Abstract
To characterize early B-cell precursors in humans, we correlated immunoglobulin heavy chain (IgH) gene rearrangement status with the CD34, CD19, and CD10 cell surface markers. Highly purified adult bone marrow (BM) cell fractions were obtained by two successive rounds of flow cytometric cell sorting, and IgH rearrangements were analyzed by polymerase chain reaction (PCR) amplification. Complete VDJH rearrangements were observed in the CD34+ CD19+ fraction, but not in the more immature CD34+ CD19− fraction. About one quarter of these rearrangements had an open reading frame, thus potentially permitting the synthesis of a μ chain. Partial DJH rearrangements were detected in both CD34+ CD19+ and CD34+ CD19− subsets, although they were less abundant in the latter. When triple labeling was used to better characterize the CD34+ CD19− population, DJH rearrangements were found to be present in the CD34+ CD10+ CD19− fraction, but not in the more primitive CD34+ CD10− CD19−. These results indicate that IgH gene rearrangements occur in CD34+ BM cells and that they initiate in immature progenitors expressing the CD10, but not yet the CD19 surface antigen. Finally, the presence of IgH gene rearrangements in CD34+ BM cells provides a useful marker of clonality to evaluate the possible involvement of these cells in various B-cell lymphoid malignancies.
B-LINEAGE LYMPHOPOIESIS is a complex process that occurs first in the fetal liver and then in the bone marrow (BM).1 It can be characterized by the sequential acquisition of intracellular and surface markers. Among these, the production of a surface B-cell antigen receptor seems to be a crucial step because the inability to do so in knock-out mice impairs B-cell development at an early stage.2 The synthesis of immunoglobulins (Ig) necessitates the coordinate rearrangement of the Ig genes, which are encoded by multiple germline DNA segments (V, D, and J).3 In most instances, the heavy (H) chain locus rearranges first and involves the assembly of a DH segment to a JH segment followed by the recombination of a VH segment to the DJH join. A productive VDJH rearrangement is thought to stop further recombination at the H chain locus and to trigger those at the light (L) chain loci, most often κ followed by λ.4 5
Numerous studies in mice have been permitted to correlate the acquisition of surface markers with the status of Ig gene rearrangements and to clearly identify the early stages of B-cell differentiation.6-10 According to Hardy's model,6-8 four fractions (termed A to D) can be identified in the precursor B220 (CD45)+, surface (s) lgM− BM cells, depending on their differential expression of the CD43 (leukosialin), heat stable antigen (HSA), and BP-1 surface antigens. Stage A (CD43+ HSA−BP-1−) is the earliest stage (pre-pro–B) and consists of cells initiating DH to JH recombinations. Cells in fraction B (early pro-B, CD43+ HSA+ BP-1−) have undergone DJH rearrangement at their IgH loci, and a minority of them also have undergone VDJH rearrangement. In the next stage (fraction C, CD43+ HSA+ BP-1+), the late pro-B cells undergo VH to DJH recombinations and a minor fraction of them VL to JL recombination. The last fraction (D, CD43− HSA− BP1+) consists of small resting pre-B cells with rearranged H and L chains loci, but no conventional slg expression. The latter appears in the immature B slgM+ cell fraction, precursor of the mature sIgM+ IgD+ B cells.
A somewhat different classification system has been proposed by Rolink et al9 10 using cell-size, c-kit, and CD25 surface expression. The first stage of B-cell differentiation is called pre-B I and consists of c-kit+ CD25− cells with DJH rearranged Ig genes. The following stage includes c-kit− CD25+ large pre-B II cells with VDJH rearrangements. L chain gene rearrangements appear in the next stage, called small pre-B II (c-kit− CD25+ sIgM−), which precedes the appearance of the immature B cells (c-kit− CD25+ sIgM+).
Although early B-cell differentiation in man probably proceeds along similar lines,11 the precise correlation between the apparition of Ig gene rearrangements and differentiation markers has been studied in much less detail. Pre-B cells have been identified by their expression of cytoplasmic (c) μ chain without L chain12 and of surface CD10 and CD19 antigens. It is now known that in both humans and mice, small amounts of μ H chain are also expressed on the surface of pre-B cells, in association with a surrogate light (ΨL) chain, product of the λ5/14.1 and Vpre-B genes.13 Earlier B-cell progenitors (pro-B stage) express the CD34 antigen, a 115 kD sialo-glycoprotein also present on hematopoietic stem cells and early commited progenitors.14 Pro-B cells also express the CD10 and CD19 antigens.11,15-17 Although early studies18 on leukemic cells indicated that CD19 appeared before CD10, later analyses11,15,17 of normal cells showed that CD10 expression can precede that of CD19. The CD34+ CD10+ CD19− cell population may include very early B-cell progenitors, as well as progenitors of other hematopoietic lineages.17,19 Conversely, cell culture experiments have confirmed that CD34+ CD19+ CD10+ cells represented B-lymphoid committed precursors.17,20,21 Although the vast majority of these cells do not contain cIg, a small proportion (less than 10%) has been found to be cIg+ by multiparameter flow cytometric analysis.21 It is likely that a fraction of this population has undergone productive IgH gene rearrangements. However, the extent and type of IgH rearrangements in these precursors remains to be determined.
We have, therefore, attempted to correlate the status of IgH rearrangements with expression of the CD34, CD10, and CD19 differentiation markers. Our study shows that IgH rearrangement do occur in CD34+ precursor cells, as complete VDJH rearrangements are found in the CD34+ CD10+ CD19+ compartment, and partial DJH rearrangements can already be detected at the earlier CD34+ CD10+ CD19− stage.
MATERIALS AND METHODS
Cell preparation. Human BM was obtained, after informed consent, from normal adult volunteer donors for allogenic transplantation. BM mononuclear cells were diluted in cold Hank's Balanced Salt Solution containing 2% (vol/vol) fetal calf serum and antibiotics (HFA) and mononuclear cells were isolated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation (density = 1.077).
Immunofluorescence staining and cell sorting. Cells were washed and incubated (107/mL) in HFA with anti-CD34 (HPCA-2, Becton Dickinson, San Jose, CA), fluorescein isothiocyanate (FITC), anti-CD19 (B4, Coulter Clone, Margency, France), phycoerythrin (PE) monoclonal antibodies (MoAb). In some experiments, anti-CD34–FITC and anti-CD10 (J5, Coulter Clone)-PE MoAb were used for three-color labeling in conjunction with anti-CD19–biotin revealed by streptavidin allophycocyanin (APC). After washing, cells were suspended in HFA and then sorted on a FACStar-Plus (Becton Dickinson) cytometer. To ensure maximum purity, the sorted cells were subjected to a second identical sort. Purity of cells was then assessed by analyzing an aliquot.
DNA preparation. Cell fractions collected in phosphate-buffered saline (PBS) were centrifuged and the pellets resuspended in 40 μL of lysis buffer consisting of 0.5% Tween 20, 0.5% Nonidet-P40, 1× PCR buffer (see below), and 2 mg/mL proteinase K. After 2 hours incubation at 37°C, the proteinase K was inactivated by heating at 95°C for 10 minutes.
PCR analysis of Ig gene rearrangements. Polymerase chain reaction (PCR) amplification of complete VDJH rearrangements was performed using a 5′ consensus framework 3 (FR3) primer [ACTCTCGAGACAC(G/A)GC(C/T)(G/A)TGTATTACTG] and a 3′ consensus JH primer (AACTGCAGAGGAGACGGTGAC).22 For detection of incomplete rearrangements, the 5′ primers consisted of a mixture of consensus primers for each of the seven main DH gene segment families23 (DHQ52 : AGAACCACTGTGCTAACTGG, DXP : TCTGTGTCACTGTGGTATTAC, DA : TGTGTGACTACAGTAACTAC, DK : TGTCAGACTGTGGTGGATA, DN : GTCACAGTGG(G/A)GTATAGCAGC, DM : TCACAG(C/T)GGGTATAAC(T/C)GGA, DLR : GTGTCACTGTGAG(A/G/C)ATATTGT). As previously described,24 these primers were designed to anneal to the 5′ part of the coding sequence of the DH gene, as well as the upstream recombination sequence, so that only DH genes with unrearanged 5′ end could be amplified in the conditions defined below. For DJH PCR, the 3′ JH oligonucleotide used was identical to that for VDJH PCR. An equivalent of 2,000 cells was used for each amplification in a 50-μL volume containing 10 mmol/L Tris-HCl pH 8.3, 50 mmol/L KCl, 2.5 mmol/L MgCl2 , 0.2 mmol/L deoxynucleotide triphosphate (dNTP), 1 mmol/L primers, and 1 U Taq Polymerase (Boehringer Mannheim, Meylan, France). Amplification consisted of 35 cycles of 45 seconds at 94°C, 45 seconds at 55°C, and 45 seconds at 72°C, and was performed in a PTC-200 thermal cycler (MJ-Research, Prolabo, Fontenay-s/Bois, France). PCR products were electrophoresed in 1.5% agarose gels, denatured in alkaline buffer, and transferred to Hybond N nylon membranes (Amersham, Les Ulis, France). The membranes were hybridized with a 32P-labeled internal JH oligonucleotide probe (CA(G/T)GGT(G/C/T)CCT(C/T)GGCCCCAG) for 2 to 8 hours at 46°C in 5× SSPE (1× SSPE is 0.15 mol/L sodium chloride, 10 mmol/L sodium phosphate, 1 mmol/L EDTA), washed in 5× SSPE, 0.1% sodium dodecyl sulfate (SDS) at 40°C to 46°C and autoradiographed for 12 to 36 hours. The RAG-1 gene primers (5′: AGGAATTAACTCACAAACTGC and 3′: GCCATGAAGAGCAGTGAATTA) were used as control for DNA integrity.
Sequencing of PCR products. PCR products were cloned directly into the pCR-Script vector (Stratagene, La Jolla, CA). Clones harboring recombinant plasmids with inserts of appropriate size, based on restriction analysis, were sequenced in both directions using the dideoxy chain termination method with T7 DNA Polymerase (Pharmacia, Uppsala, Sweden).
RESULTS
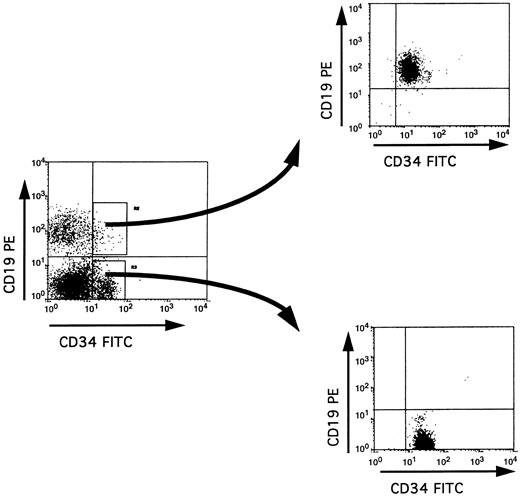
Purification of BM CD34+ subpopulations. The purification procedure used in our study involved two successive rounds of cell sorting, thus achieving a very high degree of purity of the sorted cells (>99%) (Fig 1). Because this was obtained at the expense of a low yield of cells (especially for the double positive CD34+ CD19+ or CD34+ CD10+ cells), we performed PCR analyses on cell lysates from 2,000 cells. A total of 11 BM samples was analyzed.
Isolation of CD34+ CD19+ and CD34+ CD19− populations by cell sorting. Low density mononuclear cells from adult BM were labeled with anti-CD34–FITC and anti-CD19–PE, and cells within the lymphoid blast window were analyzed before or immediately after double cell sorting on a FACStar Plus flow cytometer. The purity of the sorted cells in each fraction was at least 99%.
Isolation of CD34+ CD19+ and CD34+ CD19− populations by cell sorting. Low density mononuclear cells from adult BM were labeled with anti-CD34–FITC and anti-CD19–PE, and cells within the lymphoid blast window were analyzed before or immediately after double cell sorting on a FACStar Plus flow cytometer. The purity of the sorted cells in each fraction was at least 99%.
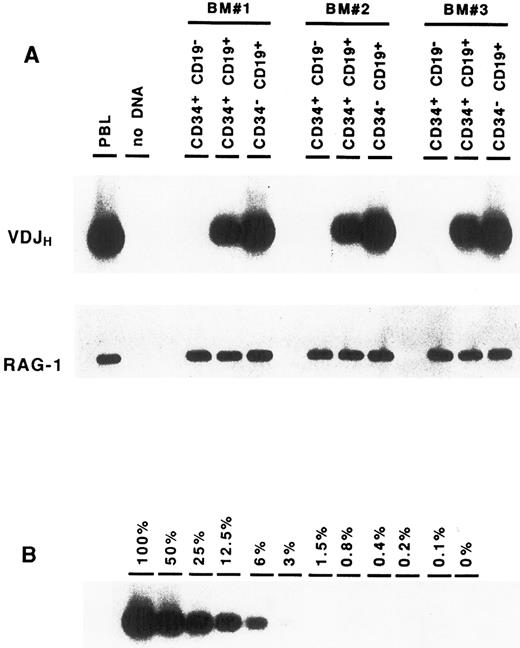
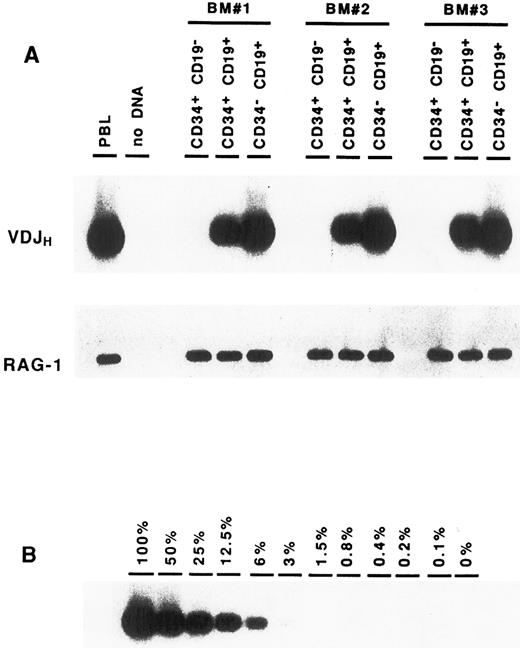
Complete VDJ IgH rearrangements are detected in CD34+ CD19+ cells. In a first series of experiments, BM cells were sorted solely on the expression of the CD34 antigen. DNA amplification was performed on cell lysates using consensus primers for the FR3 and JH regions, which are able to detect the vast majority of IgH rearrangements in normal B cells.22 Analysis of these CD34+ cells showed the presence of IgH gene rearrangements (data not shown). To identify more precisely the progenitor cells carrying these IgH gene rearrangements, BM cells were sorted according to their expression of CD34 and CD19. As can be seen in Fig 2A, IgH VDJ rearrangements could be detected consistently in the CD34+ CD19+ precursor population. Although this PCR assay is not quantitative, it should be noted that the intensity of the smear is somewhat less intense than that seen in the CD34− CD19+ mature B cells, suggesting that not all of the cells have undergone VDJH recombination. In contrast, no VDJH rearrangement was observed within the CD34+ CD19− population. The presence of Taq polymerase inhibitors or problems with DNA integrity could easily be ruled out, as amplification of a control gene (RAG-1) was positive in all fractions. To exclude the possibility that the VDJH rearrangements observed in the CD34+ CD19+ cells were due to contamination from mature B lymphocytes, CD34− CD19+ B cells were diluted in nonlymphoid cells (keratinocytes), and PCR analysis was performed in similar conditions. As can be seen in Fig 2B, the presence of at least 25% of contaminating B cells would be necessary to produce a signal of comparable intensity to that observed in the CD34+ CD19+ fraction. These results indicate that complete VDJ IgH rearrangements can be detected in BM CD34+ cells, and that they occur within the CD34+ CD19+ subpopulation.
Detection of VDJH rearrangements in BM cell fractions. DNA isolated from purified cell fractions (A) or dilutions of CD34− CD19+ B cells in keratinocytes (B) were amplified with consensus FR3 and JH primers. PCR products were subjected to Southern blot hybridization using an internal consensus JH oligonucleotide probe. Amplification of the RAG-1 gene served as control for DNA integrity.
Detection of VDJH rearrangements in BM cell fractions. DNA isolated from purified cell fractions (A) or dilutions of CD34− CD19+ B cells in keratinocytes (B) were amplified with consensus FR3 and JH primers. PCR products were subjected to Southern blot hybridization using an internal consensus JH oligonucleotide probe. Amplification of the RAG-1 gene served as control for DNA integrity.
VDJH rearrangements in CD34+ CD19+ progenitors are potentially functional. The presence of IgH rearrangements in the CD34+ CD19+ fraction prompted us to determine whether they could possibly encode an H chain protein. PCR products from three distinct CD34+ CD19+ BM cells were cloned and sequenced in both directions. Nucleotide sequence analysis showed that 12 of 45 rearrangements had an open reading frame (Table 1). This proportion (27%) is consistent with the one third frequency expected from random rearrangements.3 25 This suggests that a portion of IgH rearrangements present in the CD34+ CD19+ cells could direct the synthesis of μ protein.
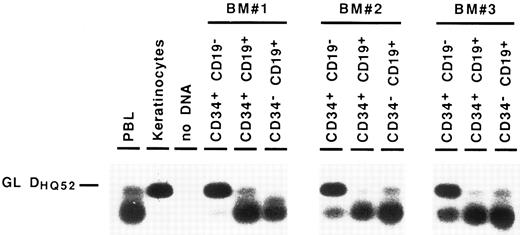
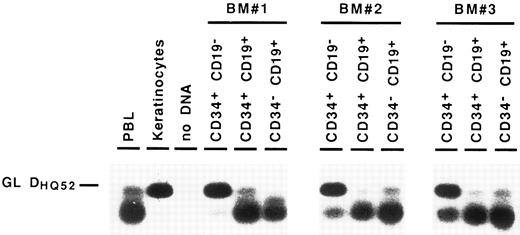
Detection of partial IgH rearrangements in CD34+ CD19− cells. Because IgH rearrangements are known to proceed in an orderly fashion, with DJH rearrangement preceding VDJH rearrangement,4,5 we attempted to detect these partial IgH rearrangements in the different BM subpopulations. For this purpose, we performed DNA amplification with a 3′ consensus JH primer and 5′ primers consisting of a mixture of oligonucleotides specific for the seven main DH gene families.23 These primers were designed to overlap the 5′ region of the coding sequence of the DH gene and the upstream recombination signal sequence, so that they could not amplify DH segments incorporated into VDJH rearrangements.24 Under such conditions, partial DJH rearrangements were observed in the CD34− CD19+ B cells, as well as in CD34+ CD19+ progenitors. As can be seen in Fig 3, they were also detected repeatedly in the most immature CD34+ CD19− cell fraction, albeit with a lower intensity. Further proof that these rearrangements were indeed DJH recombinations was obtained by sequencing of PCR products (data not shown). These results show that IgH gene rearrangements are initiated in very early BM progenitors.
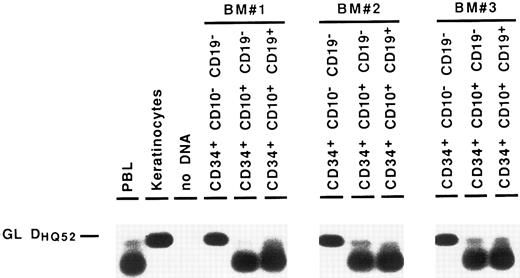
Detection of partial DJH rearrangements in BM cell fractions. DNA from cell lysates was amplified with a mixture of consensus primers for most of DH gene families and a consensus JH primer. PCR products were visualized by Southern blot hybridization using an internal consensus JH probe. GL DHQ52 indicates the amplification, from a germline IgH locus, of the DNA segment located between the most 3′ DH gene (DHQ52 ) and the most 5′ JH gene (JH1 ), producing a 160-bp product.
Detection of partial DJH rearrangements in BM cell fractions. DNA from cell lysates was amplified with a mixture of consensus primers for most of DH gene families and a consensus JH primer. PCR products were visualized by Southern blot hybridization using an internal consensus JH probe. GL DHQ52 indicates the amplification, from a germline IgH locus, of the DNA segment located between the most 3′ DH gene (DHQ52 ) and the most 5′ JH gene (JH1 ), producing a 160-bp product.
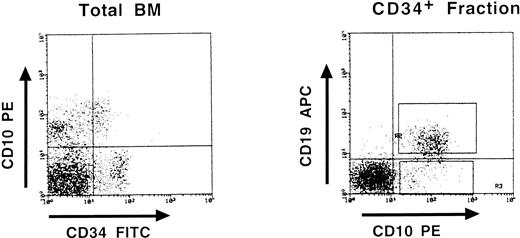
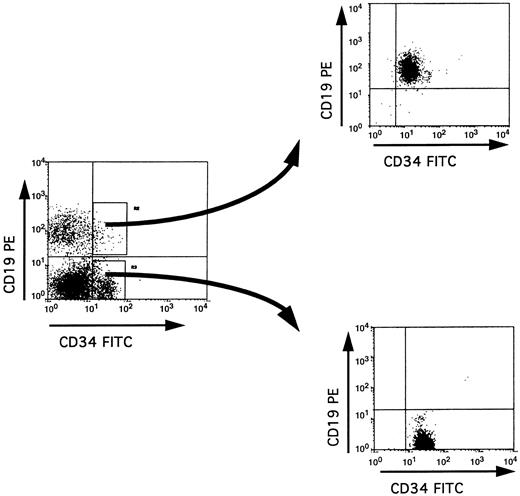
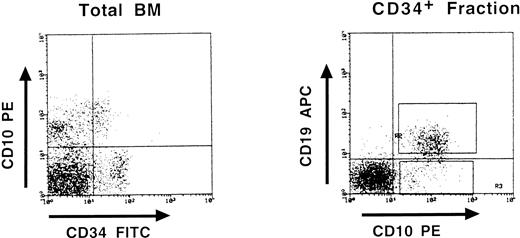
Partial DJH rearrangements are present in the CD34+ CD10+ CD19− fraction. The CD34+ CD19− fraction represents a complex population of progenitors for various blood cell lineages, as well as hematopoietic stem cells. To identify more precisely which subpopulation might harbor the partial DJH rearrangements, BM cells were analyzed and sorted using triple labeling with anti-CD34, anti-CD19, and anti-CD10 MoAb. As can be seen in Fig 4, a minor population of CD34+ CD10+ CD19− cells could be identified, whereas no clear population was detected in the CD34+ CD10− CD19+ window. This confirms earlier studies showing the appearance of CD10 before that of CD19 on normal BM cells.11,15 17 Analysis of DJH rearrangements on sorted populations showed that they were present, as expected, in the CD34+ CD10+ CD19+ fraction, but also to a lesser extent in the CD34+ CD10+ CD19− fraction (Fig 5). In contrast, no DJH rearrangements were seen in the CD34+ CD10− CD19− fraction. These results indicate that among the CD34+ CD19− BM progenitors, it is the subpopulation expressing the CD10 antigen, which has undergone partial IgH rearrangement.
Three-color fluorescence analysis of BM cells labeled with anti-CD34–FITC, anti-CD10–PE, and anti-CD19–APC. This representative experiment shows the existence of a CD34+ CD10+ CD19− population. Boxes on the right panel represent the window gates used during the sort procedure.
Three-color fluorescence analysis of BM cells labeled with anti-CD34–FITC, anti-CD10–PE, and anti-CD19–APC. This representative experiment shows the existence of a CD34+ CD10+ CD19− population. Boxes on the right panel represent the window gates used during the sort procedure.
Detection of partial DJH rearrangements in BM CD34+ fractions. Amplification of the germline DHQ52-JH1 DNA segment (GL DHQ52 ) served as an internal control for DNA integrity.
Detection of partial DJH rearrangements in BM CD34+ fractions. Amplification of the germline DHQ52-JH1 DNA segment (GL DHQ52 ) served as an internal control for DNA integrity.
DISCUSSION
Whereas the early steps of B-cell differentiation have been extensively studied in the mouse, in terms of phenotypic markers and gene rearrangement status,6-10 they have received less attention in humans. In this work, we have used multiparameter flow cytometry combined with PCR amplification of IgH gene rearrangements to characterize these B-cell progenitors in human adult BM.
Because our study is based on PCR analyses of a small number of cells (2,000), it was important to exclude the possibility that the Ig gene rearrangements detected in CD34+ progenitors were due to the presence of contaminating mature B cells. This is very unlikely for the following reasons: first, the cell purification procedure included two successive rounds of cell sorting, which yielded a purity greater than 99%; secondly, dilution experiments of purified B cells in nonlymphoid cells indicated that the level of contamination had to be at least 25% to produce a signal of comparable intensity to that seen in the CD34+ CD19+ fraction; finally, the fact that partial DJH rearrangements, but not VDJH rearrangements, were detected in the CD34+ CD19− and CD34+ CD10+ CD19− populations further ruled out the possibility of mature B-cell contamination in our cell fractions.
Pro-B cells express the CD34, CD10, and CD19 antigens, but lack cIg, while pre-B cells are CD34− and cIg+. It has, therefore, been generally admitted that Ig gene rearrangements are initiated in pro-B cells to be fully rearranged and to produce a μ chain in the following pre-B stage. Our study shows that pro-B cells have, in fact, already completed VDJH rearrangements. Although our PCR assay is not quantitative, it can be estimated that it is not a minority of cells that have undergone such rearrangements. Interestingly, about one quarter of these rearrangements were in frame, thus potentially permitting the synthesis of a μ chain. This raises the question whether μ chains could be already produced in some pro-B cells. Previous studies have produced conflicting results concerning this issue. According to Pontvert-Delucq et al,17 only very rare (1%) CD34+ CD10+ CD19+ adult BM cells express cIg, while Dittel et al21 have detected cIg in about 10% of CD34+ CD10+ cells, a proportion that would roughly correlate with our results. In pre-B cell, the μ chains are also expressed at low level on the cell surface in association with a ΨL chain composed of the products of the Vpre-B and λ5/14.1 genes.13,26,27 Although a complex formed by this ΨL chain and a protein of 125 kD, termed ΨH, has been described by some investigators on both human and murine pro-B cells,28-32 no surface expression of μ chains has so far been reported at this stage of B-cell development. It would, therefore, seem that if μ chains can be produced in pro-B cells from productive VDJH rearrangements, they either cannot be exported to the cell surface or their surface expression is below the level of detection.
Because VDJH rearrangements were detected in CD34+ CD19+, but not in CD34+ CD19− cells, the VH to DJH recombination is concomittant with the expression of CD19. This might be purely fortuitous or reflect the fact that both events are part of the same differentiation program. Alternatively, it might indicate that expression of CD19 favors the completion of IgH rearrangements. A mouse model in which the CD19 gene has been inactivated invalidates this hypothesis, as mutant animals could produce mature B cells in their BM.33 However, the role of CD19 on the regulation of IgH gene rearrangements cannot be totally ruled out because the ligation of CD19 on human pro-B cells has been shown to prevent the downregulation of the RAG genes induced by interleukin (IL)-7.34
Our study provides evidence for the presence of partial DJ IgH rearrangements in CD34+ CD10+ CD19− cells. This confirms and expands earlier findings of DHQ52 rearrangements in human fetal CD34+ CD19− BM cells.35 The nature of these CD34+ CD10+ CD19− progenitors is unclear. Because IgH rearrangements proceed from DJH to VDJH , they must contain B-cell precursors. The demonstration that some CD34+ CD10+ cells can differentiate in vitro into pre-B cells is also in keeping with this notion.21,36 CD10 antigen expression, however, is not restricted to B cells, as it is also found on T-cell progenitors.37 Interestingly DJH rearrangements have been observed occasionaly in normal T cells,38 and may correspond to some of the IgH gene rearrangements detected by Southern blot in a proportion of T-cell acute leukemia.39 On the other hand, Pontvert-Delucq et al17 have identified a population of CD34+ CD10+ CD19− cells that coexpress the CD33 antigen and which only generate macrophagic colonies in culture. Furthermore Galy et al19 have shown that CD34+ CD10+ Lin− cells could give rise to T and B lymphocytes, as well as natural killer (NK) and dendritic cells. Thus, whether the fate of this CD34+ CD10+ CD19− population with partial IgH rearrangements is restricted to the B-cell lineage, or whether it can also give rise to other hematopoietic cells remains to be elucidated.
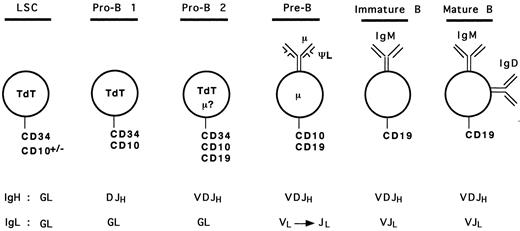
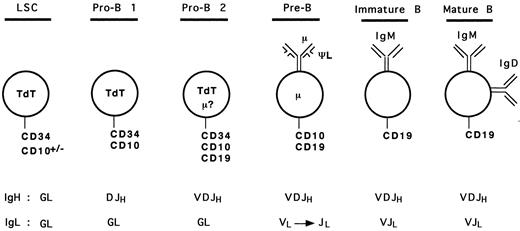
Based on our analyses and results from previous studies, we can propose a hypothetical model for human early B-cell differentiation (Fig 6). B-cell progenitors would arise from a lymphoid stem cell expressing surface CD34 and the nuclear terminal deoxynucleotidyl transferase (TdT) enzyme, as it has been shown that bone marrow CD34+ TdT+ cells lacking lineage specific surface antigen may represent the earliest progenitors committed to the lymphoid differentiation pathway.36,40,41 Some of these cells may also express CD10 because CD34+ CD10+ progenitors can generate lymphocytes, NK, and dendritic cells.19 The first step towards B-cell lineage differentiation (pro-B 1 stage) would correlate with acquisition of DJH rearrangements and surface expression of CD10. These CD34+ CD10+ CD19− DJH+ pro-B 1 cells observed in our study are likely to overlap with the recently described CD34+ CD10+ CD19− IL-7–receptor α chain+ population, which shows the ability to differentiate into CD34− CD10+ CD19+ cells in short-term cultures.36 As discussed above, these cells may represent a heterogeneous population of precursors, with a potential to generate other lineages (such as T cells). Further commitment to the B lineage would be represented by the CD34+ CD10+ CD19+ cells with VDJH rearrangements (pro-B 2 stage). As mentioned earlier, some of these cells may express a cytoplasmic μ chain, but probably no surface μ chain. Both pro-B 1 and pro-B 2 cells are expected to express the nuclear TdT enzyme, as shown in previous studies.15-17,36,40,41 The loss of CD34 and TdT, and the presence of cμ chain define the ensuing pre-B cells.12 At this stage, the pre-B cells undergo rearrangements of the L chain gene loci5 and express, on at least a subpopulation, the μ-ΨL pre-B receptor. Because of conflicting results concerning surface expression of the ΨL chain on different populations of B-cell precursors,26-29 42 it is presented only as a component of the pre-B receptor in our model (Fig 6), as all investigators agree that at least a subset of pre-B cells express it. Loss of the pre-B receptor with progressive disappearance of CD10, and expression of sIgM followed by sIgM and IgD represent immature and mature B cell stages, respectively.
Model of human early B-cell differentiation. LSC, lymphoid stem cell; GL, germline.
Model of human early B-cell differentiation. LSC, lymphoid stem cell; GL, germline.
This model of human B-cell differentiation parallels those described in the mouse6-10 to a certain extent. The pro-B 1 stage corresponds to Hardy's fraction B, while pro-B 2 corresponds to the fraction C. In a recent study, Ghia et al42 have analyzed Ig gene configuration in human BM B-lineage cells using single cell PCR. They found DJH rearrangements in CD34+ CD10+ CD19+ cells and VDJH rearrangements were inferred from the presence of a cμ chain in CD34− CD19+ cells. Based on these results, as well as analyses of IgL gene configuration, Vpre-B, TdT, and RAG gene expression, cell size and cycle status, they concluded that the human B-cell developmental pathway is very similar to that which they had described in the mouse.9 10 Thus, the CD34+ CD10+ CD19+ compartment represented the equivalent of the mouse pre-B I stage, while the CD34− CD10+ CD19+ cμ+ cells represented the equivalent of the large and small pre-B II stages. The status of IgH gene rearrangements observed in our study rather indicates that the murine pre-B I fraction (DJH rearranged) corresponds to the CD34+ CD10+ CD19− cells and the pre-B II fraction (VDJH rearranged) to the CD34+ CD10+ CD19+ cells. The fact that these investigators performed a PCR assay only for DJH rearrangements, but not for VDJH rearrangements, in the CD34+ CD10+ CD19+ cell fraction, and that they did not identify a CD34+ CD10+ CD19− population may explain the discrepancies between the two studies.
The finding of VDJH rearrangements in the CD34+ CD19+ BM cells also has important implications in malignant hematology. Autologous transfusion of CD34+ cells is currently being used to minimize the reinfusion of more mature tumor cells. There is evidence, however, that in some cases, the CD34+ cells are already involved in the malignant process. For instance, highly purified CD34+ CD19+ BM fractions from patients with follicular lymphoma were found to demonstrate bcl-2/IgH rearrangement.43 Similarly, trisomy 12 was observed in CD34+ cells from patients with chronic lymphocytic leukemia.44 However, many patients lack characteristic chromosome aberration or genetic abnormalities accessible to molecular analyses. Because IgH gene rearrangements represent clonal markers of B-cell proliferations, they provide tools to assess the clonality of CD34+ cells in these proliferations. They should prove useful for the evaluation of the possible involvement of CD34+ cells in various B-cell lymphoid malignancies.
ACKNOWLEDGMENT
The authors thank N. Azar (Centre de Transfusion Sanguine) for providing bone marrow samples, and E. Macintyre for critical reading of the manuscript.
Address reprint requests to Frédéric Davi, MD, PhD, Département d'Hématologie, Hôpital Pitié-Salpêtrière, 47 Bld de l'Hôpital, 75013 Paris, France.