Abstract
The factor XII gene from 31 unrelated factor XII-deficient patients from Germany, Switzerland, and Austria was screened for mutations at the genomic level. Several novel mutations were detected and their absence in a control group of 74 healthy unrelated individuals was checked. Most changes are in the serine protease domain affecting the catalytic triad His-393–Asp-442–Ser-544; two missense mutations, R398Q (arginine 398 to glutamine; gene bank accession no. U71276) and L395M (leucine 395 to methionine; gene bank accession no. U71277), are close to the active site histidine at position 393. Another mutation detected in a cross-reacting material (CRM)-positive female with a history of three abortions affects the active site aspartic acid by changing it to asparagine (D442N; gene bank accession no. U71275). The novel mutation G570R (glycine 570 to arginine; gene bank accession no. U71274) giving rise to a CRM-positive phenotype is located next to Cys571, which forms a vital disulfide bridge. Two mutations are causing reading frame shifts: one single basepair deletion in exon 12 [exon 12: 10590(DelC); gene bank accession no. U71278] and one acceptor splice site mutation [exon 14: 11397(G → A); gene bank accession no. L43615]. The putative regulatory mutation exon 1:−8 (g → c) in the upstream region of the gene is associated with an aberrant Taq I restriction site allele in intron B of the gene (gene bank accession no. X80393).
THE BIOLOGIC ROLE of factor XII (FXII) is not yet fully understood. This plasma protein is like other factors of the coagulation cascade a member of the serine proteases. The activated FXII (FXIIa)-induced activation of FXI is the crucial step in contact system-mediated coagulation activation. FXIIa is involved in the initiation of the coagulation cascade by cleaving prekallikrein. Kallikrein, in turn, cleaves the inactive zymogen factor XII to yield α-FXIIa and β-FXIIa. This latter process is accelerated by binding of the inactive zymogen FXII to a negatively charged surface via the N-terminal region of the protein. The coagulation cascade results in the formation of fibrin. Covalent cross-links between the fibrin strands by the action of FXIIIa finally stabilize the network. To outbalance this system, the end product fibrin has to be dissolved. Again, FXII is involved in the process of fibrinolysis. α-FXIIa cleaves prekallikrein to form kallikrein that then cleaves pro-urokinase to form urokinase. The latter finally can activate plasminogen to plasmin, which then can dissolve the fibrin clot. FXII is also an activator of the kinin system via kallikrein.1
Furthermore, FXII is thought to be involved in the downregulation of the FC receptor of monocytes, release of interleukin-1 (IL-1) and IL-6 from monocytes, aggregation and degranulation of neutrophils, and activation of C1.2
Hereditary deficiency of FXII does not result in drastic symptoms in vivo, but is rather detected by chance during in vitro testing due to prolonged activated partial thromboplastin times (APTT). Homozygous or compound heterozygous carriers of two defective FXII alleles exhibit almost no FXII-activity (<1%) as compared with normal human plasma, whereas heterozygotes display intermediate activity. In almost all patients with low levels of activity (<1% as compared with normal human plasma), no FXII antigen is detectable. These persons are referred to as cross-reacting material (CRM)-negative. With the purified FXII protein from the rare subgroup of CRM-positive patients, it was possible to identify two mutations on the protein level.3,4 The cloning of the FXII cDNA by Cool et al5 and Tripodi et al,6 the isolation of the gene by Cool and MacGillivray,7 and the chromosomal localization on chromosome 5q33-qter by Royle et al8 made it possible to study molecular aberrations in the gene. The gene consists of 13 introns and 14 exons covering 12 kb, and the liver-specific transcription of the gene at several start points gives rise to an approximately 2-kb mRNA. Several putative protein domains show high homology to the fibronectin and tissue-type plasminogen activator. In several CRM-negative FXII-deficient patients, an additional Taq I restriction site in intron B was reported by Bernardi et al.9 The presence of this restriction site was identified to represent a T → C transition 224 bp upstream of exon 3 (exon 3, −224 t → c) and shown to be associated with a mutation in the 5′ flanking region of the gene, exon 1:−8 (g → c), by Hofferbert et al.10 Schloesser et al11 found in 5 of 12 unrelated patients a novel acceptor splice site mutation in exon 14 resulting in a frameshift for exon 14 sequences.
To elucidate the molecular basis of their FXII deficiency, we studied the DNA sequence of Swiss, Austrian, and German patients and their relatives by polymerase chain reaction (PCR).
SUBJECTS, MATERIALS, AND METHODS
Blood samples from 74 healthy control subjects (group D), from 16 German unrelated cases with low FXII activity (group A), from a total of 32 Swiss individuals (group B, 13 index cases and their family relatives), 1 Austrian individual (case no. C-82), and one Austrian family (index patient no. C-17, 5 individuals, group C) were collected. DNA was prepared and PCR was performed as described by Schloesser et al.11,12 The PCR strategy was based on both cDNA and genomic DNA sequences6,7 (Fig 1, primer sequences provided upon request). Sequencing of PCR products was performed with the Taq-Dye Terminator protocol from Applied Biosystems (Foster City, CA) on a 373 or 377 Automatic Sequencer using 100 to 200 ng of purified PCR fragments. For the German patients, FXII activity and antigen and other coagulation parameters were determined as described by Braulke et al13 and Lutze et al.14 For the Swiss group, the analysis was performed as described by Lämmle et al.15 For the Austrian family, the tests were performed as described by Halbmayer et al.16
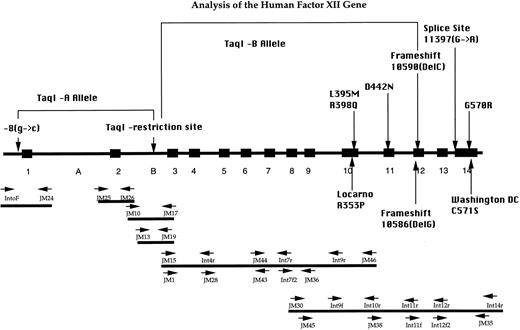
PCR strategy for analysis of the FXII gene. Exon-intron organization, the localization of primers, and the positions of the sequence alterations are depicted. The brackets indicate the association between the different Taq I-alleles and their associated mutations in the 5′ upstream region and exon 12, respectively. The mutations R353P,4 10586(DelG),17 and C571S3 depicted in the lower part were not detected in our group of patients.
PCR strategy for analysis of the FXII gene. Exon-intron organization, the localization of primers, and the positions of the sequence alterations are depicted. The brackets indicate the association between the different Taq I-alleles and their associated mutations in the 5′ upstream region and exon 12, respectively. The mutations R353P,4 10586(DelG),17 and C571S3 depicted in the lower part were not detected in our group of patients.
RESULTS
The different groups of individuals were tested for mutations by PCR and automatic fluorescent sequencing (Table 1). Patient A-148, a German female with a history of three abortions, searched for medical advice and was included in this study. For all other patients, their FXII deficiency was detected by chance. For these 31 FXII-deficient individuals, a number of 57 mutations can be expected, because 5 individuals had intermediate enzyme activites indicating the presence of only one mutated FXII allele (Table 1, patients no. 27 through 31). The number of mutations causing FXII deficiency for each patient was estimated from the FXII activity and, if available, the antigen value. Each mutation found was considered as either relevant or not by its putative biologic effect and by its relative frequency in patients and healthy controls (see below). The results are presented in Tables 1 and 2 and in Figs 1-6.
Three Different Taq I Alleles in Intron B Are Associated With Different Mutations
The Taq I-A, -B, and -C alleles. Bernardi et al9 showed that an aberrant Taq I allele in intron B is associated with FXII deficiency, but they could not explain the molecular basis or the mechanism behind how this sequence change could influence the FXII activity. We analyzed the sequence around the Taq I restriction site in intron B and could identify the sequence changes.10 This aberrant Taq I restriction site is present due to an T → C transition changing the wild-type sequence 5′-ggtcttga tct to 5′-ggtctcga tct. We termed this mutation exon 3: −224 (t → c), because the change is at position −224 upstream of exon 3. This allele has a frequency in our group of 0.72 (41 of 57 FXII chromosomes; Table 2). Interestingly, the sequence of the Taq I restriction site is part of an Alu repeat belonging to the subfamily Sb0.10 This Alu repeat is present in all our cases and controls analyzed and is not a result of chromosomal rearrangements. In all cases analyzed, the Taq I alleles confer a CRM-negative phenotype.
The Taq I-A allele. A high percentage of our patients was identified as carriers of the Taq I restriction site in intron B. As shown by Hofferbert et al,10 the presence of this site is associated with the presence of a mutation located in the 5′ upstream part of the FXII gene: the exon 1: −8 (g → c) mutation. We term this association the Taq I-A allele. As can be seen in Table 2, 38 of 41 Taq I alleles (93%) belong to this group. Neither mutation was found in 74 healthy controls. Individuals homozygous for this allele are CRM-negative, ie, no FXII antigen can be detected. A family pedigree demonstrates the association and the inheritance of both alleles, the exon 3: −224 (t → c), ie, the Taq I restriction site, and the 5′-upstream mutation, exon 1: −8 (g → c) (Fig 2). In our group of FXII-deficient patients, this mutation is not associated with any abnormal bleeding symptoms. This allele is the most frequent with a frequency of 0.66 (38 of 57 chromosomes; Table 2).
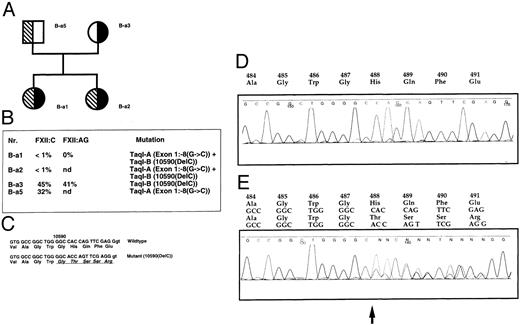
Pedigree of family B-a with the Taq I-A and Taq I-B alleles associated with the mutations exon 1: −8 (g → c) and exon 12: 10590 (Del C), respectively. Patients B-a1, B-a3, and B-a5 did not show any strong thrombotic symptoms. Patient B-a2 suffered from a superficial thrombophelitis during her pregnancy. (A) Pedigree and sequence of the single basepair deletion. Solid symbols, carriers of the Taq I-A allele/exon 1:−8 (g → c); hatched symbols, carriers of the Taq I-B allele/exon 12: 10590(Del C). (B) Coagulation parameters. (C) Comparison of wild-type and mutated sequences. (D) Fluorescence sequence analysis of wild-type exon 12 PCR products. (E) Analysis of an individual B-a1. Mutated and wild-type exon 12 sequences are superimposed. The arrow indicates the deletion of a single base at nucleotide position 10590.
Pedigree of family B-a with the Taq I-A and Taq I-B alleles associated with the mutations exon 1: −8 (g → c) and exon 12: 10590 (Del C), respectively. Patients B-a1, B-a3, and B-a5 did not show any strong thrombotic symptoms. Patient B-a2 suffered from a superficial thrombophelitis during her pregnancy. (A) Pedigree and sequence of the single basepair deletion. Solid symbols, carriers of the Taq I-A allele/exon 1:−8 (g → c); hatched symbols, carriers of the Taq I-B allele/exon 12: 10590(Del C). (B) Coagulation parameters. (C) Comparison of wild-type and mutated sequences. (D) Fluorescence sequence analysis of wild-type exon 12 PCR products. (E) Analysis of an individual B-a1. Mutated and wild-type exon 12 sequences are superimposed. The arrow indicates the deletion of a single base at nucleotide position 10590.
The Taq I-B allele. Interestingly, in two Swiss families with FXII deficiency, one of the Taq I alleles is associated with a single basepair deletion in exon 12 [exon 12:10590 (DelC); Fig 2 and Table 1]. This mutation is close to the single base deletion at position 10586 described by Kemptner et al17 (10586DelG; Fig 1). The pedigree of family B-a shows that individuals B-a1 and B-a2 inherited their Taq I-A allele from their father, whereas the Taq I-B allele associated with the deletion in exon 12 was from their mother. This deletion results in a 1-bp frameshift and consequently would severely affect the resulting protein: the amino acid sequence encoded by the DNA sequences downstream of this mutation would be completely different from the wild-type. Vital protease domains would be abolished and no functional protein can be expected (Fig 2). The associated allele (Taq I-B allele) seems to be rather rare, with an estimated allele frequency of about 0.035 among all chromosomes (Table 2).
The Taq I-C allele. For the last remaining carrier of the Taq I restriction site, case A-89, no other mutation was found. It is possible that, in this case, an associated gene lesion is located in the noncoding or regulatory sequences. Because case A-89 exhibits less than 1% of FXII:C, both FXII alleles seem to be defective. So far, except for the Taq I restriction site in intron B, we did not find any associated gene alteration. Assuming that the Taq I restriction site is not per se causative for the FXII deficiency but rather a marker for a FXII gene defect, case A-89 has probably two mutations not detected in our studies.
The Splice Site Mutation in Exon 14 (11397 G → A)
This mutation was described by Schloesser et al.11 In the German group, this mutation was the most frequent with one homozygous woman (case A-90) and 5 independent heterozygous individuals, whereas only one independent heterozygote was detected in the Swiss group (case B-m2, Table 1). These FXII-deficient patients show neither abnormal bleeding nor thrombotic symptoms and were detected by chance. The homozygous patient A-90 is a 65-year-old mother of four children with a FXII:C of less than 1% and FXII:Ag less than 1%. The family pedigree of patient A-98, a heterozygous carrier, is presented in Fig 3. As shown by transcript analysis, the mutation results in a frameshift in the FXII-mRNA as the acceptor site of exon 14 is shifted 1 nucleotide downstream. This mutation is an example for a cryptic splice site usage as described by Krawczak et al.18 The sequence ccgacccagGGTGATT is changed to ccgacccaaGGTGATT. In the original report, the mutated nucleotide at position 11397 was erroneousely assigned to nucleotide 11396. Because of this mutation, the derived amino acid sequence encoded by wild-type exon 14 and the translational stop codon are lost. Again, as for the Taq I-B allele and its associated mutation 10590 (DelC), this deletion results in a CRM-negative phenotype (Table 1). The overall allele frequency was 0.14 (Table 2).
(A) Pedigree of case A-98. The splice site mutation exon 14:11397 (G → A) (solid symbols) and exon 10: L395M (9988 C → A) (hatched symbols), a mutation close to His393. (B) Clotting parameters and genotypes. (C) Genomic sequence and derived amino acid sequence of wild-type and mutated exon 14.11 (D) Automated fluorescence sequence for the reverse wild-type sequence around L395. (E) Automated fluorescence sequence for the reverse mutated sequence around L395M.
(A) Pedigree of case A-98. The splice site mutation exon 14:11397 (G → A) (solid symbols) and exon 10: L395M (9988 C → A) (hatched symbols), a mutation close to His393. (B) Clotting parameters and genotypes. (C) Genomic sequence and derived amino acid sequence of wild-type and mutated exon 14.11 (D) Automated fluorescence sequence for the reverse wild-type sequence around L395. (E) Automated fluorescence sequence for the reverse mutated sequence around L395M.
Two Mutations in Exon 10: L395M (9988 C → A) and R398 Q (9998G → A) Close to His393
Both missense mutations are located in exon 10 of the gene close to the codon for the active site His393. For the exon 10: L395M (9988 C → A), the nucleotide exchange results in the exchange of leucine (with the codon CTG) at position 395 to methionine (with the codon ATG) in the mature protein. As can be seen in Tables 1 and 2, this mutation was only found in one family (mother, index case A-98) and not in controls. Two of the sons, cases A-99 and A-104, inherited this mutation from her (Fig 3). This maternal allele must confer a CRM-negative phenotype, because case A-98 has FXII:C of less than 5% and FXII:Ag of 5% and is compound heterozygous for the splice site mutation exon 14: 11397 (G → A) and L395M (9988C → A). Her sons, cases A-99 and A-104, both display FXII:C of 22% and 23% and a matching FXII:Ag of 23% and 28%, respectively.
Because the mother A-98 is a carrier of two defective FXII chromosomes, their father must be a carrier of two intermediate alleles of unknown nature, both conferring a phenotype of FXII:C and FXII:Ag of approximately 25%. This holds also true for the remaining four children, all having inherited the splice site mutation from the mother. Their FXII:C and FXII:Ag data fit well with the assumption of two paternal alleles conferring 25% FXII:C and FXII:Ag each. So far, we were not able to identify the corresponding underlying mutations.
The second mutation, R398Q (9998G → A), was found in one Austrian family (case C-17 and C-20; Fig 4). The amino acid arginine at position 398 is replaced by glutamine. The mutation also confers a CRM-negative phenotype. Brother and sister (C-17 and C-20) are both compound heterozygous for Taq I-A and R398Q. This mutation was detected in the son of C-17 (case C-19) and the son of C-20. The other son (case C-18) inherited the Taq I-A allele from his father.
(A) Pedigree of case C-17. Mutation R398Q (hatched symbols) and Taq I-A associated with exon 1: −8 (g → c) (solid symbols). (A) Pedigree of case C-17 with a CRM-negative phenotype. For all individuals C-17 through C-21, the protein C, protein S, AT III, and plasminogen levels were in the normal range, and the factor V-Leiden mutation was excluded.39 Patient C-20 suffered from recurrent deep vein thrombosis and pulmonary embolism, and C-17 had a stroke and peripheral vascular disease. (B) Clotting parameters and genotypes. (C) Automatic sequence analysis of wild-type exon 10 (forward). (D) Automatic sequence analysis of a heterozygous patient C-17. Mutated exon 10: R398Q(9998 G → A) and wild-type sequence. (E) Reverse sequence of patient C-17. The arrow indicates the mutation.
(A) Pedigree of case C-17. Mutation R398Q (hatched symbols) and Taq I-A associated with exon 1: −8 (g → c) (solid symbols). (A) Pedigree of case C-17 with a CRM-negative phenotype. For all individuals C-17 through C-21, the protein C, protein S, AT III, and plasminogen levels were in the normal range, and the factor V-Leiden mutation was excluded.39 Patient C-20 suffered from recurrent deep vein thrombosis and pulmonary embolism, and C-17 had a stroke and peripheral vascular disease. (B) Clotting parameters and genotypes. (C) Automatic sequence analysis of wild-type exon 10 (forward). (D) Automatic sequence analysis of a heterozygous patient C-17. Mutated exon 10: R398Q(9998 G → A) and wild-type sequence. (E) Reverse sequence of patient C-17. The arrow indicates the mutation.
A Mutation of the Codon for the Active Site Aspartic Acid 442: D442N 11372 (G → A)
In one 34-year-old woman (A-148; Table 1) with a history of three abortions we detected this novel mutation (Fig 5). The genomic DNA sequence CAC GAC CTG coding for His-Asp-Leu is changed to CAC AAC CTG coding for His-Asn-Leu. This mutation giving rise to a CRM-positive phenotype is interesting, because it directly affects the catalytic aspartic acid conserved in all serine proteases (data available upon request).7 19-28
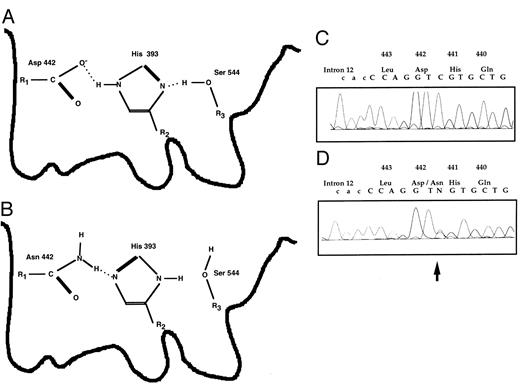
The mutation D442N(10372 G → A) in the active site of the human FXII serine protease detected in case A-148, a woman with three recurrent abortions and a CRM-positive phenotype. Her coagulation parameters were as follows: prothrombin time, 92%, partial thromboplastin time 27.6 seconds, 29.9 seconds; thrombin time, 17.8 seconds; fibrinogen (Clauss), 3.2 g/L; FII, 109%; FV, 97%; FVII, 89%; FX, 120%; FVIII, <120%; FIX, <120%; FXI, 111%; FXII:C, 63%; FXII:Ag, 100%; protein S (total), 135%; protein S (free), 130%; protein C, 110%; AT III, 107%. No antiphospholipid antibodies and no factor V Leiden mutation.39 (A) Schematic structure of the active site formed by the catalytic triad of Asp442, His393, and Ser544 (according to chymotrypsin; see Fersht31 ). (B) The replacement of Asp442 by Asn gives rise to a dysfunctional structure. (C) Reverse wild-type sequence. (D and E) Heterozgous form: reverse mutated and wild-type sequences are superimposed. The arrow indicates the mutation.
The mutation D442N(10372 G → A) in the active site of the human FXII serine protease detected in case A-148, a woman with three recurrent abortions and a CRM-positive phenotype. Her coagulation parameters were as follows: prothrombin time, 92%, partial thromboplastin time 27.6 seconds, 29.9 seconds; thrombin time, 17.8 seconds; fibrinogen (Clauss), 3.2 g/L; FII, 109%; FV, 97%; FVII, 89%; FX, 120%; FVIII, <120%; FIX, <120%; FXI, 111%; FXII:C, 63%; FXII:Ag, 100%; protein S (total), 135%; protein S (free), 130%; protein C, 110%; AT III, 107%. No antiphospholipid antibodies and no factor V Leiden mutation.39 (A) Schematic structure of the active site formed by the catalytic triad of Asp442, His393, and Ser544 (according to chymotrypsin; see Fersht31 ). (B) The replacement of Asp442 by Asn gives rise to a dysfunctional structure. (C) Reverse wild-type sequence. (D and E) Heterozgous form: reverse mutated and wild-type sequences are superimposed. The arrow indicates the mutation.
The Mutation G570R (11482 G → C) in Exon 14
In one CRM-positive FXII-deficient patient from Austria (C-82; Table 1 and Fig 6), the codon for glycine 570 was changed from G GC to the codon for arginine, C GC.
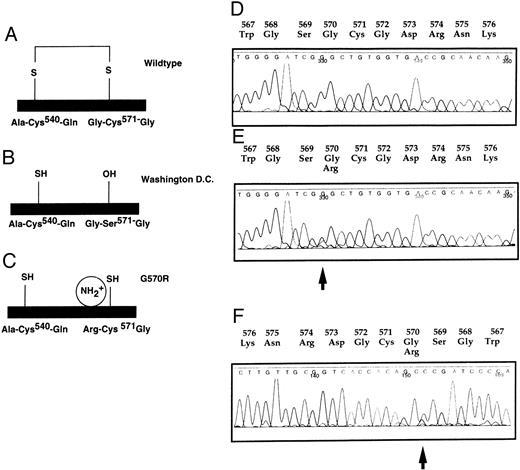
Mutation in the C-terminal part. G570R (11482 G → C) found in case C-82 giving rise to a CRM-positive phenotype (FXII:C 10%, FXII:Ag 74%). (A) Wild-type protein structure of the light chain. (B) No disulfide bond formed in FXII Washington D.C. (C) Hypothetical model for FXII G570R. No disulfide bond in putative protein structure of FXII G570R. (D) Wild-type DNA sequence. (E) Heterozygous C-82. Wild-type and mutated sequences are superimposed. (F ) Reverse sequence of C-82. The arrow indicates the mutation.
Mutation in the C-terminal part. G570R (11482 G → C) found in case C-82 giving rise to a CRM-positive phenotype (FXII:C 10%, FXII:Ag 74%). (A) Wild-type protein structure of the light chain. (B) No disulfide bond formed in FXII Washington D.C. (C) Hypothetical model for FXII G570R. No disulfide bond in putative protein structure of FXII G570R. (D) Wild-type DNA sequence. (E) Heterozygous C-82. Wild-type and mutated sequences are superimposed. (F ) Reverse sequence of C-82. The arrow indicates the mutation.
Interestingly, this sequence change is located next to the FXII Washington D.C. mutation C571S that also gives rise to a CRM-positive phenotype.3
DISCUSSION
Twenty-nine of our 31 patients with FXII deficiency are CRM-negative, ie, there is no immunologic reactive protein detectable in their plasma. This would mean that an event either on the transcriptional, the translational, or the posttranslational levels affects the synthesis, the stability, or the transport of the corresponding molecules (primary transcripts, mRNA, or protein). These events can be traced down to molecular deviations, ie, mutations, in the gene itself or in other factors regulating these processes. One of these other factors might be located on chromosome 6, because Pearson et al29 reported a reduced FXII level in an individual with a 6p deletion.
Mutations Causing Frameshifts
For three mutations of the FXII gene, namely the splice site mutation exon 14:11397 (G → A)11 that causes a 1-bp deletion and subsequently a frameshift in exon 14, the deletion in exon 12 :10590(DelC), and the deletion in exon 12:10586(DelG) found only in one patient described by Kemptner et al,17 it can be deduced that the protein is not stable as the position of the translational stop codon is altered. Consequently, matching FXII:C and FXII:AG values should be expected (Table 1).
The Taq I Restriction Sites and Their Associated Mutations
Because different mutations are associated with the Taq I alleles in intron B, it is difficult to decide whether the Taq I or the associated mutation is the gene defect causing FXII deficiency. The associated mutations, ie, exon 1:-8 (g → c) and exon 12: 10590 (DelC), are both located on the same chromosome as their correseponding Taq I restriction sites in intron B. This was clearly shown by the analysis of two pedigrees (Figs 2 and 4 and Table 1). For the exon 1: −8 (g → c) mutation, a regulatory effect on the transcriptional activity is more likely.10 Because the biologic impact of the deletion in exon 12 on the protein is without doubt severe enough to extinguish all enzymatic activity, a possible effect of point mutations in the Alu repeat located in an intronic region is difficult to evaluate. However, Vansant and Reynolds30 reported a regulatory function of an Alu repeat, similar to the Alu repeat in intron B of the human FXII gene, for the mouse keratin k18 gene. A possible regulatory function of these Alu repeats is speculative, because Alu repeats are rather abundant in the human genome (every 3 to 4 kb), and any regulatory macromolecule targeted to that specific binding site in the Alu repeat in intron B of the FXII gene would be binding to the vast number of unspecific sites.
The Missense Mutations L395M and R398Q
In the case of the mutation L395M and R398Q, the mechanisms leading to FXII deficiency are still unclear. It remains unclear how both mutations L395M (9988 C → A) and R398Q (9998G → A) would affect the stability of the FXII mRNA or the protein; as for the index cases A-98 and C- 17, no FXII antigen was detected (Table 1 and Figs 3 and 4). It can be speculated that the mutations most likely lead to a rapid degradation of the protein and/or steric stress on the active site, including histidine at position 393. Also, the efficiency of the two kallikrein cleavage sites at positions Arg334 and Arg343 might be affected, thereby preventing the processing and activation of the protein. This would be a similar situation as for the mutation in FXII Locarno (Fig 1). Kremer Hovinga et al4 reported that the amino acid substitution of arginine 353 by proline resulted in an inactive protein because it was not cleavable by kallikrein at position arginine 353/valine 354. However, it seems more likely that these amino acid changes at positions 395 and 398 would affect the integrity of the active site. For serine proteases in general and for FXII, the amino acids His393, Asp442, and Ser544 form the catalytic triad, a structure that is involved in substrate binding and hydrolysis via a so-called charge relay system. The carboxyl group of the aspartate acts on the histidine residue that, in turn, activates the serine (Fig 5). The serine residue forms the covalent bond with the substrate. Changes in the geometry and charge distribution would affect the enzyme's kinetics and thermodynamics. For the L395M (9988 C → A), it is most likely that, due to the amino acid exchange, the bulky sulfur of the Met-395 adjacent to His-393 will affect the geometry of the active site. This leucine residue at position 395 is conserved for human, bovine, and guinea pig FXII.7,20,21 For R398Q (9998G → A), we can also expect a distortion of the protein structure, because the change from Arg-398 to Gln-398 results in the loss of a positive charge. Furthermore, the amino acid sequence including His-393, Leu-395, and Arg-398 is conserved in the known FXII proteins from cattle and guinea pig and for other serine proteases.7 20-26
The Missense Mutation D442N
For the D442N mutation in which an active site residue of the catalytic triad His-Asp-Ser characteristic for serine proteases is changed, the molecular defect on the enzyme's activity is obvious.7,19-28 As outlined below, model studies on other proteins clearly showed the impact of mutations in this region. Therefore, the kinetics of the charge relay system should be drastically altered, decreasing the rate constant of substrate turnover by at least a factor of 10.31 Because patient A-148 is heterozygous for D442N (11372 G → A), this expected decrease in activity is in agreement with the FXII activity of 64%. Konvalinka et al32 reported site-directed mutagenesis experiments on immunodeficiency virus type 1 proteinase. This enzyme contains two copies of the triplet Asp-Thr-Gly in the active center with the threonine adjacent to the catalytic aspartic acid. Changing this threonine in human immunodeficiency virus type 1 proteinase to a serine resulted in a mutant enzyme with an approximately 5- to 10-fold lower activity. Sprang et al19 compared the x-ray structure of genetically engineered wild-type and mutant rat trypsin, the latter carrying the corresponding amino acid asparagine instead of aspartic acid, termed D102N trypsin. Interestingly, because both images derived from crystals of the wild-type and the mutant form were superimposable within experimental error, no significant geometric difference in the active site pocket formed by the catalytic triad His-Asp-Ser could be shown. Kinetic studies performed by Craik et al33 on both forms confirmed that the Michaelis constant (KM ) was not changed. Because this constant is an indicator for the substrate binding and because its value is mainly determined by the enzyme-substrate dissociation constant, the lowered activity of the enzyme is not due to lowered substrate affinity. On the other hand, the rate constant for the reaction with an ester substrate was 4 orders of magnitude less than the unmodified protein. Craik et al33 discuss their findings in the light of the function of the catalytic aspartic acid, which acts on the neighboring histidine. For the serine proteases in general, the active site histidine can accept a proton from serine, whereas for the mutant form D102N (in the chymotrypsin numbering system) or for the FXII mutant D442N this would not be possible.
For patient A-148 it is unclear whether this unusual FXII mutation can be regarded as the cause of her recurrent abortions that occurred twice in week 12 and once in week 14 of gestation. Several other thrombotic risk factors could be excluded, because the values for protein S (>135%), protein C (110%), and ATIII (107%) were normal; antiphospholipid antibodies were excluded and no factor V-Leiden mutation was detected by PCR analysis. Because this patient is CRM-positive and levels of inactive protein with an altered activity are present, it could be possible that this protein could interfere with the biologic pathways due to a slightly altered substrate specificity.
The Missense Mutation G570R
For the FXII G570R mutation and the FXII Washington D.C. mutation (C571S),3 both causing a CRM-positive phenotype, a similar mechanism of action, ie, lack of an essential disulfide bridge, may be operative. In the latter, the essential intramolecular disulfide bridge between Cys-540 cannot be formed due to the lack of Cys-571 (Fig 6). Miyata et al3 speculate that, therefore, the overall conformation of the protein is distorted in such a way that the enzyme is inactive. Therefore, we can expect a similar effect for the G570R mutation. The glycine residue adjacent to the cysteine and the surrounding sequence is conserved in other serine proteases, with the exception of chymotrypsin.3,7,20,21,24-26,34 35 The bulky positively charged arginine at position 570 in the mutant protein might prevent the formation of this essential disulfide link. The only heterozygous carrier detected, patient C-82, a 65-year-old man from Austria, did not show any thrombotic symptoms.
From our study it can be seen that the phenotype of FXII deficiency is based on several different molecular deviations. Whether there is an association of specific FXII gene defects with thrombophilia remains to be studied further.13,16 36-38
This speculation is based on case A-148, a woman with recurrent abortions, a heterozygous carrier of the mutation D442N with CRM-positive phenotype. It would be interesting to further study patients with a CRM-postive phenotype, because all biologic interactions of the FXII protein are still unclear. Although the Austrian familiy showed 2 patients with thromboembolic events (C-20 with deep vein thrombosis and pulmonary embolism and C-17 with stroke and peripheral vascular disease), none of the German and Swiss CRM-negative patients are symptomatic. Thus, it is most likely that the complete lack of FXII protein may not be a cause for thrombosis or other symptoms.
ACKNOWLEDGMENT
The authors thank all of the patients and their families for their cooperation. René Heise and Stefanie Schwager are thanked for excellent technical assistance. We are grateful for helpful contribution by Prof Dr M. Fischer, Dr V. Aumann, Dr U. Mittler, and Dr J.U. Wieding.
Supported by Grant No. 94.021.1. from the Wilhelm Sander Stiftung, by Grant No. 5008 from the Jubiläumsfond der Oesterreichischen Nationalbank, and by Grant No. 32-36443.92 from the Swiss National Science Foundation.
Address reprints requests to Manfred Schloesser, PhD, Institut fuer Humangenetik der Universitaet Goettingen, Gosslerstr. 12 d, D-37073 Goettingen, Germany.