Abstract
We have conducted a pilot study to investigate the role of high-dose therapy and autologous bone marrow/stem cell transplantation (ASCT) during first complete or partial remission in 52 patients with poor-risk aggressive lymphoma. There were 42 patients with intermediate-grade or immunoblastic lymphoma who were considered to be high (60%) and high-intermediate risk (40%) groups at diagnosis based on the age-adjusted International Prognostic Index (IPI) and 10 patients with high-grade, SNCCL (small non-cleaved cell, Burkitt's, and non-Burkitt's), who at presentation had poor-risk features defined as elevated serum lactate dehydrogenase level, stage IV, and bulky mass ≥10 cm. The median age was 34 years (range, 16 to 56 years). Thirty-nine were transplanted in first complete remission and 13 in first partial remission after conventional therapy. Conditioning regimens consisted of total body irradiation (TBI) administered as a single fraction 750 cGy in 3 patients and in fractionated doses for a total of 1,200 cGy in 44 patients, in combination with 60 mg/kg etoposide and 100 mg/kg cyclophosphamide. Five patients with prior radiotherapy received 450 mg/m2 carmustine instead of TBI. Stem cell sources were either bone marrow and/or peripheral blood. No in vitro purging was used. All patients engrafted. Two SNCCL patients died of venoocclusive disease at 25 days and acute leukemia at 27 months posttransplantation. There were six relapses at 1.5 to 12.8 months posttransplantation. At a median follow-up of 44 months (range, 1 to 113 months), the estimated 3-year overall survival (OS) and disease-free survival (DFS) for all patients was 84% (95% confidence interval [CI], 70% to 92%) and 82% (95% CI, 68% to 91%), respectively. In the subset of patients with intermediate-grade and immunoblastic lymphoma, the 3-year DFS was 89% (95% CI, 74% to 96%) for all patients, 87% (95% CI, 67% to 96%) for high-risk patients, and 92% (95 CI, 61% to 99%) for high-intermediate risk patients. The 3-year OS and DFS for SNCCL patients were identical at 60% (95% CI, 30% to 84%). These results suggest that high-dose therapy and ASCT during first remission may improve the survival and prognosis of patients with poor-risk intermediate- and high-grade lymphoma. A prospective randomized study comparing high-dose therapy and ASCT with conventional chemotherapy in IPI high-risk patients with aggressive non-Hodgkin's lymphoma should be undertaken.
ALTHOUGH APPROXIMATELY 40% of the patients with advanced stage intermediate- and high-grade non-Hodgkin's lymphoma (NHL) can be cured with combination chemotherapy, the majority still die of their disease. Several prognostic variables have been identified by several groups of investigators in an attempt to identify a subset of patients with aggressive NHL who have a poor prognosis and are unlikely to be cured with conventional chemotherapy1 and, therefore, may be a candidate for more aggressive therapy. A variety of clinical features have been consistently associated with a poor prognosis in aggressive NHL, including features that reflect the growth and invasive potential of the tumor (tumor stage, serum lactate dehydrogenase [LDH] level, and number of extra nodal sites), the patient's response to tumor (performance status), and the patient's ability to tolerate therapy (age and performance status).2 The International Prognostic Index (IPI) model has been developed based on age, tumor stage, serum LDH level, performance status (PS), and number of extra nodal sites.3 This IPI separates patients at the time of diagnosis into four risk groups: low, low-intermediate, high-intermediate, and high, with predicted 5-year survival rates of 73%, 51%, 43%, and 26%, respectively. For patients younger than 60 years of age, an age-adjusted IPI based on stage, LDH level, and performance status identified four risk groups with predicted 5-year survival rates of 83%, 69%, 46%, and 32%, respectively. Therefore, the IPI and the age-adjusted IPI can be used to identify patients with higher risks of relapses who may be candidates for more innovative therapy.
The IPI was specially developed to predict the outcome in patients with diffuse mixed, diffuse large-cell, or large-cell immunoblastic lymphoma (International Working Formulation categories' F, G, and H). This index has not been routinely applied to patients with high-grade SNCCL (small noncleaved cell, Burkitt's, and non-Burkitt's). However, several adverse prognostic features that are either direct or indirect measures of tumor burdens have also been identified in patients with SNCCL, including an elevated LDH level greater than 500 IU/L, an unresected tumor bulk measuring greater than 10 cm, and advanced stage with bone marrow (BM) involvement.4 5 Patients with these poor prognostic features had a 2-year relapse-free survival (RFS) of only 29%. Treatment of such high-risk patients with intensive therapy such as high-dose chemo/radiotherapy and BM transplantation (BMT) may be appropriate in an attempt to prevent both systemic and central nervous system relapses.
A small subset of patients with primary mediastinal large-cell lymphoma (MLCL) who at presentation have pleural effusion, elevated LDH level, and multiple sites of extranodal involvement represent another group of poor-risk aggressive NHL.6 These patients have an approximately 80% chance of relapses after initial response to induction chemotherapy and only a 20% chance for long-term disease-free survival (DFS). In addition, the risk of relapse is extremely high in patients who have residual mass with persistent gallium 67 avidity after treatment. Given the poor outcome and the high risk of relapse in these patients, it seems appropriate to explore the role of high-dose therapy and autologous BM/stem cell transplantation (ASCT) during first complete remission (CR)/partial remission (PR) in these poor-risk patients with MLCL.
We have previously reported the results of our pilot study of high-dose therapy and ASCT as consolidation therapy during first CR/PR in 20 patients with poor-risk intermediate- and high-grade lymphoma.7 In that study, the poor-risk features included elevated LDH level, bulky mass greater than 10 cm, advanced stage III or IV, and two or more extranodal sites. Our preliminary results were encouraging, with a DFS of 84% at a median follow-up of 34 months. Since the introduction of the IPI in 1993, we have used the IPI as criteria for selection of patients with aggressive lymphoma and included only high-risk and high-intermediate risk patients for high-dose therapy and ASCT during first CR/PR. We extended our study to include patients with poor-risk SNCCL and primary MLCL with poor-risk features as outlined earlier. We report here the results of high-dose therapy and ASCT during first CR/PR in 52 patients with poor-risk lymphoma, including the 20 patients reported previously.7
PATIENTS AND METHODS
Selection of patients. Patients were selected for this study based on the following eligibility criteria: (1) age between 15 and 60 years; (2) a confirmed histologic diagnosis of high-grade and intermediate-grade NHL; (3) poor prognostic factors at diagnosis included stage III or IV, LDH level greater than one times normal, and ECOG performance status 2 through 4 or at diagnosis were considered to be high (3 risk factors) and high-intermediate risk (2 risk factors) groups based on an age-adjusted IPI; (4) attain a CR or PR to first-line standard conventional chemotherapy; (5) normal renal, hepatic, pulmonary, and cardiac function; and (6) negative human immunodeficiency virus antibody.
Patients were referred for transplant evaluation after having received some if not most of the induction therapy by the community hematologists and medical oncologists. High-risk patients were eligible for high-dose therapy and ASCT after they had achieved PR or CR with conventional chemotherapy. High-intermediate risk patients were eligible for ASCT only if they had achieved a PR with conventional chemotherapy. Patients with primary MLCL with or without sclerosis who at diagnosis had elevated LDH level with bulky mediastinal mass greater than 10 cm associated with a pleural effusion on chest radiography or computer tomography or who had persistent mediastinal mass with positive posttreatment gallium 67 (67Ga) scans were also eligible for ASCT after achieving a first CR or PR. In addition, SNCCL patients were eligible for ASCT after achieving a first CR or PR who at presentation had all of these following factors: elevated LDH level greater than 500 IU/L, unresectable bulky mass greater than 10 cm, and stage IV with BM involvement. In our study, PR was defined as greater than 50% to 75% reduction of tumor mass with or without abnormal 67Ga scans. CR was defined as the complete disappearance of all measurable and evaluable disease or greater than 75% reduction of tumor mass with negative 67Ga scans.
Patient characteristics. Between May 1987 and January 1997, 52 patients with intermediate-grade, immunoblastic lymphoma (IL) and SNCCL underwent high-dose chemo/radiotherapy or high-dose chemotherapy followed by ASCT during first CR/PR at the City of Hope National Medical Center. All patients were treated with induction chemotherapy for a minimum of three cycles or until they had achieved a CR or minimal disease state. Some patients also received radiotherapy to the area of bulky disease at the time of presentation due to airway obstruction, superior vena cava syndrome, or spinal cord compression. All patients were informed of the investigational nature of this study and informed consent was obtained from each patient in accordance with institutional guidelines.
The patients' characteristics at diagnosis for intermediate grade and IL (group I) are shown in Table 1. There were 42 patients: 30 men (71%) and 12 women (29%). The median age was 33.5 years (range, 16.5 to 56 years). Stages at diagnosis included 5 in stage II (12%), 8 in stage III (19%), and 29 in stage IV (69%). Histology included 21 diffuse large-cell (50%), 5 diffuse mixed (12%), 6 follicular large-cell (14%), and 10 IL (24%). Thirty-three patients (79%) had extranodal involvement, including pleura in 12, gastrointestinal (GI) tract in 10, lung in 6, liver in 5, BM in 3, bone in 4, kidney in 5, and skin in 2. Sixteen patients (38%) had ≥2 sites of extranodal involvement. Serum LDH levels were elevated in 35 patients (83%) and were normal in 7 patients (17%). ECOG PS included 1 in 5 patients (12%), 2 in 18 (43%), and 3 in 19 (45%). Twenty-nine patients (70%) had bulky diseases ≥10 cm. Based on the age-adjusted IPI, 25 patients (60%) were in the high-risk group and 17 (40%) were in the high-intermediate group. The median number of cycles of combination chemotherapy administered before ASCT was 6 (range, 3 to 10). Ten patients received 12 weeks of MACOP-B (methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin) before ASCT. Details regarding induction chemotherapy regimens used before ASCT are provided in Table 1. Five patients (12%) also received involved field radiotherapy to sites of bulky disease as part of their initial therapy. The median time from diagnosis to ASCT was 6.2 months (range, 3 to 12 months). Thirty-two were transplanted in first CR (76%) and 10 in first PR (24%).
Thirteen patients with primary MLCL comprised a subset of patients in group I. All patients had bulky mass ≥10 cm at diagnosis, 11 had an elevated LDH level, 8 had pleural effusions at presentation, 4 had ≥2 extranodal sites, and 4 had a positive posttreatment 67Ga scan. According to the age-adjusted IPI, 7 patients were in the high-intermediate and 6 were in the high-risk group. Their characteristics are shown in Table 2.
The characteristics of 10 patients with SNCCL are shown in Table 3. There were 7 men and 3 women. The median age was 38 years (range, 22.5 to 56 years). All but 2 had stage IV disease. All patients also had extranodal involvement, with 80% having ≥2 extranodal sites, including GI tract (6), liver (4), pleura (3), BM (3), bone (1), kidney (1), and skin (1). ECOG PS at diagnosis were PS 2 (4 patients) and PS 3 (6 patients). Serum LDH levels were elevated in 8 (80%). According to the age-adjusted IPI, there were 8 patients in the high and 2 in the high-intermediate risk group. Prior combination chemotherapy used before ASCT is listed in Table 3. One patient also received involved field radiation to a bulky abdominal mass. At the time of ASCT, 7 patients were in first CR, whereas 3 patients with residual bulky mass were considered to be in first PR. The median time from diagnosis to BMT was 5.8 months (range, 3.6 to 12.2 months).
Preparative regimen. Unless precluded by previous radiotherapy, all patients received a total body irradiation (TBI)-based regimen. These included fractionated TBI of 1,200 cGy at 120 to 200 cGy per fraction with 50% transmission lung blocks from days −8 to −5 (44 patients) or single-dose TBI at 750 cGy (3 patients) in combination with 60 mg/kg etoposide on day −4 and 100 mg/kg cyclophosphamide on day −2. Another 5 patients with prior radiation treatment received 450 mg/m2 carmustine administered as either a single infusion over 2 hours or divided over 3 days instead of TBI.
Autologous marrow harvest, cryopreservation, and reinfusion. All patients had undergone bilateral iliac crest BM biopsies that showed no microscopic evidence of lymphomatous involvement at the time of BM harvesting and at BMT. In addition, cytogenetic studies and immunophenotyping were normal in all patients. Patients with prior BM involvement at the time of diagnosis underwent reinfusion of peripheral blood stem cells (PBCSs) collected via leukapheresis procedures that have been previously described.8 Before 1989, all patients also underwent three PBSC collections and received a combination of BM and PBSCs. Beginning in 1992, PBSCs were used in preference to BM, even in those without marrow involvement. PBSCs were collected after mobilization with granulocyte colony-stimulating factor (G-CSF ) with or without chemotherapy. The marrow and PBSCs were reinfused 48 hours after completion of cyclophosphamide treatment (day 0). Since 1991, G-CSF was administered routinely after BM and PBSC reinfusion when it became commercially available.
Seven patients (13%) received BM only, 26 (50%) received a combination of BM and PBSCs, and 19 (37%) received PBSCs only. The median number of nucleated cells dose reinfused was 2.04 × 108/kg (range, 0.14 to 4.94 × 108/kg) for marrow and the PBSC mononuclear cell dose was 5.4 × 108/kg (range, 1.6 to 11.9 × 108/kg) for patients receiving PBSCs alone.
Supportive care. All patients were housed in high efficiency particulate air-filtered isolation rooms during the period of granulocytopenia. Nonabsorbable antibiotics (neomycin and oral vancomycin) for GI decontamination were administered to all patients. Trimethoprim-sulfamethoxazole was administered from day −13 to −3 and prophylaxis was reinstituted at the time of discharge and continued until 6 months posttransplantation. Empiric broad spectrum antibiotics and parenteral nutrition were used as clinically indicated. Low-dose amphotericin-B (0.1 to 0.2 mg/kg) was administered on day +1 and continued daily until the day of discharge.9 All blood components were irradiated to 1,500 cGy. Patients who were seronegative for cytomegalovirus (CMV) received CMV-seronegative blood products.
Statistical methods. Descriptive measures on continuous data were summarized as medians and ranges. Comparisons of continuous variables were made using the Wilcoxon rank-sum test. Survival analyses were performed using the product-limit method of Kaplan and Meier, with 95% confidence intervals (CI) calculated using the method of logit limits. Survival comparisons were made using the log-rank test.
RESULTS
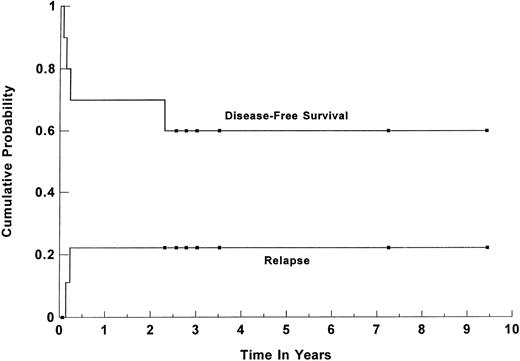
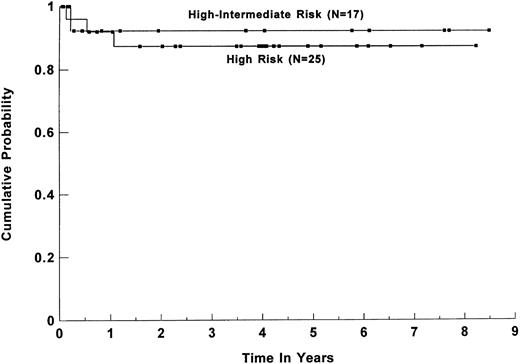
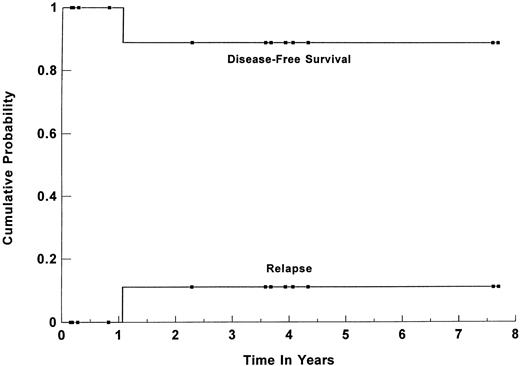
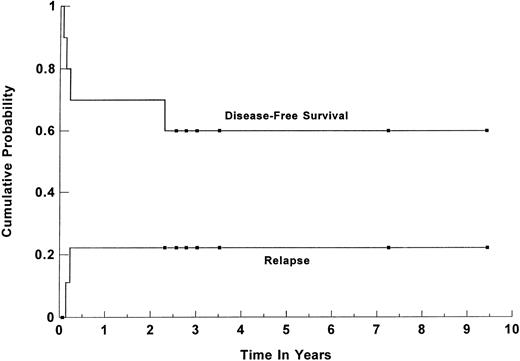
Survival. Forty-five patients (86%) are alive and disease-free at a median follow-up of 44 months (range, 1 to 113 months) for all surviving patients. The estimated 3-year overall survival (OS) and DFS for all patients were 84% (95% CI, 70% to 92%) and 82% (95% CI, 68% to 91%), respectively (shown in Fig 1). For group I, the 3-year OS and DFS were 91% (95% CI, 76% to 97%) and 89% (95% CI, 74% to 96%), respectively. When the group was separated based on the age-adjusted IPI risk group, the 3-year OS and DFS for the 25 patients in the high-risk group were 91% (95% CI, 71% to 98%) and 87% (95%CI, 67% to 96%), respectively (Fig 2). For the 17 patients in the high-intermediate risk group, the 3-year OS and DFS were 91% (95% CI, 56% to 99%) and 92% (95% CI, 61% to 99%), respectively. There was no statistically significant difference in the OS and DFS between patients who were transplanted in first CR versus first PR. For the subset of 13 patients with primary MLCL, both the estimated 3-year DFS and OS plateaued at 89% (95% CI, 50% to 98%). Their survival curve is shown in Fig 3. The 3-year OS and DFS for the 10 SNCCL patients were both 60% (95% CI, 30% to 84%). The survival curve is shown in Fig 4.
Kaplan-Meier product limit estimate of the cumulative probabilty of DFS and relapse for 52 patients with poor-risk intermediate- and high-grade lymphoma who underwent high-dose therapy and ASCT while in first CR or PR. (▪) Censored data points.
Kaplan-Meier product limit estimate of the cumulative probabilty of DFS and relapse for 52 patients with poor-risk intermediate- and high-grade lymphoma who underwent high-dose therapy and ASCT while in first CR or PR. (▪) Censored data points.
DFS for 42 patients with intermediate-grade and immunoblastic lymphoma based on the age-adjusted IPI risk group: high and high-intermediate risk group. P = .72 (log-rank test). (▪) Censored data points.
DFS for 42 patients with intermediate-grade and immunoblastic lymphoma based on the age-adjusted IPI risk group: high and high-intermediate risk group. P = .72 (log-rank test). (▪) Censored data points.
Kaplan-Meier estimate of probability of DFS and relapse for the 13 patients with poor-risk primary MLCL who were transplanted during first CR or PR. (▪) Censored data points.
Kaplan-Meier estimate of probability of DFS and relapse for the 13 patients with poor-risk primary MLCL who were transplanted during first CR or PR. (▪) Censored data points.
Kaplan-Meier estimate of probability and relapse for 10 patients with poor-risk high-grade small non-cleaved cell lymphoma undergoing high-dose therapy and ASCT during first CR/PR. (▪) Censored data points.
Kaplan-Meier estimate of probability and relapse for 10 patients with poor-risk high-grade small non-cleaved cell lymphoma undergoing high-dose therapy and ASCT during first CR/PR. (▪) Censored data points.
Relapse. Six patients (4 group I and 2 SNCCL) relapsed at 1.5 to 12.8 months posttransplantation. All relapses occurred at the previous sites of bulky disease. The 3-year probability of relapse for all patients was 13% (95% CI, 6% to 26%) and for patients with SNCCL (group II) was 22% (95% CI, 6% to 58%). For group I patients, the 2-year probability of relapse was 8% (95% CI, 1% to 39%) and 13% (95% CI, 4% to 33%) for patients in the high-intermediate and high-risk groups, respectively. There was no difference in the relapse rate between patients transplanted in first CR or first PR.
Toxicity. The major nonhematologic toxicities of the preparative regimens consisted primarily of oral and GI mucositis and skin inflammation related to high-dose etoposide and TBI. The majority of patients required continuous infusion of narcotics for pain control and parenteral nutrition support. All patients became febrile while neutropenic and required therapy with broad-spectrum antibiotics. There was 1 early death from liver veno-occlusive disease (VOD) with multiorgan failure at 25 days posttransplantation. This patient had SNCCL with liver involvement and extensive bulky abdominal masses and had received involved radiation to the abdomen before ASCT. A second patient developed mild VOD that resolved. One patient developed carmustine (BCNU)-associated interstitial pneumonitis that responded to corticosteroid. Therapy-induced myelodysplasia/acute leukemia occurred in 1 patient who subsequently died of progressive leukemia at 27 months post-ASCT. Another patient was found to have marrow cytogenetic abnormality on routine marrow study at 50 months post-ASCT. This patient is now alive 8 years posttransplantation and so far there is no morphologic evidence of myelodysplasia or acute leukemia.
Engraftment. All patients achieved a complete hematologic recovery. The median time to reach an absolute granulocyte count of greater than 0.5 × 109/L was 12 days (range, 8 to 48 days). The median time to a self-sustaining platelet count of 20 × 10 9/L was 13 days (range, 7 to 100 days). The hematopoietic recovery was significantly faster in patients receiving PBSCs with or without marrow than those receiving BM alone (P = .01).
DISCUSSION
The combination chemotherapy regimen of CHOP was first introduced as an effective regimen for treatment of aggressive NHL in 197510 and has since remained the best available combination therapy for patients with advanced stage intermediate- and high-grade NHL. Several generations of combination chemotherapy using six to eight chemotherapy drugs with differing cytotoxic mechanism have been developed and appear to produce higher CR rates and survival rates in pilot trials. However, when these regimens were compared with CHOP in prospective, randomized trials, there were no statistically significant difference in either response rates or survival rates.11 12 The CR rates remained a disappointing 44% to 56%, with the estimated 3-year DFS of 41% to 46% confirming that nearly 60% of patients with advanced stage intermediate- and high-grade NHL continue to die of progressive lymphoma. Innovative approaches are necessary to further improve their prognosis. Currently, two different strategies are being investigated, including the use of repetitive courses of dose-intensive therapy with growth factor support as an initial therapy and early high-dose therapy followed by ASCT during first CR or PR.
The IPI and the age-adjusted IPI have identified a subset of patients with high-risk aggressive lymphoma who have poor prognoses due to the increased risk of death from both a lower CR rate and a higher rate of relapse from CR.3 Our results show that early consolidation with high-dose therapy and ASCT can prevent relapse and improve survival of these high-risk patients. Our study also supports the results from the Groupe d' Etude des Lymphomes del' Adulte (GELA) randomized study reported by Haioun et al13 and suggested that dose-intensive therapy and ASCT should be considered for higher risk patients after achieving remission with conventional therapy. Our results, when compared with those of the GELA, showed a better survival (DFS 89% v 57% for ABMT and 36% for chemotherapy). The superior outcome reported in our study could be due to more intensive preparative regimens used, patient selection, and a single-center study. A prospective multicenter randomized study comparing our high-dose regimen and ASCT with conventional chemotherapy in IPI high and high-intermediate risk patients will be necessary to confirm our results.
Several pilot trials of high-dose therapy and ASCT have been reported with promising results.7,14-18 Like our study, these trials included small numbers of patients, various poor-risk factors, and different induction chemotherapy regimens and preparative regimens and the results were compared with historical controls. Therefore, the benefit of high-dose therapy and ASCT early in the course of disease can only be proven in prospective randomized trials. Recently reported randomized trials comparing conventional chemotherapy with high-dose therapy and ABMT in aggressive lymphoma have reached different conclusions,13,19,20 but none of these studies selected patients based on the poor-risk factors. In a multicenter randomized study reported by Haioun et al from the GELA study,13 patients who achieved a CR with standard induction therapy were randomized to receive either high-dose combination chemotherapy with ASCT or further standard maintenance therapy. Although there was no difference in the outcome for the whole group, the benefit of dose-intensive therapy and ABMT was shown in high-intermediate and high-risk group (2 to 3 risk factors). The 5-year DFS was significantly higher in the ABMT arm compared with the chemotherapy arm (57% v 36%, P = .01), and the 5-year survival rates also were better for ABMT (65% v 52%, P = .06). However, two other randomized studies, one from the Netherlands reported by Verdonck et al19 and a second from the Italian Cooperative group reported by Martelli et al,20 showed that no survival benefit was achieved using ABMT in patients who were either slow responders or who had only partial response to front-line chemotherapy. In the Dutch study,19 patients who did not have a near-complete response after three cycles of CHOP were randomized either to receive high-dose chemoradiotherapy and ABMT or to continue for an additional three cycles of CHOP. There were no significant differences in either overall survival or DFS. However, this study was criticized for their definition of PR, and more than 50% of the patients in the study were in the low and low-intermediate risk groups. In the Italian Cooperative Group Study,20 although there was a trend toward a lower relapse rate and a better progression-free survival for patients received ABMT, the number of patients randomized was too small to show a statistical difference and ABMT did not result in improvement in survival.
To increase dose intensity and to prevent the emergence of drug-resistant clone, high-dose sequential therapy (HDS) and hematopoietic cell support as an initial induction therapy for aggressive lymphomas has also been studied.21-23 Gianni et al22 conducted a phase III trial comparing HDS and hematopoietic stem cell support with a third generation chemotherapy regimen, MACOP-B, as induction therapy in 98 patients with poor-risk aggressive lymphoma. In their study, poor-risk features included bulky mass more than 10 cm in diameter, stage III or IV, and ECOG PS 0 to 4. Forty-eight patients were randomized to HDS arm and 50 patients to the MACOP-B arm. Initially, there was a high toxic death rate (16%) in the HDS arm,21 which led to modification of HDS regimens. After the median follow-up of 55 months, the CR rate (96% v 70%, P = .001), freedom from disease progression (84% v 49%, P < .001), and event-free survival (76% v 49%, P = .004) were significantly better in patients receiving HDS therapy when compared with those treated with MACOP-B. In addition, the overall survival at 7 years was also better in the patients receiving HDS treatment. Although the results of early high-dose intensive therapy appear promising and warrant further studies, the therapies may be limited by their potential for repetitive, severe toxicity. In another randomized study from the GELA group recently reported by Gisselbrecht et al,23 302 aggressive lymphoma patients with two IPI adverse prognostic factors were randomized to receive either a short intensive therapy for 3 cycles followed by a high-dose therapy BEAM (carmustine, etoposide, cytarabine, and melphalan) regimen and ASCT on day 60 of therapy or by standard combination chemotherapy ACVB regimen (doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone). At a median follow-up of 16 months, there was no difference in CR rate, EFS, and survival between the two arms. They concluded that early dose-intensive therapy and ASCT did not increase the CR rate or prevent early relapses. However, our study shows that the outcome of ASCT is better when performed in remission using more intensive preparative regimens. Therefore, the timing of ASCT and the intensity of high-dose regimens used may play a significant role.
Primary MLCL is a well-recognized clinico-pathologic subtype of lymphoma that is characterized by very aggressive clinical course and poor response to conventional chemotherapy, including CHOP ± radiotherapy.6 Although a higher CR rate has recently been reported with third-generation chemotherapy regimens, MACOP-B,24,25 the prognosis of patients who fail to attain CR remains very poor. A number of poor prognostic factors that predict for increase risk of relapse and decreased survival have also been identified, including the presence of pleural effusion at diagnosis, elevated LDH level, the number of involved extranodal sites, and incomplete response to therapy as evidenced by residual mass or persistent 67Ga avidity.6,25 Patients with these poor prognostic features have an extremely poor outcome, with an approximately 80% to 100% chance of relapse.6 In our study, 13 patients with MLCL who had these poor prognostic factors, in addition to being in a high- or high-intermediate risk group, underwent ASCT while in first CR/PR. One patient relapsed at 13 months posttransplantation. Twelve patients remain alive in remission with a follow-up time of 1 to 91 months (median, 42 months). Our results would support the role of early high-dose therapy and ASCT in patients with poor-risk MLCL. Because of its usefulness in detecting residual disease and its predictive value,26 a 67Ga scan should be performed routinely before and after therapy to aid in identifying patients at risk for relapse who will require alternative therapy such as high-dose therapy and ASCT.
Small noncleaved cell Burkitt's and non-Burkitt's lymphomas have different natural histories and responses to treatment. Optimal therapy for adult patients with SNCCL has not been established and, in general, the prognosis is poor when treated with conventional chemotherapy regimens used to treat diffuse large-cell lymphoma. Recently, a few studies have shown that their prognosis can be improved with the use of more intensive combination chemotherapy regimen.27-31 In a study reported by McMaster et al27 from Vanderbilt University, 20 patients with SNCCL were treated with 8 weeks of high-dose intensive regimen consisting of cyclophosphamide, etoposide, doxorubicin, vincristine, bleomycin, methotrexate with leucovorin rescue, and prednisone. Eighty percent had stage IV and 85% had two or more poor prognostic features. Seventeen patients (85%) achieved a CR and 5-year actuarial DFS was 60%. There were 2 treatment-related death in elderly patients. In another study from the National Cancer Institute reported by Longo et al,29 33 patients with small noncleaved non-Burkitt's lymphoma were treated with ProMACE-based combination chemotherapy regimens. All patients treated with ProMACE-CytaBOM achieved a CR and their overall survival was 88%. However, only a few patients had elevated LDH level or other poor prognostic factors; therefore, it remains unclear whether ProMACE-CytaBOM would be equally effective in higher risk patients. Recently, excellent results were reported by Magrath et al,31 who treated 20 adults and 21 children with an intensive program, including fractionated high-dose cyclophosphamide, doxorubicin, vincristine, high-dose methotrexate, and intrathecal cytarabine and methotrexate prophylaxis (CODOX-M) and a combination of ifosfamide, etoposide, high-dose cytarabine, and intrethecal methotrexate (IVAC). Ninty-five percent achieved a CR and there was no significant difference in event-free survival at 2 years in children (85%) compared with adults (100%). However, this regimen was also associated with significant thrombocytopenia, sepsis, and severe disabling neuropathy.
The experience of high-dose therapy and ASCT for patients with SNCCL during first CR is limited.30,32 Sweetenham et al32 reported the European Group for Blood and Bone Marrow Transplantation (EBMT) experience of high-dose therapy and ASCT in 117 adults with Burkitt's and Burkitt's-like NHL. Because of the retrospective nature of this study, the criteria for patient selection for ASCT during first CR were not given in detail. However, among the 70 patients transplanted in first CR, approximately one-third of the patients had stage IV and bulky mass greater than 10 cm and 57% had B symptoms at presentation. With a median follow-up of 23 months, the 3-year actuarial OS and progression-free survival rates were 72% and 73%, respectively. Our results in 10 patients with SNCCL treated with high-dose therapy and ASCT are comparable with the results reported from the EBMT, although our study included only poor-risk patients. Given these results, it remains to be answered which therapy offers the best chance for cure in advanced stage SNCCL; proof of superiority would require a comparative randomized trial. Unfortunately, such a comparative study may not be feasible due to the rarity of this lymphoma. Ultimately, the choice of therapy will depend on the long-term follow-up results of these studies in a larger number of patients and the short-term and long-term complications associated with each treatment.
Therapy-induced secondary myelodysplastic syndrome (MDS) and ANLL have now been recognized as a serious complication of high-dose therapy and ABMT. The reported incidence ranges from 4% to 10% and several risk factors for development of MDS/ANLL have been reported from different series.33-35 Therapy-induced MDS/ANLL has also been reported in patients receiving dose-intensive chemotherapy without ASCT such as high-dose CHOP in lymphoma.36 Potential strategies to minimize the risk of MDS after ASCT includes early transplantations before stem cell injuries from repetitive courses of cytotoxic therapy, routine cytogenetic analysis before transplant, and modification of high-dose regimens. Two of these strategies were used in our study, including routine cytogenetic studies and early transplantations after achieving a CR. Despite this, 2 patients (4%) in our study developed clonal cytogenetic abnormality and ANLL and 1 died of progressive leukemia. Therefore, alternative strategies such as sensitive assays to detect DNA damage and gene marking studies could be undertaken to help understand the developmental process of therapy-induced MDS.
In conclusion, our results suggest that high-dose therapy and ASCT may improve the survival and prognosis of patients with poor-risk intermediate- and high-grade lymphoma. A prospective randomized study comparing high-dose therapy and ASCT with conventional chemotherapy during remission in patients in high- and high-intermediate IPI risk group are required to confirm our findings.
ACKNOWLEDGMENT
The authors dedicate this manuscript to Henry Rappaport, MD, Distinguished Pathologist, for his scientific contributions and devotion to the field of hematology. The authors thank Mudra Nathawani, Annette Brown, and Lisa Yonemoto for their help in data collection and Chris Kohna for assistance with manuscript preparation.
Supported by Grants No. NCI PPG CA 30206 and NCI CA 33572.
Address reprint requests to Auayporn Nademanee, MD, Department of Hematology and Bone Marrow Transplantation, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010.