regimen. Recently, granulocyte colony-stimulating factor (G-CSF ) has been investigationally applied to mobilize PBSCs for allogeneic transplantation17-19; however, the use of G-CSF in high doses is associated with adverse effects such as general fatigue and bone pain.20
We have now investigated whether FL and G-CSF exhibit synergistic effects on the mobilization of PBSCs. We administered FL and G-CSF, alone or in combination, to mice and then assayed for the presence of colony-forming cells (CFCs) in the PB, bone marrow (BM), and spleen, as well as for colony-forming unit-spleen (CFU-S) and preCFU-S in the PB.
MATERIALS AND METHODS
Mice
Five- to 6-week-old C57BL/6J mice, with body weight of 20 to 25 g, were obtained from Clea Japan (Osaka, Japan) and maintained in our animal facility.
Growth Factors
Chinese hamster ovarian (CHO) cell-derived FL was kindly provided by Dr Stewart Lyman (Immunex, Seattle, WA). Recombinant mouse (rm) stem cell factor (SCF ), rm granulocyte-macrophage CSF (GM-CSF ), and rm interleukin-3 (IL-3), as well as recombinant human (rh) G-CSF and rh erythropoietin (Epo) were kindly provided by Kirin Brewery (Tokyo, Japan).
Cell Harvesting
Mice were injected subcutaneously with rh G-CSF at a dose of 250 μg/kg alone or in combination with CHO cell-derived FL at a dose of 20 μg/kg for 5 days. PB was collected before and 3, 5, 7, and 9 days after the onset of treatment, and the number of white blood cells (WBCs) was determined. For all mice, 10 or 15 minutes after the injection of heparin (50 to 100 U), PB was collected from the orbital plexus and pooled before and 3, 5, 7, and 9 days after the onset of treatment. At each time point, one mouse in each group was killed by cervical dislocation, and both tibiae, both femora, and the spleen were dissected. BM cell suspensions were obtained by flushing the bones with 1 mL of Iscove's modified Dulbecco's medium (IMDM) supplemented with 1% bovine serum albumin (BSA) (Sigma, St Louis, MO). Spleen cell suspensions were prepared by mincing the tissue with scissors, passing it through a 21-gauge needle, and then filtering through a 70-μm nylon cell strainer (Becton Dickinson, Franklin Lakes, NJ). PB, BM, and spleen cell suspensions were layered over Ficoll-Metrizoate (Lymphoprep; Nakarai Tesque, Kyoto, Japan) and centrifuged at 1,700 rpm for 30 minutes at 4°C. Cells at the interface were removed and washed twice with phosphate-buffered saline containing 1% BSA. After the last wash, the cell pellet was suspended in IMDM containing 1% BSA.
Committed Progenitor Cell Assay
CFCs, including colony-forming unit GM (CFU-GM), burst-forming unit-erythroid (BFU-E), and CFU-Mix, were estimated by the standard methylcellulose method. One-milliliter suspensions of 5 × 104 to 2 × 105 nucleated PB cells, 1 to 2 × 104 nucleated marrow cells, or 5 × 104 to 1 × 105 nucleated spleen cells were plated in triplicate or quadruplicate. Cultures were supplemented with 20% fetal calf serum (GIBCO, Gaithersburg, MD), 2% 1-glutamine (Nissui, Tokyo, Japan), 5 × 10−5 M 2-mercaptoethanol (Nakarai Tesque, Kyoto, Japan), 1% iron-saturated transferrin (5 × 10−7 mol/L; Sigma, St Louis, MO), rmSCF (100 ng/mL), rmIL-3 (100 U/mL), rmGM-CSF (50 ng/mL), rhG-CSF (50 ng/mL), and rhEpo (2 U/mL). The plates were incubated for 7 days at 37°C in a fully humidified 5% CO2-air atmosphere, and colonies containing more than 50 cells were scored using an inverted microscope.
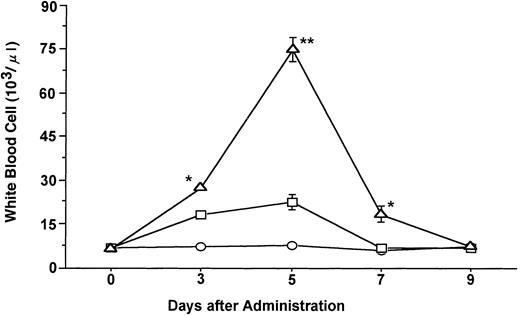
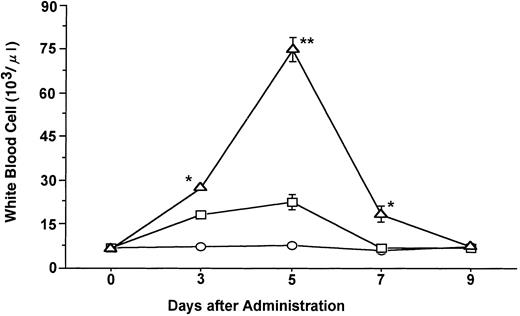
Time course of changes in the mean ± SE of total WBCs during the administration of FL at 20 μg/kg (○), G-CSF at 250 μg/kg (□), and FL plus G-CSF (▵) for 5 days. Points that differ significantly from data for G-CSF administration on the same day are marked: **P < .005, *P < .05.
Time course of changes in the mean ± SE of total WBCs during the administration of FL at 20 μg/kg (○), G-CSF at 250 μg/kg (□), and FL plus G-CSF (▵) for 5 days. Points that differ significantly from data for G-CSF administration on the same day are marked: **P < .005, *P < .05.
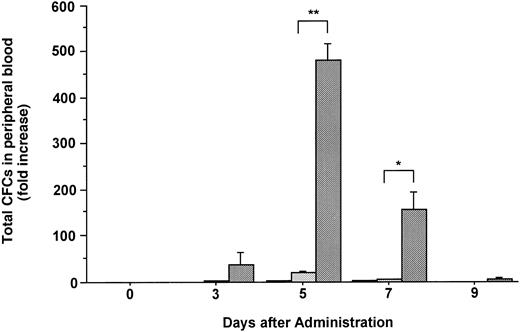
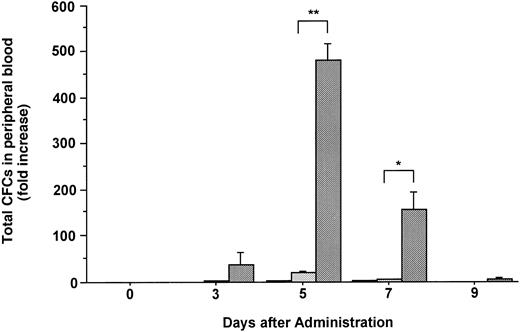
Changes in the number of total CFCs in the PB during the administration of FL at 20 μg/kg (□), G-CSF at 250 μg/kg (), and FL plus G-CSF () for 5 days. Data are mean ± SE and are expressed as fold increases relative to mice before treatment. Points that differ significantly from data for G-CSF administration on the same day are marked: **P < .005, *P < .05.
Changes in the number of total CFCs in the PB during the administration of FL at 20 μg/kg (□), G-CSF at 250 μg/kg (), and FL plus G-CSF () for 5 days. Data are mean ± SE and are expressed as fold increases relative to mice before treatment. Points that differ significantly from data for G-CSF administration on the same day are marked: **P < .005, *P < .05.
CFU-S Assay
For each data point, three to four recipient mice were irradiated with 9-Gy x-rays to prevent the production of endogenous spleen colonies as reported previously.16 Irradiated mice were injected intravenously via the tail vein with 1 to 3 × 105 nucleated blood cells within several hours after the completion of irradiation. The mice were killed by cervical dislocation 12 days later, and the spleen was excised and fixed in Tellyesniczky's fixative. The number of macroscopic spleen colonies was then scored.
PreCFU-S Assay
We investigated whether the combination of FL and G-CSF could mobilize preCFU-S into PB. A spleen from one of three mice that had been irradiated and injected with nucleated blood cells 12 days earlier was excised. Spleen cell suspensions were prepared as described above. After the number of CFU-S (day 12) in other spleens of recipient mice was scored, the single cell suspension was diluted and injected further into irradiated mice (0.3 to 1 colony per mouse). The number of daughter colonies (preCFU-S) was scored 12 days later. The number of colonies generated per CFU-S (day 12) was calculated as described previously,21 and the number of preCFU-S was estimated as total number of preCFU-S per milliliter of PB.
Statistic analysis.Data are expressed as mean ± SE and were analyzed by Student's t-test. A P value of < .05 was considered statistically significant.
RESULTS
Effects of FL and G-CSF on Circulating WBCs
Administration of G-CSF at a dose of 250 μg/kg induced an approximately threefold increase in the total number of WBCs after 5 days of treatment as compared with the number present before treatment (Fig 1). FL at a dose of 20 μg/kg had no effect on the number of WBCs, whereas the combination of FL (20 μg/kg) and G-CSF (250 μg/kg) increased the number of these cells to a markedly greater extent than did G-CSF alone (P < .005). The percentage of neutrophils in the WBC fraction was increased slightly more by the combination of FL and G-CSF than by G-CSF alone. Immature cells, myelocytes and metamyelocytes, were also more increased in the PB of mice given FL and G-CSF (data not shown).
Effects of FL and G-CSF on the Mobilization of Committed Progenitor Cells Into the Circulation
The number of hematopoietic committed progenitor cells in the PB was increased 2.2- and 21.3-fold after 5 days of treatment with FL or G-CSF, respectively (Fig 2). In contrast, the combination of FL and G-CSF caused an approximately 480-fold increase in the total number of CFCs in PB at the same point, which was statistically significant as compared with those given the same dose of G-CSF alone (P < .005). Although various types of CFCs (CFU-GM, BFU-E, and CFU-Mix) were increased in blood, the administration of G-CSF alone or in combination with FL mobilized predominantly CFU-GM (Table 1).
Effects of FL and G-CSF on Committed Progenitor Cell Numbers in the BM and Spleen
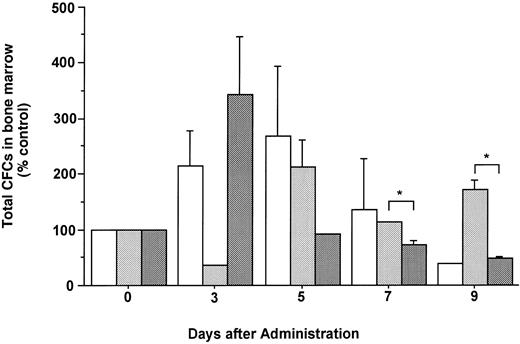
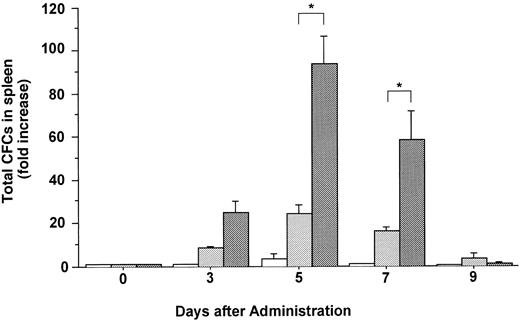
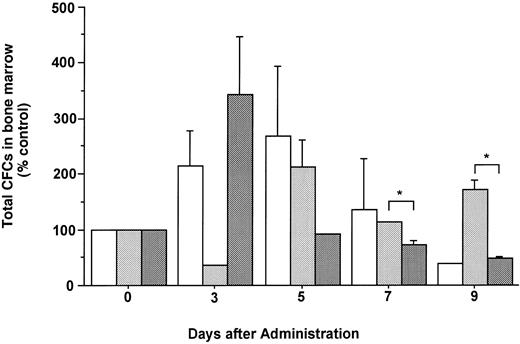
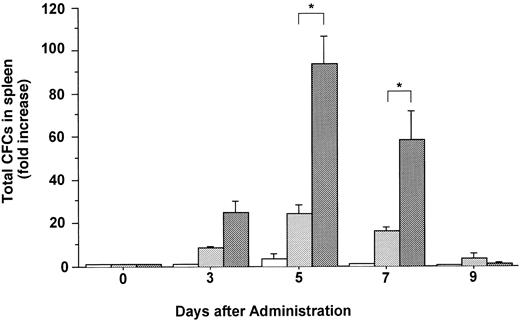
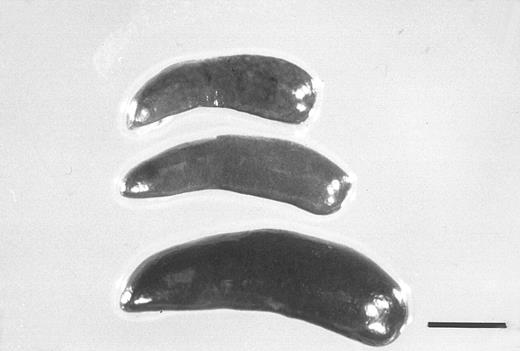
The total number of CFCs in the BM (femora and tibiae) showed a maximal, 2.7-fold increase after 5 days of treatment with FL (Fig 3). The number of CFCs was decreased 3 days after and showed a twofold increase 5 days after the onset of treatment with G-CSF. In mice treated with both FL and G-CSF, the total number of CFCs in BM showed a maximal, 3.4-fold increase after 3 days and then decreased to below control values. The total number of CFCs in the spleen showed a 24.2-fold increase after 5 days of treatment with G-CSF (Fig 4), at which time the spleen was enlarged (Fig 5). The administration of both FL and G-CSF induced marked splenomegaly (Fig 5) and a 93.7-fold increase in the number of total CFCs in spleen 5 days after the onset of treatment (Fig 4) (P < .05).
Changes in the number of total CFCs in the BM during the administration of FL at 20 μg/kg (□), G-CSF at 250 μg/kg (), and FL plus G-CSF () for 5 days. Data are mean ± SE and are expressed as a percentage of total CFC numbers before cytokine administration. Points that differ significantly from data for G-CSF administration on the same day are marked: *P < .05.
Changes in the number of total CFCs in the BM during the administration of FL at 20 μg/kg (□), G-CSF at 250 μg/kg (), and FL plus G-CSF () for 5 days. Data are mean ± SE and are expressed as a percentage of total CFC numbers before cytokine administration. Points that differ significantly from data for G-CSF administration on the same day are marked: *P < .05.
Changes in the number of total CFCs in the spleen during the administration of FL at 20 μg/kg (□), G-CSF at 250 μg/kg (), and FL plus G-CSF () for 5 days. Data are mean ± SE and are expressed as fold increases relative to mice before treatment. Points that differ significantly from data for G-CSF administration on the same day are marked: *P < .05.
Changes in the number of total CFCs in the spleen during the administration of FL at 20 μg/kg (□), G-CSF at 250 μg/kg (), and FL plus G-CSF () for 5 days. Data are mean ± SE and are expressed as fold increases relative to mice before treatment. Points that differ significantly from data for G-CSF administration on the same day are marked: *P < .05.
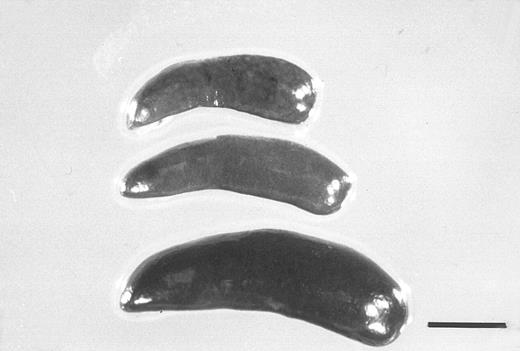
Spleens from a control mouse (top) and from mice treated for 5 days with G-CSF (250 μg/kg) alone (middle), or together with FL (20 μg/kg) (bottom). The bar indicates 5 mm.
Spleens from a control mouse (top) and from mice treated for 5 days with G-CSF (250 μg/kg) alone (middle), or together with FL (20 μg/kg) (bottom). The bar indicates 5 mm.
Effects of FL and G-CSF on CFU-S (Day 12) in PB
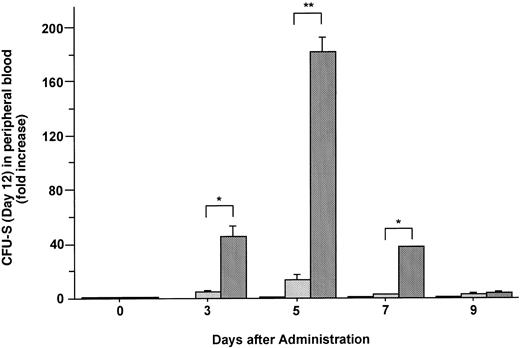
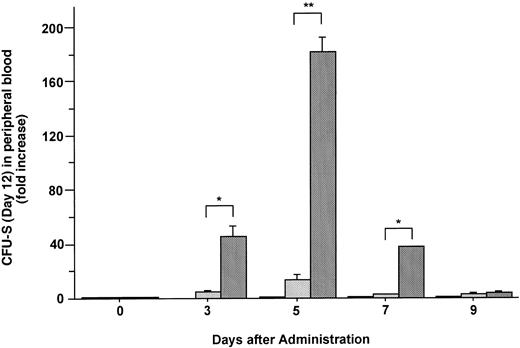
The formation of spleen colonies in the irradiated mice injected with mobilized cells was also measured. Increases in the mean number of CFU-S (day 12) in the blood of mice injected with FL, G-CSF, and the combination of these two factors are plotted as fold increases (relative to control mice analyzed on the same day) in Fig 6. Administration of FL alone did not mobilize CFU-S (day 12) into the blood. Although G-CSF alone did cause an increase of 13.1-fold in CFU-S (day 12), the combination of G-CSF and FL caused an increase of 182-fold in PB CFU-S (day 12) 5 days after the onset of treatment (Fig 6) (P < .005).
Changes in the number of CFU-S (day 12) during the administration of FL at 20 μg/kg (□), G-CSF at 250 μg/kg (), and FL plus G-CSF () for 5 days. Data are expressed as mean ± SE fold increases relative to mice before treatment. Points that differ significantly from data for G-CSF administration on the same day are marked: **P < .005, *P < .05.
Changes in the number of CFU-S (day 12) during the administration of FL at 20 μg/kg (□), G-CSF at 250 μg/kg (), and FL plus G-CSF () for 5 days. Data are expressed as mean ± SE fold increases relative to mice before treatment. Points that differ significantly from data for G-CSF administration on the same day are marked: **P < .005, *P < .05.
Effects of FL and G-CSF on preCFU-S in PB
The mobilization of preCFU-S after administration of G-CSF alone or in combination with FL is shown in Table 2. While G-CSF alone induced a 315-fold increase in preCFU-S in the PB, the combination of FL and G-CSF induced a 1,048-fold increase in the number of preCFU-S in the blood 5 days after the onset of treatment. Thus, the combination of FL and G-CSF strikingly mobilized preCFU-S into the blood.
DISCUSSION
In a previous study we showed that FL can mobilize primitive and committed hematopoietic progenitor cells into the blood in a dose-dependent fashion and that the administration of FL alone at 20 μg/kg mobilizes only small numbers of primitive and committed hematopoietic progenitor cells into blood.16 Therefore, we investigated whether a low dose of FL would have synergistic effects on the mobilizing effect obtained with G-CSF. In mice, FL synergistically enhances the leukocytosis by G-CSF. Moreover, the combination of FL and G-CSF increased the number of more immature granulocytes present in the PB than did either cytokine alone. Although the administration of FL at a low dose of 20 μg/kg did not mobilize CFCs or CFU-S into blood, it significantly potentiated the increase in the number of CFCs in PB and spleen as well as the number of CFU-S (day 12) in the PB induced by G-CSF. The administration of FL mobilized various lineages of CFC into the blood,16 22 whereas approximately 94% of the CFCs mobilized either by G-CSF alone or by FL plus G-CSF were CFU-GM (Table 1). One possibility for this is that the dose of FL was too low to influence the types of CFCs mobilized by G-CSF treatment. Therefore, it would be interesting to study types of CFCs mobilized by higher doses of FL with G-CSF. The number of CFC in the BM was not significantly increased by the administration of FL or G-CSF either alone or in combination. The number of CFC in the BM was maximal after 3 days of treatment with both FL and G-CSF, and subsequently decreased through 9 days after the onset of treatment. In mice injected with G-CSF, the number of these cells was below control values and then increased to as much as twice control values 3 and 5 days, respectively, after the onset of treatment. A peak number of CFCs in the BM of mice treated with FL was apparent after 5 days.
These observations suggest that the mechanism of CFC mobilization by G-CSF plus FL differs from that induced by FL or G-CSF alone. Roberts and Metcalf23 showed that hematopoietic progenitor cells in the BM first move to the PB or spleen and then proliferate in mice treated with G-CSF alone. We have previously shown that redistribution as well as in vivo expansion of hematopoietic progenitor cells appear to contribute to the mobilization of these cells induced by FL in mice16 because the administration of FL alone led to a slight increase in the number of CFC in BM. It is likely that primitive and committed hematopoietic progenitor cells first proliferate more rapidly in the BM of mice treated with G-CSF plus FL than in that of mice treated with either cytokine alone, with the maximal number of HPCs in the BM apparent after 3 days of treatment. These cells then move into the PB and then into the spleen, resulting in maximal numbers being apparent at these sites after 5 days. The number of CFC in the BM of mice treated with both FL and G-CSF decreased to below control values 7 and 9 days after the onset of treatment. One possible explanation for this finding is that the hematopoietic stem cells in the BM of mice treated with both these agents move into the PB or the spleen after hematopoietic stem cells proliferate and differentiate into progenitor cells more than these cells do in mice treated with either agent alone, resulting in a decrease of progenitor cells in the BM. It is therefore likely that the splenomegaly apparent in mice treated with FL and G-CSF results from the movement of the expanded progenitor cells from the BM to the spleen and from the further proliferation of these cells in the spleen.
We also examined the mobilization of preCFU-S into the blood. Whereas G-CSF mobilized preCFU-S, the administration of a low dose of FL potentiated the increase in the number of mobilized preCFU-S by G-CSF. Because preCFU-S may be closely related to long-term reconstituting cells, these findings suggest that FL synergistically enhances the G-CSF-induced mobilization of hematopoietic stem cells into the blood of mice.
Recently, SCF has also been shown to mobilize hematopoietic stem cells and progenitor cells into the PB of primates,24 and clinical trials monitoring this effect of SCF alone or in combination with G-CSF are underway.25-27 However, several subjects in these trials developed respiratory symptoms, including hoarseness, cough, and laryngospasm,26 presumably because SCF induces the proliferation and maturation of mast cells.28,29 Because FL does not affect the growth of mast cells or their degranulation,30 its in vivo administration would be expected to induce fewer and less masked allergic reactions than SCF. The combination of FL with G-CSF may thus prove useful for harvesting PBSCs for transplantation. Further studies are warranted to determine whether FL with G-CSF could have synergistic effects on the mobilization of progenitors in humans.
Address reprint requests to Eishi Ashihara, MD, Second Department of Medicine, Kyoto Prefectural University of Medicine, 465 Kawaramachi Hirokoji, Kamigyoku, Kyoto 602, Japan.