Abstract
Human platelets were found to contain myosin phosphatase consisting of a 38-kD catalytic subunit of protein phosphatase type 1δ, a 130-kD myosin-binding subunit (MBS) and a 20-kD subunit, all of which cross-reacted with antibodies against these subunits of smooth muscle myosin phosphatase. Anti-MBS antibody coimmunoprecipitated RhoA and Rho-kinase of human platelets. Platelets MBS is a substrate for Rho-kinase and phosphorylation of MBS decreases the activity of myosin phosphatase. Treatment of intact platelets with 9,11-epithio-11,12-methano-thromboxane A2 led to a dramatic increase in phosphorylation of MBS and a significant decrease in the activity of myosin phosphatase. These findings suggest a putative mechanism for agonist-induced regulation of myosin phosphatase activity in platelets.
THE 20-kD LIGHT CHAIN of myosin (MLC) is rapidly phosphorylated, and then dephosphorylated during platelet activation.1,2 Phosphorylation of 20-kD MLC is causally related to platelet shape change, contraction, and secretion.1-3 When the 20-kD MLC is phosphorylated by Ca2+/calmodulin-dependent MLC kinase, myosin can interact with actin because phosphorylation increases actin activation of platelet myosin ATPase.4 A good temporal correlation exists between MLC phosphorylation and myosin association with the cytoskeleton in platelet activation.1 We identified the catalytic subunit isozymes (α, γ, δ) of protein phosphatase-1 (PP1) and the catalytic subunit of protein phosphatase-2A (PP2A) in human platelets.5 We found that the association of PP1 with the Triton-insoluble cytoskeleton occurred on agonist-induced platelet activation, even in the absence of aggregation, whereas the translocation of PP2A depended on aggregation.6 Thus, the interaction of PP1 with the cytoskeleton may play a role in agonist-induced formation of the contractile cytoskeleton by regulating the phosphorylation state of cytoskeletal myosin.
Myosin phosphatase of smooth muscle is composed of a 38-kD PP1δ catalytic subunit, a 130-kD and a 20-kD component, which are thought to be regulatory components involved in targeting the phosphatase to its substrate.7-9 The 130-kD subunit is a myosin-binding subunit (MBS), which targets the catalytic subunit to myosin.8,9 A growing body of evidence suggests that smooth muscle myosin phosphatase is regulated under physiologic conditions.10,11 Thiophosphorylation of the 130-kD MBS resulted in a fivefold decrease in phosphatase activity and an increased Ca2+ sensitivity of force output in α-toxin-permiabilized portal vein strips.12,13 Rho is involved in the GTP-enhanced Ca2+ sensitivity of smooth muscle contraction.14,15 It was recently reported that overexpression of RhoA or dominant activated RhoA (RhoAv14 ) in NIH 3T3 cells increases phosphorylation of MBS and 20-kD MLC.16 Kimura et al showed that GTP-Rho activates Rho-associated kinase (Rho-kinase), then Rho-kinase phosphorylates MBS and myosin phosphatase is thereby inactivated.17 Left unanswered is the key question of whether MBS phosphorylation occurs in response to external stimuli in intact cells and therefore would be a possible mechanism for regulation of myosin phosphatase. Here, we report that Rho-kinase phosphorylates platelet MBS and inactivates platelet myosin phosphatase in vitro. We also demonstrate that a stable thromboxane A2 analog 9,11epithio-11,12-methano-thromboxane A2 (STA2 ) induces MBS phosphorylation and inactivation of myosin phosphatase in intact platelets, thus raising the possibility that this may be attributed in part to 20-kD MLC phosphorylation.
MATERIALS AND METHODS
Preparation of human platelet suspension and measurement of aggregation. Human platelets (109/mL) were resuspended in Tyrode-HEPES Buffer that contained a final concentration of 0.14 mol/L NaCl, 2.7 mmol/L KCl, 1 mmol/L MgCl2 , 0.1% D-glucose, 3.75 mmol/L NaH2PO4 , 15 mmol/L HEPES, pH 7.5. Washed platelets (400 μL) were stimulated by STA2 at 1 μmol/L for various times in the aggregometer (37°C), and aggregation was monitored photometrically, as described.2 5 The reaction was quenched with ice-cold lysis buffer (1% NP-40, 20 mmol/L Tris-HCl, pH 7.5, 0.15 mol/L NaCl, 2 mmol/L EDTA, 4 mmol/L phenylmethylsulfonyl fluoride [PMSF], 200 μg/mL leupeptin, 2 mmol/L sodium vanadate) and used for immunoprecipitation.
Measurement of protein phosphorylation in intact platelets. The platelet suspension (400 μL) prelabeled with 18.5 MBq/mL [32P] orthophosphate (at 30°C, for 60 minutes) was stimulated with 1 μmol/L STA2 in the aggregometer.2 5 At various times, the reaction was terminated by adding the ice-cold lysis buffer. Phosphoproteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The gel was stained with Coomassie blue, dried, and subjected to autoradiography, using Kodak X-Omat AR film with an intensifying screen at −80°C.
Immunoprecipitation. Immunoprecipitation of platelet lysates was performed after clarification of samples by centrifugation at 10,000g for 1 hour, and the soluble fraction precleared with protein A Sepharose beads, was incubated with 5 μL of specific antibodies against MBS9 at 4°C for 1 hour. The immune complex was precipitated by adding protein A-Sepharose (Pharmacia Biotech AB, Uppsala, Sweden), incubating for an additional 1 hour at 4°C, then washing the beads three times with lysis buffer. The sample was then split into two portions; one was used for immunoblotting, and the other was processed further for in vitro phosphatase assay.
Immunoblot analysis. Immunoblot analysis was carried out as described.6,18 Polyclonal antibodies against the catalytic subunit isozymes (α and δ) of PP1, 130-kD MBS, the 20-kD subunit, and Rho-kinase were obtained by immunizing rabbits with synthesized fragments of respective proteins and used after purification.5,6 Quantitative estimation of the level of MBS in human platelets was performed densitometrically by scanning the immunoreactive band after immediately photographing the visualized band.6 18 The signal was then compared with that of known amounts of the purified recombinant MBS. Routinely, at least three different amounts of MBS were included as standards to quantify the level of platelet MBS.
Kinase assay. In vitro kinase reactions using recombinant glutathione S-transferase (GST)-Rho-kinase were carried out in 50 μL of the reaction mixture 40 mmol/L Tris-HCl, pH 7.5, 2 mmol/L EDTA, 1 mmol/L dithiothreitol, 6.5 mmol/L MgCl2 , 0.1% CHAPS, 10 nmol/L okadaic acid (OKA; Wako Pure Chemicals, Osaka, Japan), 20 μmol/L [γ-32P]ATP, 25 μL of the immunoprecipitate, and 8 ng of GST-Rho-kinase, which is constitutively active.19 The kinase reaction was initiated by adding the immunoprecipitate. Reactions were carried out at 30°C for the times indicated and terminated by the addition of SDS sample buffer. Phosphoproteins were separated by SDS-PAGE. The gel was stained with Coomassie blue, dried, and subjected to autoradiography, using Kodak X-Omat AR film with an intensifying screen at −80°C. Quantitative estimation of the level of phosphorylation was carried out densitometrically with a Fujix BAS2000 Bioimager (Fuji Photo Film Co, LTD, Tokyo, Japan) or a Molecular Dynamics Scanning Densitometer (Sunnyvale, CA) in conjugation with ImageQuant program run on a Dell Personal Computer (Austin, TX) by scanning the radioactive band.18 The area of an individual peak was measured above background in densitometric tracing and was expressed as an arbitrary unit.
Measurement of phosphatase activity. Activities of PP1, PP2A, protein phosphatase-2B (PP2B), and protein phosphatase-2C (PP2C) were determined with [32P]-phosphorylated MLC of chicken gizzards as a substrate.20 PP1 and PP2A activities were measured in a reaction mixture (50 μL) containing 20 mmol/L Tris-HCl, pH 7.4, 50 mmol/L NaCl, 0.1 mmol/L EGTA, 2 mmol/L sodium vanadate, ±10 nmol/L OKA, ±a heat stable phosphatase inhibitor-2, 4 mmol/L PMSF, and 200 μg/mL leupeptin. PP2A was completely inhibited by 10 nmol/L OKA, whereas PP1 was hardly affected at this concentration. PP1 can be taken as the activity that is sensitive to the inhibitor-2 and PP2A can be taken as the activity that is blocked by 10 nmol/L OKA.5 PP2B and PP2C activities were determined as the activity in the presence of 0.5 μmol/L CaCl2 , 5 mmol/L MgCl2 , 0.5 μmol/L OKA, 1 μmol/L calmodulin, and ±0.15 mmol/L trifluoperazine.21 PP1 and PP2A are completely inhibited at 0.5 μmol/L OKA, therefore this activity was taken as the total activity of PP2B and PP2C. Trifluoperazine, 0.15 mmol/L, was added to completely inhibit PP2B, but PP2C was unaffected by trifluoperazine, even at 0.15 mmol/L.22 PP2B activity was determined by subtracting PP2C (the activity in the presence of 0.15 mmol/L trifluoperazine) from total activity. Assays were performed in duplicate, and the cpm measured in blank assays lacking cell sample was subtracted from the total cpm. The phosphatase activity was indicated as nanomoles released 32Pi per minute in cell samples.
RESULTS AND DISCUSSION
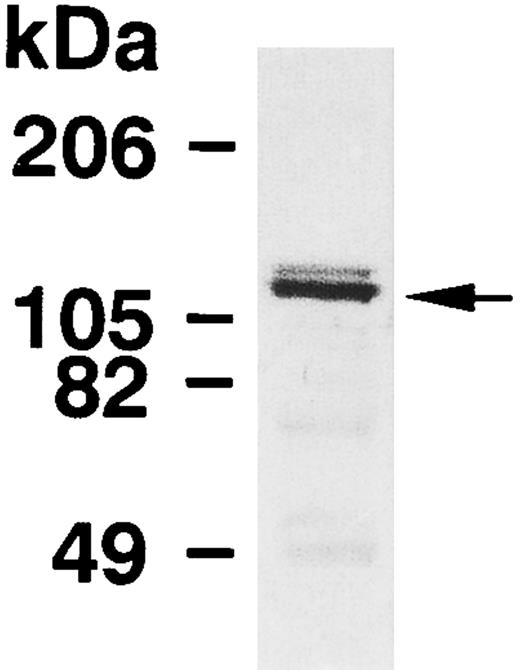
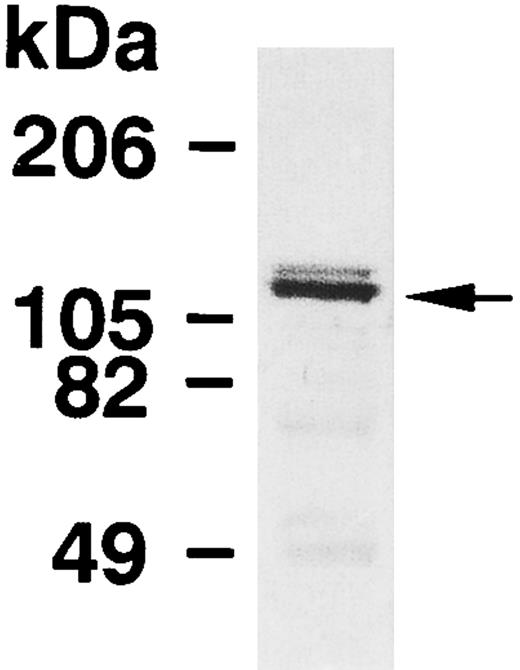
The expression of MBS in human platelets was examined by immunoblot analysis using a polyclonal antibody specific against smooth muscle MBS. As shown in Fig 1, the antibody detected a major band with 130-kD, as noted in the smooth muscle.7-9 To investigate whether the immunoreactive protein at 130-kD is MBS of human platelets, we analyzed immunoprecipitates with anti-MBS antibody of platelet extract (Fig 2A). The procedure was to submit the Protein A precipitates to SDS-PAGE followed by immunoblotting with polyclonal antibodies against the PP1 catalytic subunit and the 20-kD subunit of myosin phosphatase. The PP1δ-isoform and 20-kD subunit of myosin phosphatase were detected at 38-kD and at 20-kD, respectively (Fig 2A), while the PP1α-isoform was not detected. MBS was also detected at a major band with a 130-kD in the anti-PP1δ antibody immunoprecipitate.
Immunoblot analysis of MBS in human platelets. Platelet lysates were applied to SDS-PAGE followed by immunoblots using the antibody against MBS as described in the Materials and Methods.
Immunoblot analysis of MBS in human platelets. Platelet lysates were applied to SDS-PAGE followed by immunoblots using the antibody against MBS as described in the Materials and Methods.
Immunodetection of myosin phosphatase in human platelets. Platelet lysates were immunoprecipitated with the antibody against MBS as described in the Materials and Methods. (A) Immunoprecipitates with anti-MBS antibody (a) or the preimmune serum (b) were immunoblotted with anti-MBS antibody. Immunoprecipitates with anti-MBS antibody were immunoblotted with antibodies against PP1δ catalytic subunit and the 20-kD subunit, respectively. The immunoprecipitate with the antibody against PP1δ catalytic subunit was immunoblotted with anti-MBS antibody. IP, immunoprecipitation antibody used; IB, immunoblotting antibody used; Ig, cross-reacted immunoglobulin. (B) Immunoprecipitates with anti-MBS antibody were assayed for phosphatase activity, using [32P]-MLC as substrate. Each phosphatase activity was determined in triplicate as described in Materials and Methods.
Immunodetection of myosin phosphatase in human platelets. Platelet lysates were immunoprecipitated with the antibody against MBS as described in the Materials and Methods. (A) Immunoprecipitates with anti-MBS antibody (a) or the preimmune serum (b) were immunoblotted with anti-MBS antibody. Immunoprecipitates with anti-MBS antibody were immunoblotted with antibodies against PP1δ catalytic subunit and the 20-kD subunit, respectively. The immunoprecipitate with the antibody against PP1δ catalytic subunit was immunoblotted with anti-MBS antibody. IP, immunoprecipitation antibody used; IB, immunoblotting antibody used; Ig, cross-reacted immunoglobulin. (B) Immunoprecipitates with anti-MBS antibody were assayed for phosphatase activity, using [32P]-MLC as substrate. Each phosphatase activity was determined in triplicate as described in Materials and Methods.
We further investigated phosphatase activity of the immunoprecipitates using [32P]-phosphorylated MLC as a substrate. About 80% of total phosphatase activity in the precipitate was PP1 activity (Fig 2B). These results indicate that the immunoreactive protein at 130-kD is MBS in human platelets. We determined the amount of MBS in whole platelets using the purified recombinant MBS as the standard. The amount of MBS was 3.14 ± 0.48 ng/107 platelets (mean ± SD, n = 3). The amount of PP1δ catalytic subunit in whole platelets was 7.1 ± 0.5 ng/107 platelets (n = 6) and constituted approximately 28% of the total PP1 catalytic subunit (PP1α + PP1γ + PP1δ).6 These data suggest that MBS is present at a lower level than that of PP1δ catalytic subunit.
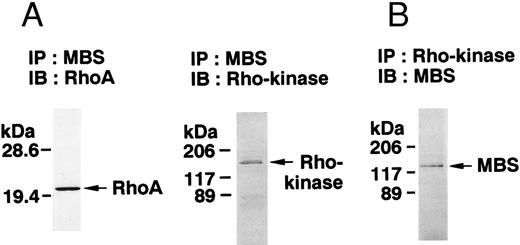
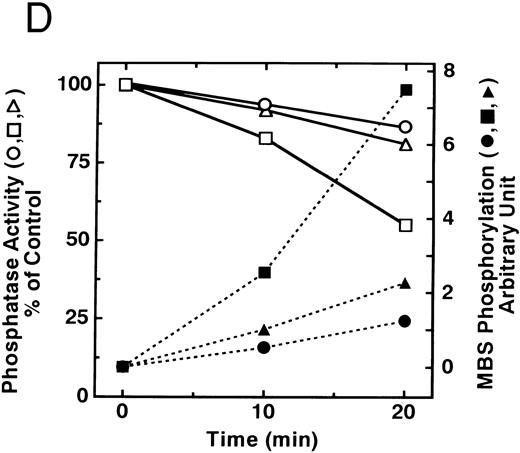
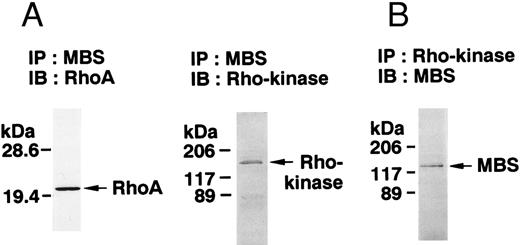
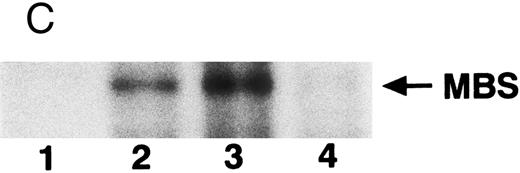
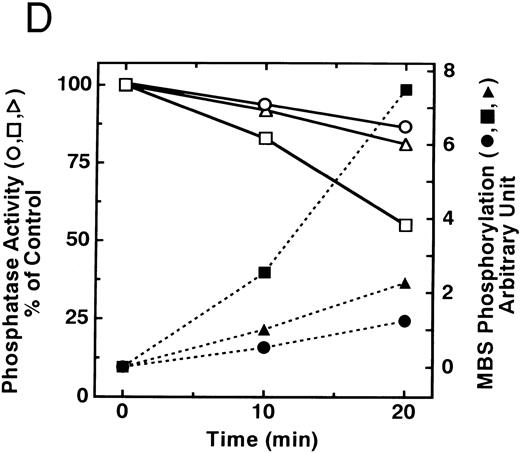
The small GTPase Rho is implicated in response to extracellular signals and is associated with cytoskeletal rearrangements.14,23 It has been reported that platelets contain a high level of RhoA protein, and that botulinum C3 exoenzyme ADP-ribosylates only Rho when added to platelet lysates.24 Three Rho targets have been identified: protein kinase N,25,26 Rho-kinase,16 which is also identified as ROK,27 and MBS of myosin phosphatase.17 Rho-kinase, a serine/threonine kinase, phosphorylates MBS from chicken gizzard smooth muscle, and consequently inactivates the activity of myosin phosphatase.17 We then studied whether Rho and Rho-kinase are functionally associated with platelet MBS. RhoA and Rho-kinase were detected in the anti-MBS immunoprecipitates, using polyclonal antibodies in human platelets, as shown in Fig 3A. The association of platelet MBS with Rho-kinase was further verified by immunoblotting to MBS in immunoprecipitate with anti–Rho-kinase antibody (Fig 3B). We next tested if platelet MBS would be phosphorylated in a cell-free system by GST-Rho-kinase, which is constitutively active,19 as is the case with smooth muscle.17 In Fig 3C, incubation with GST-Rho-kinase produced a phosphorylated band at 130-kD in immunoprecipitates with anti-MBS antibody from human platelet lysates, and the protein kinase inhibitor staurosporine inhibited its phosphorylation. Staurosporine has been found to inhibit the kinase activity of both Rho-kinase and GST-Rho-kinase.19 The 130-kD MBS was slightly phosphorylated in the absence of GST-Rho-kinase, suggesting that the anti-MBS immunoprecipitates contain endogenous Rho-kinase and possibly an unidentified kinase. There was no phosphorylation at the 38-kD band suggesting that the PP1 catalytic subunit is not phosphorylated. We then investigated the effect of MBS phosphorylation by GST-Rho-kinase on phosphatase activity of the immunoprecipitates with anti-MBS antibody (Fig 3D). The extent of phosphorylation increased as the phosphatase activity decreased. Both MBS phosphorylation and the decrease in myosin phosphatase activity were mostly dependent on the presence of GST-Rho-kinase. Addition of staurosporine led to inhibition of the GST-Rho-kinase–induced MBS phosphorylation and to a reduction in inactivation of the phosphatase activity.
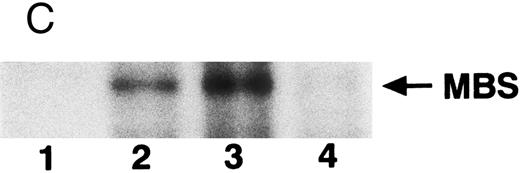
Phosphorylation of MBS and inactivation of myosin phosphatase by Rho-kinase. (A) Coimmunoprecipitation of RhoA and Rho-kinase with platelet MBS. Coprecipitated RhoA (left) and Rho-kinase (right) were determined by probing immunoblots with antibodies against respective proteins. (B) Coprecipitation of MBS with Rho-kinase from human platelets. Immunoprecipitation with anti–Rho-kinase antibody and immunoblot analysis of MBS were described in the Materials and Methods. (C) In vitro phosphorylation of platelet MBS by GST-Rho-kinase and its inhibition by staurosporine. MBS immunoprecipitates of platelet lysates were incubated with Rho-kinase in the presence of staurosporine, as described under Materials and Methods. Protein phosphorylation was analyzed by SDS-PAGE, followed by autoradiography. Lane 1, no incubation; Lane 2, 15 minutes of incubation without GST-Rho-kinase; Lane 3, 15 minutes of incubation with GST-Rho-kinase, Lane 4; 15 minutes of incubation with GST-Rho-kinase in the presence of 1 μmol/L staurosporine. The results are representative of three independent experiments. (D) Effect of MBS phosphorylation on the activity of myosin phosphatase. MBS immunoprecipitates were incubated without GST-Rho-kinase (▵, ▴), with GST-Rho-kinase (□, ▪) or with GST-Rho-kinase and 1 μmol/L staurosporine (○, •), as described in the Materials and Methods. At the indicated times, aliquots of the reaction mixture were quenched by addition of a solution containing final 10 mmol/L EGTA to stop the reaction and were kept on ice. Activity of myosin phosphatase (open symbol) was determined immediately. Phosphorylation (solid symbol) of MBS was analyzed by SDS-PAGE and autoradiography. Points, means of three separate experiments (SD < 10%).
Phosphorylation of MBS and inactivation of myosin phosphatase by Rho-kinase. (A) Coimmunoprecipitation of RhoA and Rho-kinase with platelet MBS. Coprecipitated RhoA (left) and Rho-kinase (right) were determined by probing immunoblots with antibodies against respective proteins. (B) Coprecipitation of MBS with Rho-kinase from human platelets. Immunoprecipitation with anti–Rho-kinase antibody and immunoblot analysis of MBS were described in the Materials and Methods. (C) In vitro phosphorylation of platelet MBS by GST-Rho-kinase and its inhibition by staurosporine. MBS immunoprecipitates of platelet lysates were incubated with Rho-kinase in the presence of staurosporine, as described under Materials and Methods. Protein phosphorylation was analyzed by SDS-PAGE, followed by autoradiography. Lane 1, no incubation; Lane 2, 15 minutes of incubation without GST-Rho-kinase; Lane 3, 15 minutes of incubation with GST-Rho-kinase, Lane 4; 15 minutes of incubation with GST-Rho-kinase in the presence of 1 μmol/L staurosporine. The results are representative of three independent experiments. (D) Effect of MBS phosphorylation on the activity of myosin phosphatase. MBS immunoprecipitates were incubated without GST-Rho-kinase (▵, ▴), with GST-Rho-kinase (□, ▪) or with GST-Rho-kinase and 1 μmol/L staurosporine (○, •), as described in the Materials and Methods. At the indicated times, aliquots of the reaction mixture were quenched by addition of a solution containing final 10 mmol/L EGTA to stop the reaction and were kept on ice. Activity of myosin phosphatase (open symbol) was determined immediately. Phosphorylation (solid symbol) of MBS was analyzed by SDS-PAGE and autoradiography. Points, means of three separate experiments (SD < 10%).
To observe if the phosphorylation of MBS occurs in intact human platelets, we analyzed immunoprecipitates and made use of a polyclonal anti-MBS antibody of [32P]Pi-labeled platelets before and after STA2 stimulation. The stable thromboxane analog STA2 was used to activate platelets, since thromboxane A2 initiates polyphosphoinositide metabolism, Ca2+ mobilization, and the phosphorylation of 20-kD MLC and 47-kD plekstrin following receptor occupancy.28,29 When platelets were activated with STA2 in the absence of stirring, they changed shape and secreted without aggregation.5 6 As shown in Fig 4, a phosphorylation band at 130-kD MBS was detected in the precipitates after STA2 stimulation, in non-stirred platelets. The level of MBS phosphorylation increased rapidly for up to 1 minute, reached a seven-fold higher level than at rest, then decreased after exposure to 1 μmol/L STA2 . MBS phosphorylation subsequently reached a near resting level 5 minutes after STA2 stimulation. Amounts of precipitated MBS per se remained unchanged after stimulation (data not shown). We attempted to detect phosphotyrosine at 130-kD MBS in precipitates after STA2 stimulation, using a monoclonal anti-phosphotyrosine antibody (4G10), but phosphotyrosine was not detected (data not shown). Platelet MBS was not phosphorylated in vitro by two major platelet tyrosine kinases, pp60src and p72syk. The phosphorylation band at 130-kD appeared to be at serine and/or threonine residues. These observations suggest that STA2 treatment results in an increase in serine/threonine phosphorylation at MBS in intact human platelets. Phosphorylation of the 38-kD PP1 catalytic subunit was not evident in anti-MBS antibody immunoprecipitates after STA2 stimulation.
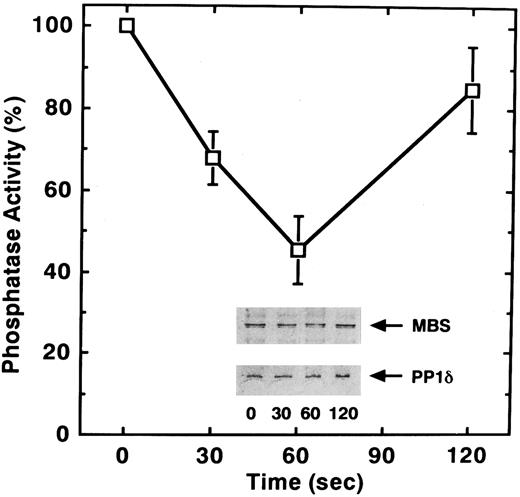
STA2-induced phosphorylation of MBS in intact platelets. The [32P] Pi-labeled platelets were stimulated with 1 μmol/L STA2 without stirring. MBS was immunoprecipitated with anti-MBS antibody, and phosphorylation of MBS was analyzed by SDS-PAGE and autoradiography (inset). Results were expressed as fold increase in the phosphorylation relative to the level at 0 seconds. Similar results were obtained in three other experiments with different donor platelets.
STA2-induced phosphorylation of MBS in intact platelets. The [32P] Pi-labeled platelets were stimulated with 1 μmol/L STA2 without stirring. MBS was immunoprecipitated with anti-MBS antibody, and phosphorylation of MBS was analyzed by SDS-PAGE and autoradiography (inset). Results were expressed as fold increase in the phosphorylation relative to the level at 0 seconds. Similar results were obtained in three other experiments with different donor platelets.
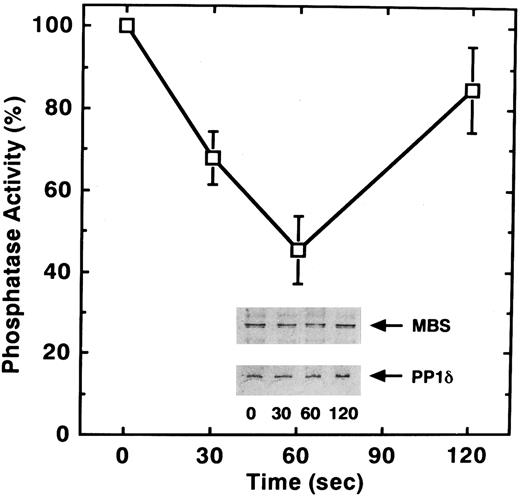
To determine if, in addition to phosphorylation, STA2 treatment of human platelets affects myosin phosphatase activity, we examined phosphatase activity of the anti-MBS immunoprecipitates after STA2 stimulation. As shown in Fig 5, STA2 treatment for 60 seconds led to a 55% decrease in myosin phosphatase activity of the precipitates relative to that for 0 seconds. STA2-induced changes in phosphatase activity appeared to parallel the extent of MBS phosphorylation (Figs 4 and 5). The amount of MBS and the coprecipitated catalytic subunit of myosin phosphatase did not change throughout the time course, judging from the immunoblot analysis (Fig 5). Phosphorylation of the MBS apparently did not dissociate the myosin phosphatase holoenzyme and the decrease in myosin phosphatase activity was not due to any change in the amount of coprecipitated catalytic subunit. These data suggest that attenuation of phosphatase activity is a reflection of the phosphorylation of MBS. It is likely that phosphorylation and dephosphorylation of MBS represent an in vivo mechanism for regulating myosin phosphatase activity in platelets.
STA2-induced inactivation of myosin phosphatase in intact platelets. Washed platelets (1 × 106/μL platelets) were stimulated with 1 μmol/L STA2 without stirring. MBS was immunoprecipitated with anti-MBS antibody and the activity of myosin phosphatase was determined immediately as described in the Materials and Methods. Data represent the means ± SE of five independent experiments. Immunoprecipitates were immunoblotted with antibodies against MBS and the PP1δ catalytic subunit (inset).
STA2-induced inactivation of myosin phosphatase in intact platelets. Washed platelets (1 × 106/μL platelets) were stimulated with 1 μmol/L STA2 without stirring. MBS was immunoprecipitated with anti-MBS antibody and the activity of myosin phosphatase was determined immediately as described in the Materials and Methods. Data represent the means ± SE of five independent experiments. Immunoprecipitates were immunoblotted with antibodies against MBS and the PP1δ catalytic subunit (inset).
By analogy to the proposed processes that regulate myosin phosphatase activity in smooth muscle,17 the present study suggests that phosphorylation of MBS and inactivation of platelet myosin phosphatase occur in vitro and in intact platelets in response to agonist stimulation. If this postulation is tenable, then this provides new evidence that myosin phosphatase is controlled in intact platelets. Upon stimulation with STA2 , intracellular Ca2+ concentration is elevated28 and GDP⋅Rho may be converted to GTP⋅Rho (activation of Rho). The rise in the Ca2+ level activates MLC kinase to increase 20-kD MLC phosphorylation, activating myosin and stimulating contractility. Activated Rho appears to inhibit myosin phosphatase possibly via the phosphorylation of MBS by Rho-kinase, which also contributes to the increase in 20-kD MLC phosphorylation, thereby amplifying and prolonging activation of MLC kinase. Rho is implicated in other physiologic functions associated with cytoskeletal rearrangements such as shape change and aggregation.14,23 Treatment with a specific Rho inhibitor C3 exoenzyme led to complete suppression of thrombin-induced platelet aggregation, whereas serotonin secretion was delayed but reached the same extent as in the control platelets.30 These data suggest that Rho plays important roles in the aggregation process rather than in secretion. To obviate the role of Rho in the aggregation, platelets were activated without stirring in the present study. Under non-aggregating conditions, agonist-induced platelet secretion was associated with Ca2+-dependent MLC phosphorylation,2 and dephosphorylation was prevented,31 which means that myosin phosphatase is probably less active under these conditions. The elevation of MLC phosphorylation could result from increased MLC kinase activity and/or from decreased myosin phosphatase activity. Collectively, our present data suggest that agonist-mediated regulation of myosin phosphatase activity, possibly via Rho-kinase, is operational in platelets, although the primary regulator of MLC phosphorylation is MLC kinase. Various agonists other than STA2 also induced MBS phosphorylation in intact platelets (data not shown). Thrombin, collagen, and A23187 stimulated MLC phosphorylation, but the rate and extent of MLC phosphorylation varied with the agonist.1 The contribution of MBS phosphorylation and its inactivation of phosphatase activity to MLC phosphorylation may vary with the agonist.
Metastatic carcinoid tumor of hindgut origin. A 50-year-old, asymptomatic woman with a retroperitoneal mass, found on routine physical examination, underwent surgery. Only formaldehyde-fixed material was available for ultrastructural studies. Despite poor preservation of subcellular details, tumor cells that were filled with round, electron-dense granules of the type found in hindgut carcinoid tumors were observed. Within 2 months of this surgery, a small primary rectal carcinoid tumor was discovered by endoscopy. Original magnification × 25,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.)
Metastatic carcinoid tumor of hindgut origin. A 50-year-old, asymptomatic woman with a retroperitoneal mass, found on routine physical examination, underwent surgery. Only formaldehyde-fixed material was available for ultrastructural studies. Despite poor preservation of subcellular details, tumor cells that were filled with round, electron-dense granules of the type found in hindgut carcinoid tumors were observed. Within 2 months of this surgery, a small primary rectal carcinoid tumor was discovered by endoscopy. Original magnification × 25,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.)
ACKNOWLEDGMENT
We thank M. Ohara for helpful comments.
Supported in part by grants for research from the Ministry of Education, Science, Sports and Culture of Japan.
Address reprint requests to Masakatsu Nishikawa, MD, The 2nd Department of Internal Medicine, Mie University School of Medicine, 2-174 Edobashi, Tsu, Mie 514, Japan.

![Fig. 2. Immunodetection of myosin phosphatase in human platelets. Platelet lysates were immunoprecipitated with the antibody against MBS as described in the Materials and Methods. (A) Immunoprecipitates with anti-MBS antibody (a) or the preimmune serum (b) were immunoblotted with anti-MBS antibody. Immunoprecipitates with anti-MBS antibody were immunoblotted with antibodies against PP1δ catalytic subunit and the 20-kD subunit, respectively. The immunoprecipitate with the antibody against PP1δ catalytic subunit was immunoblotted with anti-MBS antibody. IP, immunoprecipitation antibody used; IB, immunoblotting antibody used; Ig, cross-reacted immunoglobulin. (B) Immunoprecipitates with anti-MBS antibody were assayed for phosphatase activity, using [32P]-MLC as substrate. Each phosphatase activity was determined in triplicate as described in Materials and Methods.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3936/3/m_bl_0019f2a.jpeg?Expires=1769142047&Signature=XqINbEysnrGw1LyUYZy0vSYvp6X0sQswNcce4NNSDBlA2LFlJup1cuH~vEzNsO5RL2xn56ar48Zmm5cGTIzjgV2WBp0W6JpxamU4oVh2bMmjbL8tjBOv8f~423bbzFlItE0Vi1D52RwFguQ1iian~qCh3Nf2mQ2O1q3EVx3yUCotznpZrRP-9owk0ddJLxmSXFZ736Hws7bi~UfNw6yH3GDjU4PXKm7MM4sizav95l1~MzcXHn3tZym66qCIJASBguWEC3BbD2wdvwq2Uo~tgBGU77c2pTcc0VlKmw8KBWv0whmGsZv5-rdyII-bUHb3EQN5aCvev3qut7WgzqrObQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Immunodetection of myosin phosphatase in human platelets. Platelet lysates were immunoprecipitated with the antibody against MBS as described in the Materials and Methods. (A) Immunoprecipitates with anti-MBS antibody (a) or the preimmune serum (b) were immunoblotted with anti-MBS antibody. Immunoprecipitates with anti-MBS antibody were immunoblotted with antibodies against PP1δ catalytic subunit and the 20-kD subunit, respectively. The immunoprecipitate with the antibody against PP1δ catalytic subunit was immunoblotted with anti-MBS antibody. IP, immunoprecipitation antibody used; IB, immunoblotting antibody used; Ig, cross-reacted immunoglobulin. (B) Immunoprecipitates with anti-MBS antibody were assayed for phosphatase activity, using [32P]-MLC as substrate. Each phosphatase activity was determined in triplicate as described in Materials and Methods.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3936/3/m_bl_0019f2b.jpeg?Expires=1769142047&Signature=WLy479k7PxW4bjOdfsM5AT2-DK9LZAak5ja2yToJzqrO7W~s~dWDYN6mnyS589Hoy1cbzoCyg~W1sjnGaAEmMP2HtRB1gUZ4f5saI503NyIvd3rLPz-91XBDj-WZpcBponFcEZ8qeLZHWqeXtTCv7uhYAC-2L3MAsqCNxEZKGT~Lx86rOkufi58DzCwfeEJY7S5ZQYHvGf~AMPuenandJtCVPnd9kO0LROEq98S~G29zD5TjtX2qLf3nEnpkd-tXPsPgIm~vHHu0orcOXtfSkObY3DsuF0~5lJ9D8T-lPLSFFovoGzlTWvgVHCPBZZRDBusg1JdnlaeQVJpDDSO1IQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. STA2-induced phosphorylation of MBS in intact platelets. The [32P] Pi-labeled platelets were stimulated with 1 μmol/L STA2 without stirring. MBS was immunoprecipitated with anti-MBS antibody, and phosphorylation of MBS was analyzed by SDS-PAGE and autoradiography (inset). Results were expressed as fold increase in the phosphorylation relative to the level at 0 seconds. Similar results were obtained in three other experiments with different donor platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3936/3/m_bl_0019f4.jpeg?Expires=1769142047&Signature=SEPfuWbRwtgUBCN52sCAS7j2W1afgovSS6O4A1iWC9S3DqviRBunKIfj2Qso-gZuYnWTaqnsPzV6~NxEHdkkjbX1HinBq0JVi9jXqTvcNK21dJWsibcCPyDtTzvqe3-OkoyASjpY~4p5S~f~wSsVmlmZ22~U3fvXakJmy7hV9C1euGv91qx4GbD23wMxqfTDLbIQ9ryipp6m-I2tYHVUXJkkaiSoDs52VaxnVQ9S-VBomf-rSxPKPF8PMUAqnP0VP9WaKV3b0jRS2O~KUMPSYU609fmndkhr26lmO~Geo4stMqveVWRjwi~G22f1JMWrz3IJoUL-qi3EgUZ2~rdcxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Immunodetection of myosin phosphatase in human platelets. Platelet lysates were immunoprecipitated with the antibody against MBS as described in the Materials and Methods. (A) Immunoprecipitates with anti-MBS antibody (a) or the preimmune serum (b) were immunoblotted with anti-MBS antibody. Immunoprecipitates with anti-MBS antibody were immunoblotted with antibodies against PP1δ catalytic subunit and the 20-kD subunit, respectively. The immunoprecipitate with the antibody against PP1δ catalytic subunit was immunoblotted with anti-MBS antibody. IP, immunoprecipitation antibody used; IB, immunoblotting antibody used; Ig, cross-reacted immunoglobulin. (B) Immunoprecipitates with anti-MBS antibody were assayed for phosphatase activity, using [32P]-MLC as substrate. Each phosphatase activity was determined in triplicate as described in Materials and Methods.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3936/3/m_bl_0019f2a.jpeg?Expires=1769142048&Signature=bkvNKGY3HRuI0lBPycMI2Cim6Gcrvnurx0~Q4Dy6jmNKGgnKZ18GUat2yRhrscQlzf2mWVLGzNqeU-V0RItXbnGGxaYWTkWoVpN~nsUlAMZCxiBxKZJBYvvRUtlAvGjCja5AKPkWWlgJZL~Xv36JyijgoL1l0tKqZMg~1mh4q1AFw-b99pn1M~hwN4YfyhFWXhZlU5MoNHHf4FjIroM~k0E8H~BBdjmoP91ya5JtTxPJMeuhhraR16T97KhOIMDhStQjD3-lDfa~uQtv-f0jv8xMfrRn-G01B-788AqwcWUtjYWzdrYIDdrKgydnrwUqyA~Ym49hw0Em6dTwQ~KdBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Immunodetection of myosin phosphatase in human platelets. Platelet lysates were immunoprecipitated with the antibody against MBS as described in the Materials and Methods. (A) Immunoprecipitates with anti-MBS antibody (a) or the preimmune serum (b) were immunoblotted with anti-MBS antibody. Immunoprecipitates with anti-MBS antibody were immunoblotted with antibodies against PP1δ catalytic subunit and the 20-kD subunit, respectively. The immunoprecipitate with the antibody against PP1δ catalytic subunit was immunoblotted with anti-MBS antibody. IP, immunoprecipitation antibody used; IB, immunoblotting antibody used; Ig, cross-reacted immunoglobulin. (B) Immunoprecipitates with anti-MBS antibody were assayed for phosphatase activity, using [32P]-MLC as substrate. Each phosphatase activity was determined in triplicate as described in Materials and Methods.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3936/3/m_bl_0019f2b.jpeg?Expires=1769142048&Signature=MCNZdWA10Tyu9i0bYEgi5C2jYQJdtkzPBg~h87aIFIdv9Hl~PA~OV8YvjnLRNIVRFVgA-u1KKvvAkXP0bTHZXSrAQ8VNTueXjDBTW9QQQv32lXF5ENdGQNATe9sLlgeraFmCaZ3yxOUXxyK1LNJXooJ8uR-G9yjp4LdFWx7LYCukRX8EDQRPE8EILSWYknp7qqVI5kWkbJY7LeJh0k9NrXNPyWOLfIkzCvfjub0aUAdNxQDbQPXuLCdaWdQdxcSm5t8zH3XpogkAJzIuicvmJDVda2~fLW9qmLcUxsR1AvsKj~1HOjGeb9NpDIb9UPTLCJaAju~WAtDkydYA~iVukQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. STA2-induced phosphorylation of MBS in intact platelets. The [32P] Pi-labeled platelets were stimulated with 1 μmol/L STA2 without stirring. MBS was immunoprecipitated with anti-MBS antibody, and phosphorylation of MBS was analyzed by SDS-PAGE and autoradiography (inset). Results were expressed as fold increase in the phosphorylation relative to the level at 0 seconds. Similar results were obtained in three other experiments with different donor platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3936/3/m_bl_0019f4.jpeg?Expires=1769142048&Signature=05UCaweShYC3KoYuYn6JoEn2vmzvCeafL3TxXsR7WSmIpDg7ks8o7zS3apV-Xvudo49-EGeVwnQ2h5kjz-xqkV4gsJiaRfi0ly6l0HHJ4YC4OtSXuIfcD6-7-KSkCkdT12XeuYBsfDKQ0WmNptAj0pFNnRIex~BpG3eoibs2lEVJtiZUcXX4rHagY1E3a0dGNrdZtt3I~tHBg-EpoVQKlbahlVmPWOwBbxLGACPY4jBuXfyIKh64v85-Cy5yDNu~2MyeRS57iIDB-oR2wUa8PDURUOOoMQfrNB5V2TIZ9LMwybhiVWYthWXgF2lFJpFcijAqrJCH6DP0s2KkDPeYgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)