Abstract
Monomeric recombinant molecules prove generally unsatisfactory for in vivo use. Most biological systems are indeed multivalent either structurally, associating different chains, or functionally, when cross-linked by their ligands. Mimicking natural molecules for immune intervention implies the need for multimerizing systems to create multivalent molecules capable of interfering with physiological processing. A multivalent anti-Rh(D) recombinant protein has been designed by reconstructing the antibody binding site of a human monoclonal anti-Rh(D) antibody as a single chain Fv mini antibody, then multimerizing it by inserting at its C-terminal end the C-terminal part of the C4 binding protein (C4bp) alpha chain, which is responsible for the octamer multimerization of that molecule. This soluble multivalent recombinant molecule was functional, bound red blood cells (RBCs), agglutinated them, and did not activate complement. This demonstration model opens the way for future in vivo use of multivalent molecules associating antibody valences and other functional molecules for cell targeting, imaging, or removal of cells such as Rh(D)-positive RBCs for preventing Rh alloimmunization.
MULTIVALENT EXPRESSION of antibody combining sites allows the creation of complexes that are multivalent and can be multispecific and are, thus, susceptible to a spectrum of therapeutic and diagnostic applications.
Multimerization of chimera molecules can be conceived according to different principles. Two possible orientations are direct coupling of the entities concerned or the use of an intermediary multimerizing system through which two or more molecular species can be associated to form heteromultimers. This second approach, susceptible to more general applications is the one we have chosen for the present work. In a therapeutic perspective, to be nonimmunogenic and as inert in vivo as possible, the fragment conferring multimerizing potential should derive from a physiological constituent of human plasma and should not activate complement.
The C4 binding protein (C4bp) molecule, a normal plasma protein,1-5 is a spider-like structure (570 kD) made of 7 α-chains and 1 β-chain. Binding sites for C4bp molecules are located on α-chains, whereas the protein-S binding site is located on the β-chain. A minor form made of only 7 α-chains is also present at a lower concentration in normal human plasma. A third 5α/1β-chain molecule has also been described. The basic repetitive structure of both chains is termed short consensus repeat (SCR). Each SCR of about 60 amino acids includes two intra-chain disulfide bridges.
A minimal C4bp α C-terminal fragment lacking biological functions has been used to produce a soluble multimeric multivalent single chain Fv (scFv) anti-Rh(D) molecule capable of spontaneous multimerization through the associated C4bp fragment. These molecules are homomultimers, which maintain their capacity to recognize antigen but have a higher valence number.
We have elected to use an anti-Rh(D) human single-chain Fv as a model system for multimerization. Rh antigen D is borne by a transmembrane unglycosylated protein. Rh antigens, such as Rh(D), can be conceived as an anchoring point for heterochimeric molecules with novel properties such as enzymatic activities. The Rh(D) antigen is responsible for anti-Rh(D) alloimmunization by transfusion and, through materno-fetal immunization, is the most common cause of hemolytic disease of the newborn. Postpartum prevention of alloimmunization is at the present time affected by injection of human polyclonal anti-Rh(D). In the search for a future replacement a multimeric structure of a monoclonal derived anti-Rh(D) scFv is a first step in the construction of a heteromultimeric molecule that can serve to target Rh(D)-positive red blood cells (RBCs) to selected antibody-dependent cell-mediated cytotoxicity effector cells.
MATERIALS AND METHODS
Cloning of Anti-Rh(D) Heavy and Light Chain-Variable Region Coding Sequences and Assembly Into an scFv
A lymphoblastoid cell line, H2D5D2F5, derived from the peripheral blood lymphocytes of a hyperimmunized donor,6 and producing a human monoclonal IgG1, λ specific for erythrocyte Rh(D) antigen, was used as a source of monoclonal cells to rescue variable heavy (VH) and light (VL) regions via polymerase chain reaction (PCR) amplification.
The single-chain Fv was constructed according to the method described by Marks et al.7 Amplification of VH and VL coding fragments was effected with the specific V gene family primers.8 Paired VH and VL were assembled by PCR with a (Gly4 -Ser)3 linker, and the assembled structures were cloned into pHEN-1 vector9 (kindly provided by Prof G. Winter, M.R.C. Laboratory of Molecular Biology, Cambridge, UK). Clones were tested for expression of soluble scFv after isopropylthiogalactoside induction.7 The scFv construct from a clone with high specific reactivity to anti-Rh(D) erythrocytes was selected for construction of the chimeric protein.
Primers Used to Amplify the C-Terminal Part of the C4bp Molecule
The 174-base pair C-terminal C4bp α fragment was amplified using the following primers: 5′ primer with MYC Tag, 5′AGTGCG-G C C G C A G A A C A A A A A C T C A T C T C A G A A G A G G A T C T G-AATGAGACCCCCGAAGGCTGTGA-3′; 5′ primer without MYC Tag, 5′-AGTGCGGCCGCAGAGACCCCCGAAGGCTGTGA-3′; 3′ primer, 5′-CTCGCGGCCGCCTCGAGTTATAGTTCTTTATCCAAAGTGG-3′.
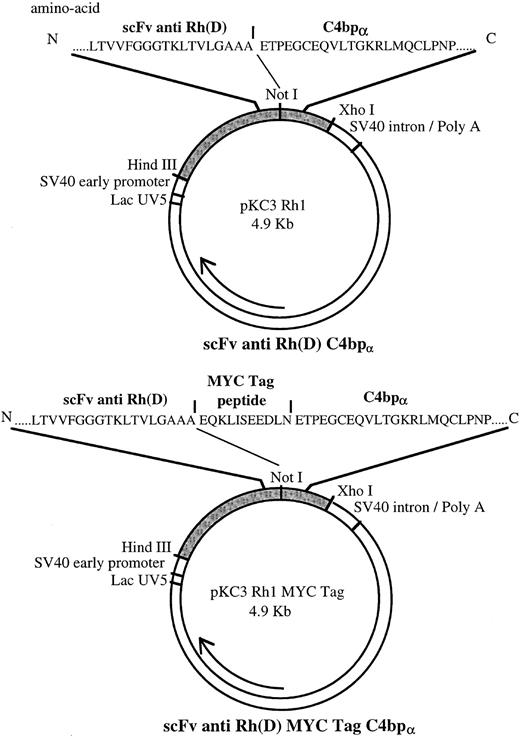
Underlined sequences represent restriction endonuclease sites. The 5′ and 3′ primers contain Not I and Xho I sites, respectively. The sequence depicted in bold characters codes for the MYC Tag peptide.10 Two different sequences were amplified. The first sequence contained a stop codon and a restriction site for Xho I at its 3′ end as well as a restriction site for Not I at its 5′ end. The second sequence also contained these flanking sequences as well as a coding sequence for the MYC Tag peptide at its 5′ end, downstream to the Not I site. This peptide allowed detection and characterization of the recombinant protein (Fig 1).
Maps of pKC3 Rh1 and pKC3 Rh1 MYC Tag plasmids coding for multimeric scFv Rh(D) and MYC Tag multimeric scFv Rh(D) respectively. Amino acid sequences of the junction areas between scFv Rh(D) and C-terminal part of C4bp α-chain are depicted above the overall plasmid.
Maps of pKC3 Rh1 and pKC3 Rh1 MYC Tag plasmids coding for multimeric scFv Rh(D) and MYC Tag multimeric scFv Rh(D) respectively. Amino acid sequences of the junction areas between scFv Rh(D) and C-terminal part of C4bp α-chain are depicted above the overall plasmid.
PCR Amplification
Genomic DNA was used as a template for PCR amplification. The reaction mixture was subjected to 30 cycles of amplification using a Gene Amp PCR System 9600 (Perkin-Elmer, Foster City, CA). Cycles were of 1 minute at 94°C, 1 minute at 56°C, and 2 minutes at 72°C. DNA was then digested with Not I and Xho I.
Cloning of the scFv Anti-Rh(D) C4bp Construct (mRh1)
Restriction enzymes and alkaline phosphatase used for cloning were purchased from Boehringer Mannheim (Meylan, France). The T4 DNA ligation kit used was from Ozyme (Montigny-Le-Bretonneux, France). The scFv construct was excised from the pHEN-1 vector by digestion at the HindIII and Not I sites, thus including the bacterial leader peptide pelB.11 The amplified C4bp fragment was digested by Not I and Xho I. These two DNA fragments were linked to a dephosphorylated pKC3 vector12 digested by HindIII and Xho I (Fig1). Escherichia coli DH5 α host strain was transformed using the pKC3 scFv anti-Rh(D) C4bp construct (mRh1).
Transfection of Chinese Hamster Ovary (CHO) Eukaryotic Cell Line and Amplification of the Transfected mRh1 Genes
Dihydrofolate reductase (dhFr) negative CHO cells (ATCC CRL-9096; American Type Culture Collection, Rockville, MD) were used for transfection.
Transfection was performed using a calcium phosphate transfection kit (5 Prime-3 Prime Inc, TEBU, Le Perray En Yvelines, France). Plasmid pKC3 scFv anti-Rh(D) C4bp or pKC3 scFv anti-Rh(D) C4bp MYC Tag (Fig 1) was cotransfected with the dhFr selective plasmid ST4.13 CHO transformed with the appropriate vectors were selected according to their ability to grow in nucleoside-free medium. The screening of positive clones was performed by direct hemagglutination. Subsequent selective cycling in the presence of increasing concentrations (0.02 to 80 μmol/L) of amethopterin (Methotrexate; Sigma, St Louis, MO), a potent inhibitor of dhFr function, resulted in an amplification of the integrated DNA and an increased expression of the multimeric scFv.
Direct Hemagglutination
Gel-test and columns were purchased from Institut J. Boy (Reims, France). Twenty microliters of a 2.5% suspension of papain-treated erythrocytes (E) were incubated 30 minutes at 37°C with 50 μL of pure or diluted supernatants of transfected cells. Agglutination was then assessed in Sepharose columns after a 1,000g centrifugation of 10 minutes at room temperature.
Immunoprecipitation
Anti-MYC Tag monoclonal antibody (MoAb)14 from MYC 1-9E10.2 (ATCC CRL-1729) cell line was purified using the octanoic acid, contra precipitation method15 and biotinylated using a hydroxysuccinimide LC biotin linker (Pierce; Interchim, Montluçon, France) according to the manufacturer's instructions.
A total of 3 × 106 cells in a 75-cm2 flask were labeled during 24 hours at 37°C using 170 μCi/mL 35S methionine-cysteine (Amersham, Les Ulis, France) in 7 mL of methionine and cysteine-free RPMI 1640 medium (ICN, Orsay, France) supplemented with 10% heat-inactivated fetal calf serum and 2 mmol/L glutamine. Magnetic beads coated with sheep antimouse IgG (Dynabeads; Dynal, Compiegne, France) were incubated with radiolabeled supernatant. After washing, immunoprecipitated proteins were analyzed in sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE) under reducing and nonreducing conditions.16
Immunofluorescence Assays: Assessment of the Fixation of mRh1 on RBCs
Twenty microliters of a 2.5% suspension of papain-treated Rh(D) positive RBCs was incubated for 45 minutes at 37°C with 50 μL of mRh1 supernatant, washed twice in phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin, then incubated with 2 μg of biotinylated anti-MYC Tag MoAb. RBCs were then washed twice and stained with a biotin-avidin–enhancing system, as previously described.17 Stained RBCs were fixed in 0.37% formaldehyde diluted in washing buffer and analyzed on a FACStar Plus (Becton Dickinson, Mountain View, CA). Papain-treated Rh(D)-negative RBCs were used as a negative control. A competition assay using the anti-Rh(D) DF5 human MoAb, from which the scFv originated, was performed to assess the specificity of binding of mRh1 to RBCs. For longitudinal testing experiments, fluorescent calibration beads (Becton Dickinson) and mouse IgG-coated beads (Qifikit; Biocytex, Marseille, France) were used to standardize day-to-day settings of the apparatus.
Complement Fixation Tests
Assessment of C activation by mRh1 using hemolytic assay. Fifty microliters of 2.5% suspension of papain-treated Rh(D)-positive RBCs were sensitized by mRh1 at 37°C for 30 minutes in 0.24 mol/L glycin, 3 mmol/L sodium phosphate (pH 6.8), 31 mmol/L NaCl, low ionic strength saline buffer including 0.15 mmol/L Ca2+, and 0.5 mmol/L Mg2+.
Fifty microliters of guinea pig serum were then added (Biomérieux, Marcy l'étoile, France). Culture medium or MoAb against glycophorin A18 were used as controls. After 30 minutes incubation at 37°C and a quick cooling in an ice-water bath, tubes were centrifuged for 10 minutes at 700g, then the 405-nm optical density of the supernatants was determined using a microplate reader (SLT, Labinstruments, Vietech, St Bonnet De Mure, France). Complement lysis tests were also performed with an additional step of 30 minutes incubation of 1.5 μg of anti-MYC Tag MoAb.
Twenty microliters of rabbit antiserum to whole IgG (Cappel, Flobio, Courbevoie, France) were then added for another 30 minutes incubation before adding guinea pig serum. Correct attachment of mRh1 on RBCs was checked by flow cytometry analysis following fluorescein isothiocyanate protein A labeling (Sigma).
Assessment of C activation by mRh1 using flow cytometry analysis. Papain-treated Rh(D)-positive RBCs were sensitized by using mRh1, anti-MYC Tag MoAb, and an affinity purified rabbit antibody against mouse IgG and IgM (heavy and light chain) Ig (Pierce). Human C5-deficient serum, twofold diluted in low ionic strength saline including 0.15 mmol/L Ca2+ and 0.5 mmol/L Mg2+, was then added, and the mixture was incubated for 30 minutes at 37°C. Human C3b deposits were revealed by fluorescein-labeled goat antiserum against human C3b (Cappel). RBCs were washed twice, stabilized in 0.37% formaldehyde PBS buffer, then analyzed on a FACStar Plus (Becton Dickinson).
RESULTS
Multivalent anti-Rh(D) recombinant proteins were obtained with C4bp and C4bp MYC Tag multimerizing fragments. They were soluble and stable in culture supernatant.
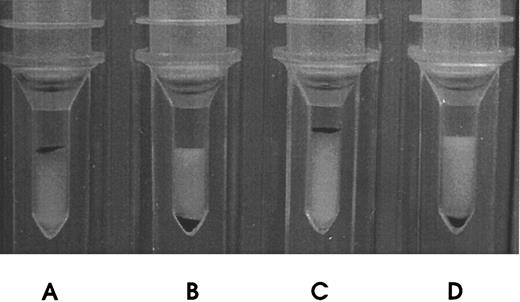
These mRh1 directly agglutinated papain-treated Rh(D)-positive RBCs, as did anti-Rh(D) MoAb DF5 (Fig 2), unlike monomeric anti-Rh(D) scFv (data not shown), and could also, less intensively, agglutinate native RBCs, which the parent DF5 MoAb did not. These recombinant multimers proved stable: Supernatants produced in the course of this work are functional by direct agglutination after 2 years at 4°C, and testing of mRh1 supernatants every week for accelerated aging during 1 month at 37°C showed no significant decrease in staining intensity in flow cytometry binding assays.
Direct hemagglutination of Rh(D)-positive RBCs by mRh1. (A) Incubation of papain-treated Rh(D)-positive RBCs with DF5 MoAb (positive control). (B) Incubation of papain-treated Rh(D)-negative RBCs with mRh1. (C) Incubation of papain-treated Rh(D)-positive RBCs with mRh1. (D) Incubation of papain-treated Rh(D)-negative RBCs with culture medium (negative control). Agglutinated RBCs remained at the top of the columns.
Direct hemagglutination of Rh(D)-positive RBCs by mRh1. (A) Incubation of papain-treated Rh(D)-positive RBCs with DF5 MoAb (positive control). (B) Incubation of papain-treated Rh(D)-negative RBCs with mRh1. (C) Incubation of papain-treated Rh(D)-positive RBCs with mRh1. (D) Incubation of papain-treated Rh(D)-negative RBCs with culture medium (negative control). Agglutinated RBCs remained at the top of the columns.
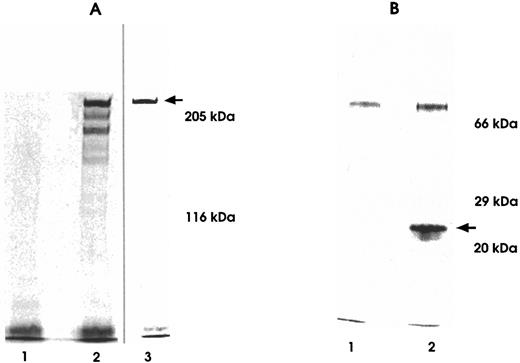
A first experiment of biosynthetic 35S labeling followed by immunoprecipitation on a bulk of mRh1-transfected cells led to a pattern made of three bands in the range of 200 to 300 kD, the higher band being largely predominant. This pattern was compatible with a mixture of 6-, 7-, and 8-valence multimers, a counterpart of the physiological pattern of C4bp multimers that consists mainly of octamers together with a few heptamers and hexamers. After cloning by limiting dilution, a clone only secreting the molecule of the highest molecular weight was chosen and subsequently developed. 35S amino-acid labeling experiments showed that a single molecular species of 270 kD apparent molecular weight on SDS-PAGE analysis was secreted by this mRh1-transfected CHO cell clone. The molecular weight of its monomeric component was assessed from SDS-PAGE under reducing conditions and was found to be 27 kD. These data suggested that the molecular formula of the multivalent molecule mRh1 was an octamer, like the natural major C4bp component (Fig 3).
SDS-PAGE analysis of 35S-labeled supernatants immunoprecipitated using anti-MYC Tag MoAb. Immunoprecipitates were run on 5% (A) and 10% (B) polyacrylamide gels under nonreducing (A-2 and A-3) and reducing conditions (B-2). Untransfected CHO cell immunoprecipitates served as negative controls (A-1 and B-1). Lane A-2 depicts a three-band heterogeneous hexamere, heptamere, and octamere profile observed under nonreducing conditions in a biosynthetic labeling experiment from a bulk of transfected cells before final cloning of cells.
SDS-PAGE analysis of 35S-labeled supernatants immunoprecipitated using anti-MYC Tag MoAb. Immunoprecipitates were run on 5% (A) and 10% (B) polyacrylamide gels under nonreducing (A-2 and A-3) and reducing conditions (B-2). Untransfected CHO cell immunoprecipitates served as negative controls (A-1 and B-1). Lane A-2 depicts a three-band heterogeneous hexamere, heptamere, and octamere profile observed under nonreducing conditions in a biosynthetic labeling experiment from a bulk of transfected cells before final cloning of cells.
The scFv MYC Tag product did not lend itself well to quantification, possibly because of the position of the tag at the center of the spider-like structure. With the same vector, other multimerized molecules (CD4 or CD16) regularly reach a stable secretion level of 1 to 3 μg/mL, which can be assumed as the secretion range of mRh1, whereas, with a more powerful vector, engineered CD46 reaches a 10 to 20 μg/mL secretion level (manuscript in preparation).
Flow cytometry analysis showed that the binding of mRh1 was strikingly better on papain-treated RBCs and during a warm incubation.
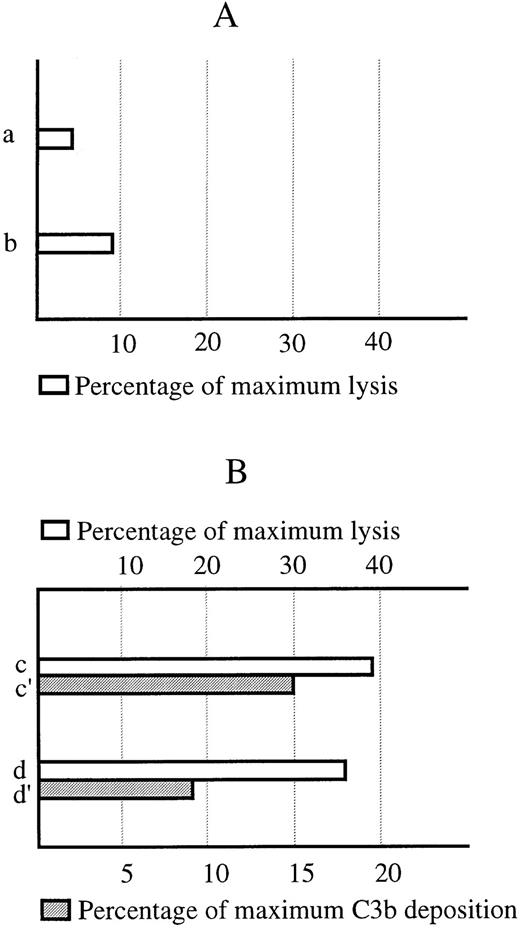
Flow cytometry competition experiments between mRh1 and human anti-Rh(D) DF5 MoAb, from which originated the binding site of mRh1, showed a dose-dependent inhibition validating the specificity of mRh1 binding on RBCs. In complement lysis experiments, no complement fixation was found on mRh1 coated RBCs whereas a significant lysis was observed when an anti-MYC Tag and an antimouse Ig dual antibody enhancing system was used as a positive control of both mRh1 binding and complement potency (Fig 4).
Lack of activation of the complement system by the mRh1. (A) Lack of complement-dependent lysis of RBCs sensitized by using mRh1. (a) mRh1-sensitized RBCs. (b) Control RBCs. (B) Positive control. (c) RBCs sensitized using mRh1 and an anti-MYC Tag and antimouse Ig enhancing system. (d) Control RBCs lacking mRh1 incubation step. (c′ and d′) Immunofluorescence controls of mRh1 and enhancing system fixation. Scale is expressed in percentage as a ratio of the sample mean fluorescence channel to that of an antiglycophorin A MoAb positive control. Hemolysis is quantified as percentage of the maximal lysis of the system when using a complement fixating antiglycophorin A MoAb.
Lack of activation of the complement system by the mRh1. (A) Lack of complement-dependent lysis of RBCs sensitized by using mRh1. (a) mRh1-sensitized RBCs. (b) Control RBCs. (B) Positive control. (c) RBCs sensitized using mRh1 and an anti-MYC Tag and antimouse Ig enhancing system. (d) Control RBCs lacking mRh1 incubation step. (c′ and d′) Immunofluorescence controls of mRh1 and enhancing system fixation. Scale is expressed in percentage as a ratio of the sample mean fluorescence channel to that of an antiglycophorin A MoAb positive control. Hemolysis is quantified as percentage of the maximal lysis of the system when using a complement fixating antiglycophorin A MoAb.
Taken together, these data showed the secretion by mRh1-transfected CHO cells of a unique soluble multimeric molecule with multivalent antibody properties, devoid of any complement fixation activity.
DISCUSSION
The use of molecules of human origin for therapy began with the use of polyclonal whole antisera. MoAb allowed production of better defined molecules, which led to reproducible and well-controlled products. However, their use remained mostly limited to situations in which the destruction of a target is needed because of the properties of the Fc fragment of Igs. Artificial structures such as single chain antibodies7,9 19-21 generally have a short half-life, have a low avidity because of their single valence, and are, in most circumstances, unable to trigger biological functions alone.
The association of different structures in a given recombinant molecule has been hampered by problems because of conformation and accessibility. Different approaches have been proposed to obtain bivalent or multivalent molecules. Chemical linkage of proteins to polyethylene-glycol or dextran is cumbersome, requiring large amounts of purified material.22 Disulfide linking of Fcγ or use of Fcμ fragments to create multivalent Ig molecules23-29 has been proposed to maintain and amplify Fc-mediated functions. However the enhancement of Fc-associated reactivity, such as antigen-independent complement activation, can be undesirable in a therapeutic context. The molecules synthesized in these systems have been heterogeneous, varying from monomers to hexamers and higher order structures. Dimerization of smaller F(ab′)2 or Fv structures has been effected through the use of amphiphatic helices, either leucine zippers or bundle-helix constructs.30,31 The former do not satisfactorily react to the introduction of a covalent link and, thus, are potentially unstable; furthermore, these molecules are not natural components of plasma and may prove immunogenic. Other approaches to dimerization of scFv have been proposed,32 such as diabodies.33 Nevertheless dimeric structures may not be optimal for given applications.
Multimeric constructions, such as those described by Ito et al,23 based on fusion to protein A, can generate complexes of Fv-protein A and IgG with variable stoichiometry or by Dübel et al,34 in which scFv are fused to a core-streptavidin structure allowed the creation of tetrameric antibodies with additional coupling possibilities because of the presence of biotin binding sites and cysteines. The use of a heteroantigenic fragment, protein A, or streptavidin carries the risk of an immune response which could seriously limit potential therapeutic applications.
The C-terminal C4bp multimerizing system fulfills the requirement of a multi-purpose multimerizing system for future in vivo use.
This study established that the C terminal part of the α-chain of C4bp is sufficient to induce polymerization during protein synthesis, and, although the expression vector codes only for monomers, multimers are assembled in the cell without necessity for secondary modifications resulting in the secretion of a unique, covalently linked soluble molecule. This research model can be further optimized to meet the needs of high-level production. Clearly, production system, vectors, leader sequence and junction area at the 5′ end of the C4bp multimerizing system have to be optimized for that purpose. The multimerizing structure originates from a normal component of human plasma avoiding immunization. It does not impair the solubility of multimeric molecules and lacks any biological function. Although only electron microscopy images will resolve the question with certainty, it seems reasonable to believe that the three multimeric forms observed before cloning were the counterpart of the physiological forms of C4bp, because their relative proportions were also in accordance with the physiological pattern: There was a major representation of structures with eight valences, a higher number than reached by any other multimerizing system. Although no definite explanation is available for the variations in the structure of multimers produced by different cell clones, they could be prone to variation in protein synthesis rate and/or to interference of other protein syntheses in the polymerization process. A possible effect of sequence variation appears less likely, because no clone only secreting either hexamers or heptamers without octamers has been detected, but this hypothesis cannot be totally ruled out. The multimerizing system provided the multimeric mRh1 with the ability to agglutinate RBCs, suggesting an improvement of the scFv binding through its multivalent nature. The lack of complement activation from mRh1-coated RBCs suggested a promising potential of this system for future in vivo harmless targeting onto cells, here considered either as transporters of various biologically active molecules or for localization by imaging of cells expressing an inappropriate antigen.
Multibodies may also be developed from this model of multi-scFv, associating different antibody binding sites against various epitopes of a given antigen, to maintain the avidity of polyclonal antisera by mimicking the cooperative effect of different antibodies in polyclonal antisera.
Future developments of this system towards heterofunctional multimeric molecules will include the use of C terminal parts of both α- and β-C4bp chains or the use of modified α-chains to control the ratio of two components in heterochimeric molecules and to modulate the polymerization process. These heterofunctional multimeric molecules will either retain the lack of complement activation properties of this system, for example for enzyme replacement therapy or imaging, or will incorporate complement activating or cell attracting molecules to promote the clearance of targeted cells. In the future, new recombinant molecules for clearing Rh(D)-positive RBCs and preventing Rh alloimmunization may be designed from this system.
Address reprint requests to J.H.M. Cohen, MD, Laboratoire d'Immunologie, Hôpital Robert Debré, 51092 Reims, Cedex, France.