Abstract
Hepatocyte growth factor-scatter factor (HGF-SF ) mediates mito-, moto-, and morphogenic effects through the MET receptor, a membrane bound tyrosine kinase. HGF-SF/MET signaling is mitogenic for a large number of epithelial and endothelial cells and activates organ regeneration. HGF-SF transcripts have been detected in various myeloid cell lines. Therefore, the potential role of HGF-SF/MET signaling for circulating cells of the immune system, especially under conditions of inflammation, was evaluated. Several B-lymphoid and myeloid cell lines were found to express HGF-SF or c-met transcripts, while activity of both genes was mutually exclusive with the exception of low level coexpression in two B-cell lines. HGF-SF transcripts were present in low quantities in freshly isolated peripheral blood mononuclear cells (PBMNCs). In contrast, c-met expression was not detected in freshly isolated cells from peripheral blood, but was induced in monocytes by activation of monocytic or T-cell function. HGF-SF incubation led to an increased c-fos steady state transcript level in myeloblastic K562 cells and moderately promoted cell viability of freshly isolated preactivated monocytes. c-met expression is thus established in activated monocytes, in particular under conditions resembling inflammation, making these cells accessible to functional effects of HGF-SF.
HEPATOCYTE growth factor-scatter factor (HGF-SF ) is a multifunctional and pleiotropic cytokine of approximately 90 kD.1 HGF-SF exerts multiple stimulatory effects on different target cell types, in that it is mitogenic, motogenic, and morphogenic for epithelial and vascular endothelial cells as well as for melanocytes.1-4 In addition, there is evidence that HGF-SF is neurotrophic for specific subpopulations of central nervous system neurons,5 and at higher concentrations cytotoxic for a variety of tumor cell lines.6 Furthermore, HGF-SF appears to be an essential factor in the fetal morphogenesis of the placenta and the liver,7,8 as well as an important positive regulator of liver regeneration after injury.9-12 It has also been speculated that HGF-SF may play a role in tumor progression by stimulating tumor cell invasion and metastasis.13
All functions thus far attributed to HGF-SF can be mediated by the MET tyrosine kinase, the high affinity HGF-SF receptor. MET is the product of the c-met gene and is known to activate different intracellular signaling pathways through an intracytoplasmic multifunctional-docking domain.14-18 Costimulatory effects of HGF-SF together with granulocyte-macrophage colony-stimulating factor (GM-CSF ), interleukin-3 (IL-3), or erythropoietin have been demonstrated on blast and colony-forming units from bone marrow, suggesting that HGF-SF/MET signaling may serve as a hematopoietic regulator.19-22 Furthermore, moderate functional effects have been reported in B cells, T cells, and granulocytes after stimulation with HGF-SF.23-25
To further elucidate the potential role of HGF-SF in myeloic and lymphocytic cells, we have analyzed the expression and potential function of HGF-SF, as well as of c-met using a representative panel of permanent cell lines and primary cell cultures under different inducing conditions. Our results show the presence of HGF-SF and c-met expression in several myeloid cell lines. c-met is activated in peripheral blood monocytes after in vitro stimulation, rendering them functionally responsive to HGF-SF stimulation. These results suggest that activated monocytes in vivo respond to increased concentrations of HGF-SF as observed under conditions of inflammation.
MATERIALS AND METHODS
Cell culturing and isolation of cells from peripheral blood. The permanent cell lines described in Table 1 were obtained from the Deutsche Sammlung für Mikroorganismen und Zellkulturen (Braunschweig, Germany); cell line U937 was kindly provided by B. Fleischer (Hamburg, Germany). Peripheral blood mononuclear cells (PBMNCs) were separated by centrifugation over a Ficoll-Hypaque density gradient (Biochrom, Berlin, Germany) from heparinized peripheral blood samples obtained from healthy individuals. PBMNCs were recovered from the interface and washed twice in phosphate-buffered saline (PBS). For a number of experiments, the PBMNCs were cultured for up to 7 days in RPMI 1640 medium (Biochrom) containing 10% fetal calf serum (FCS).
Monocytes were isolated by immunomagnetic separation using antibody-coated magnetic beads (Deutsche Dynal, Hamburg, Germany) according to the manufacturer's protocol.26 Briefly, a highly monocyte enriched fraction (purity >85%) was obtained by negative selection, extracting T cells with anti-CD2 and B cells with anti-CD19 antibody-coated beads. Natural killer (NK) cells were bound to antibodies against CD16 (Immunotech, Marseille, France) and CD56 (Dako, Copenhagen, Denmark) and then extracted with antimouse IgG-coated beads (Dynal).
Stimulation of cultured cells. Permanent cell cultures (see Table 1) were incubated with 1% dimethyl sulfoxide (DMSO) (Sigma; Deisenhofen, Germany), 1 ng/mL 12-0-tetradecanoylphorbol-13-acetate (TPA; Sigma), 100 nmol/L 2-0-dibutyryladenosin-′5′cyclic monophosphate (dbcAMP; Sigma), or recombinant human HGF-SF (30 ng/mL)27 under otherwise constant medium conditions for 24 hours. The following substances were used for stimulation of isolated PBMNCs and highly monocyte enriched fractions: pokeweed mitogen (PWM; 0.8 μg/mL, Sigma), phytohemagglutinin (PHA; 2.4 μg/mL; Sigma), and lipopolysaccharide (LPS; 50 ng/mL, Sigma).
Plasmids and probes. The following cDNAs were used for hybridization: a 2.2-kb BamHI-Kpn I-fragment, containing the coding region of the human HGF-SF cDNA cloned into pGEM3Z (Promega, Madison, WI; plasmid pGhHGF ), a 1.3-kb EcoRI fragment covering the cDNA part coding for the cytoplasmic part of the human MET-receptor cloned into pGEM3Z (plasmid pGhosI); hybridization for the human c-fos RNA was performed with a 2.1-kb cDNA fragment cloned into pSPT 18/19.28 A cDNA probe for human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control in rehybridization of the filters.29 The purified cDNA fragments were labeled by primer extension using [32P] deoxycytidinetriphosphate (dCTP) for Northern hybridization.30 Single-stranded [35S] uridinetriphosphate (UTP)-labeled riboprobes were prepared by linearization of the plasmid downstream of the inserted cDNA fragment and subsequent in vitro transcription using the respective phage polymerases.31
Isolation of RNA and Northern hybridization. Isolation of total cellular RNA was performed according to the method of Chirgwin et al.32 Poly[A]+ RNA was purified from total cellular RNA using oligo-[dT]–cellulose.31 A total of 5 μg poly[A]+ RNA or 20 μg total cellular RNA was separated in 1% denaturing formaldehyde agarose gels. After gel electrophoresis, the RNA was transferred by capillary transfer onto Hybond N or Hybond N+ membranes (Amersham, Buckinghamshire, UK) using 20× standard saline citrate (SSC) and immobilized by 5-minute shortwave-ultraviolet (UV) treatment.31 The filters were prehybridized for greater than 4 hours followed by overnight hybridization with 2 × 107 cpm of the respective [32P] dCTP-labeled cDNA fragment in 20 mL hybridization buffer (50% formamide, 12.5% dextrane sulfate, 5× SSC, 1× Denhardt's solution, 50 mmol/L NaH2PO4 , and 0.25 mg/mL salmon sperm DNA). After the hybridization reaction, the filters were washed under stringent conditions (1× SSC at 65°C) and exposed to Kodak X-OMAT-AR films (Eastman Kodak, Rochester, NY) at −70°C using intensifying screens. Densitometric analyses of Northern hybridizations were performed with a Wincam 2.1 gel imaging system (Cybertech, Berlin, Germany).
Combined in situ hybridization/immunocytology. Cytospins containing 105 PBMNCs/slide were prepared. In situ hybridization reactions were performed according to a previously described protocol with [35S]-labeled cRNA probes, prepared by in vitro transcription from a human c-met cDNA.33 Complete immunocytology was then performed by the avidin-biotin-complex (ABC) method with rabbit polyclonal antihuman CD3 or mouse monoclonal antihuman CD68 antibodies (Dako), respective secondary polyclonal antibodies, horseradish peroxidase coupled streptavidin, and the chromogen diaminobenzidine. After completion of immunocytology, the slides were dehydrated, coated with photoemulsion, and exposed at 4°C for 2 weeks. Autoradiography was developed in Kodak D-19 and fixed in Kodak rapid fixer (Eastman Kodak). The cytospins were counterstained with Meyer's hemalum and analyzed by light microscopy. The analysis was performed by two investigators independently, and photomicrographs were taken with a Leica Diaplan microscope (Leica, Bensheim, Germany). The results of combined immunocytology/in situ hybridization experiments were analyzed semiquantitatively by evaluating 100 cells in three different areas of each cytospin. Because the procedure resulted in a moderate diffuse grain background over the whole cytospin, only the cells containing significant accumulations of grains were considered to be positive (see Fig 3C).
c-met Expression in activated CD68+ monocytes obtained from normal PBMNCs. Combined in situ hybridization/immunocytology was performed with a c-met [35S] RNA-probe together with T-cell and monocyte-specific antibodies. Cell surface staining was performed with a rabbit polyclonal antihuman CD3 antibody (A) and a mouse monoclonal antihuman CD68 antibody (B and C). Note the specific hybridization signal (accumulation of black grains) in the CD3-negative cell population (A) and the CD68-positive cell population (B, large arrows: markedly positive cells; arrowhead: slightly positive cell; small arrows: negative cells). (C) Shows negative control hybridization with a c-met sense probe in combination with immunocytology for CD68.
c-met Expression in activated CD68+ monocytes obtained from normal PBMNCs. Combined in situ hybridization/immunocytology was performed with a c-met [35S] RNA-probe together with T-cell and monocyte-specific antibodies. Cell surface staining was performed with a rabbit polyclonal antihuman CD3 antibody (A) and a mouse monoclonal antihuman CD68 antibody (B and C). Note the specific hybridization signal (accumulation of black grains) in the CD3-negative cell population (A) and the CD68-positive cell population (B, large arrows: markedly positive cells; arrowhead: slightly positive cell; small arrows: negative cells). (C) Shows negative control hybridization with a c-met sense probe in combination with immunocytology for CD68.
Monocyte viability assay. Highly monocyte enriched cell fractions were isolated as described above and seeded onto nonadherent teflon jars (Savillex Corp, Minnetonka, MI) at a density of 2 to 3 × 106 cells/mL in RPMI 1640 medium (Biochrom) supplemented with 2.5 % FCS and prestimulated with PHA and LPS for 17 hours at 37°C. Afterwards, the cells were washed twice and seeded onto 96-well flat bottom plates, each well containing 200 μL RPMI 1640 supplemented with 1% FCS at 37°C. Stimulations with recombinant HGF-SF (8 to 100 ng/mL) were performed for 72 hours. The cell numbers were measured by colorimetric assay (EZ4U nonradioactive proliferation assay, Biomedica, Vienna, Austria) according to manufacturer's instructions.34 Each experiment was performed with separate monocyte preparations from at least three different healthy donors and measured in triplicate.
RESULTS
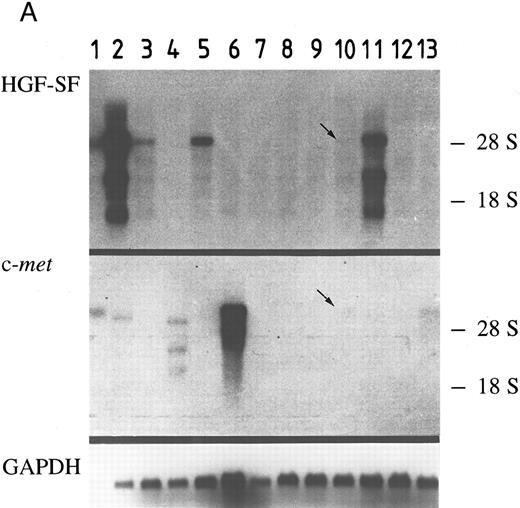
HGF-SF and c-met expression in myeloid and B-lymphoid cell lines does not correlate with differentiation. A panel of 12 myeloid and lymphoid cell lines (six myeloid, four B-lymphoid, and two T-lymphoid lines) was analyzed to determine whether HGF-SF and c-met are expressed in the lymphocytic and myeloic cell compartment and whether expression of both genes is limited to certain hematopoietic lineages or stages of maturation. The results (Fig 1 and Table 1) showed HGF-SF and c-met expression in some of the myeloid and B-lymphoid cell lines, whereas the T-cell lines expressed neither HGF-SF nor c-met transcripts. In separate experiments performed under nonstimulating conditions, the B-cell line, Raji, was positive for c-met transcripts, while the T-cell line, Jurkat, was negative for both HGF-SF and c-met transcripts (data not shown). All myeloid cell lines expressed either HGF-SF (KG-1, HL-60, HEL, MM6) or c-met (K562, U937), although expression of one gene appeared to exclude expression of the other. Furthermore, a tendency towards higher expression of HGF-SF was noted in less differentiated (ie, immature) myeloid cell lines, and expression of HGF-SF was thus highest in the myeloblastic cell line, KG-1. In B-lymphoid cell lines, low level expression of HGF-SF and c-met transcripts coexisted in cell lines, REH and OPM-2, while the cell line, JVM-2, with high HGF-SF expression, was c-met negative and neither HGF-SF nor c-met transcripts were detected in cell line, MN-60. Transcripts of approximately 6 kb, 3 kb, and 1.5 kb were present in all HGF-SF positive cell lines, with the full-size 6 kb transcript being the predominant and the 1.5 kb transcript the least abundant species. c-met transcripts of approximately 7 kb were found in cell lines, U937 and REH, while cell line U937 also expressed a second transcript of 5 kb. In contrast, the myeloblastic cell line, K562, showed c-met transcripts of 5.5 kb and subgenic species of 3.5 kb and 2.5 kb at similar quantities.
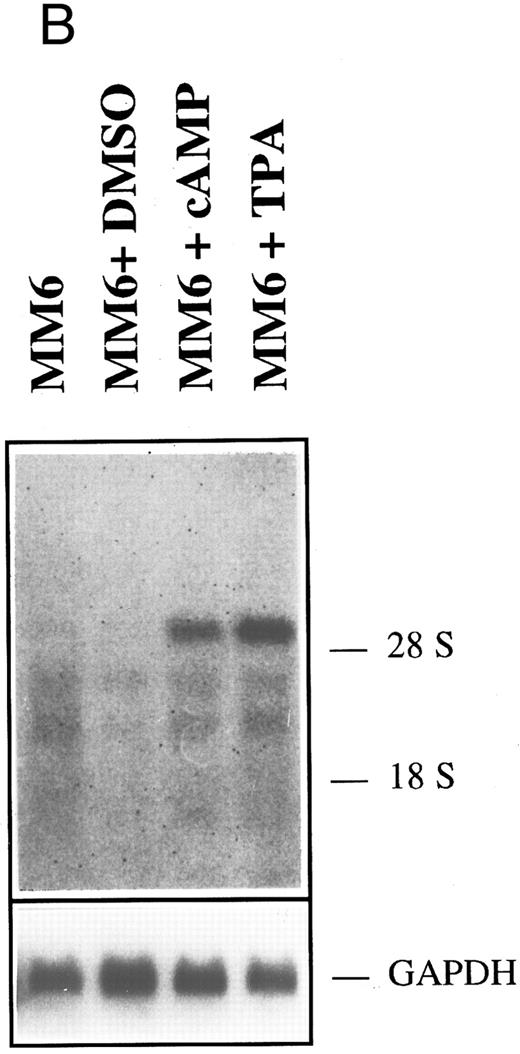
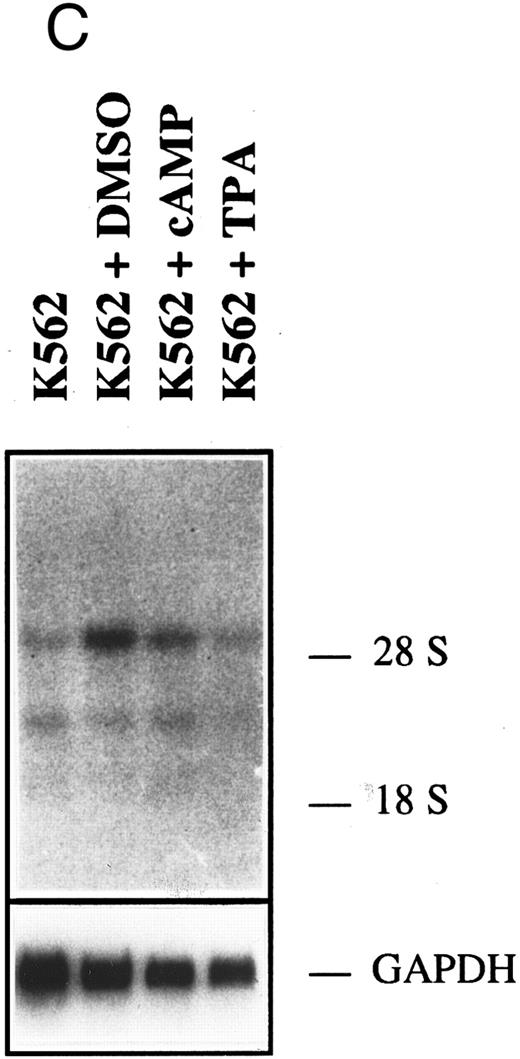
HGF-SF and c-met expression in myeloid and lymphoid cell lines. (A) Northern hybridization for HGF-SF (above) and c-met transcripts (middle) in human myeloid and lymphoid cell lines: embryonic lung fibroblasts (control) (1), KG-1 (2), HL-60 (3), K562 (4), HEL (5), U937 (6), M(ono)M(ac)6 (7), Molt-3 (8), SKW-3 (9), REH (10), JVM-2 (11), MN-60 (12), and OPM-2 (13). Faint bands in lane 2 (KG-1) of c-met hybridization represent a remnant signal from hybridization for HGF-SF. In lanes 10 and 13 (HGF-SF ) and 10 (c-met ) faint signals (arrows) were detectable only after extended exposure times (see also Table 2). Rehybridization for GAPDH is shown below. (B) HGF-SF expression in cell line M(ono)M(ac)6 without (MM6) and after stimulation with dimethylsulfoxide (MM6 + DMSO), cyclic AMP (MM6 + cAMP), and phorbol ester (MM6 + TPA). (C) c-met expression in cell line K562 without (K562) and after stimulation with DMSO (K562 + DMSO), dbcAMP (K562 + cAMP) and TPA (K562 + TPA). Rehybridizations for GAPDH in (B) and (C) are shown below.
HGF-SF and c-met expression in myeloid and lymphoid cell lines. (A) Northern hybridization for HGF-SF (above) and c-met transcripts (middle) in human myeloid and lymphoid cell lines: embryonic lung fibroblasts (control) (1), KG-1 (2), HL-60 (3), K562 (4), HEL (5), U937 (6), M(ono)M(ac)6 (7), Molt-3 (8), SKW-3 (9), REH (10), JVM-2 (11), MN-60 (12), and OPM-2 (13). Faint bands in lane 2 (KG-1) of c-met hybridization represent a remnant signal from hybridization for HGF-SF. In lanes 10 and 13 (HGF-SF ) and 10 (c-met ) faint signals (arrows) were detectable only after extended exposure times (see also Table 2). Rehybridization for GAPDH is shown below. (B) HGF-SF expression in cell line M(ono)M(ac)6 without (MM6) and after stimulation with dimethylsulfoxide (MM6 + DMSO), cyclic AMP (MM6 + cAMP), and phorbol ester (MM6 + TPA). (C) c-met expression in cell line K562 without (K562) and after stimulation with DMSO (K562 + DMSO), dbcAMP (K562 + cAMP) and TPA (K562 + TPA). Rehybridizations for GAPDH in (B) and (C) are shown below.
Following stimulation with agents known to induce either granulocytic (DMSO, dbcAMP)35,36 or monocytic (TPA)37-39 differentiation of myeloid cells, none of the initially negative cell lines showed neoexpression of HGF-SF or c-met. The observed changes in expression did not correlate with the known differentiation inducing potential of the employed substances (Table 1, Fig 1B and C). DMSO reduced HGF-SF expression in all HGF-SF positive cell lines, while TPA increased steady state levels of HGF-SF, with the exception of cell line, HEL, where the effect could not be conclusively demonstrated. dbcAMP incubation did not significantly alter HGF-SF transcript levels and an increase in expression was detected only in the low HGF-SF expressing cell lines, MM6 and OMP-2. DMSO incubation led to increased transcript levels of c-met in myeloid cell lines, in contrast to a slight or absent stimulatory effect of dbcAMP and TPA (TPA is stimulatory in cell line U937).
The presence of HGF-SF and c-met transcripts in some myeloid and lymphoid cell lines raised the possibility that both genes may be expressed in normal cells from peripheral blood. Therefore, we analyzed freshly isolated PBMNCs from healthy individuals. While low level HGF-SF expression was observed immediately after isolation, HGF-SF expression was absent after 5 days of primary culture. In PBMNCs from healthy individuals, no expression of c-met was found, either in freshly isolated cells or in cells cultured for 5 days. In contrast, a marked induction of c-met expression was achieved when PBMNCs were incubated with the T-cell stimulus PHA. This effect was further augmented when PHA was added together with the B-cell stimulus PWM and the monocyte activator LPS, while PWM alone did not exert a significant stimulatory effect (Fig 2B).
HGF-SF and c-met expression in primary cell cultures derived from peripheral blood cells. Northern hybridization analysis of total RNA (20 μg) from PBMNCs isolated from peripheral blood of healthy individuals for HGF-SF (A) and c-met (B) (lanes: respective positive controls (K562 or JVM-2), primary isolated PBMNCs from two healthy individuals (PBMNC [1] and [2]), primary culture of PBMNCs after 5 days (PBMNC [5D]), PBMNCs stimulated with PHA (PHA), PWM (PWM), or PHA, PWM, and LPS (PHA + PWM + LPS). Rehybridization for GAPDH is shown below. (C) Kinetic analysis of c-met expression under different conditions of stimulation. (), 1 and 2 hours; (▪), 6 hours; (□), 20 hours. Data represent evaluation of two independent Northern hybridizations with highest expression arbitrarily set to 1.
HGF-SF and c-met expression in primary cell cultures derived from peripheral blood cells. Northern hybridization analysis of total RNA (20 μg) from PBMNCs isolated from peripheral blood of healthy individuals for HGF-SF (A) and c-met (B) (lanes: respective positive controls (K562 or JVM-2), primary isolated PBMNCs from two healthy individuals (PBMNC [1] and [2]), primary culture of PBMNCs after 5 days (PBMNC [5D]), PBMNCs stimulated with PHA (PHA), PWM (PWM), or PHA, PWM, and LPS (PHA + PWM + LPS). Rehybridization for GAPDH is shown below. (C) Kinetic analysis of c-met expression under different conditions of stimulation. (), 1 and 2 hours; (▪), 6 hours; (□), 20 hours. Data represent evaluation of two independent Northern hybridizations with highest expression arbitrarily set to 1.
Kinetic analyses by Northern hybridization (Fig 2C) confirmed a significant induction of c-met expression by PHA and LPS compared with negative controls. A difference in the kinetics of stimulation was observed for PHA and LPS. While maximal c-met expression after PHA stimulation was noted after 6 hours with lower expression after 20 hours, c-met activation after LPS stimulation was moderate after 6 hours and markedly increased after 20 hours. c-met expression levels were even higher following PHA/LPS costimulation. Further stimulatory experiments were performed using costimulation with PHA and LPS over a period of 17 hours.
Combined immunocytology (with T-cell marker, CD3 or monocyte marker, CD68) together with in situ hybridization for c-met was performed on cytospins of PHA- and LPS-activated PBMNCs to determine the c-met neoexpressing cell population (Fig 3). Quantitation showed that approximately 98% of the cells exhibiting specific accumulations of the c-met hybridization signal are CD68-positive by immunocytology (Table 2, Fig 3B). In contrast, CD3-positive cells did not show significant specific c-met hybridization (Fig 3A). The intensity of c-met expression in CD68-positive cells varied considerably, ranging from high to low levels, as well as absence of expression. Eighteen percent of the CD68-positive cells were positive for c-met after PHA and 27% after PHA/LPS stimulation for 17 hours. Control hybridizations with a c-met sense probe showed a moderate background, but no signal accumulation, especially not above CD68-positive cells (Fig 3C). These experiments show that the vast majority of c-met expressing PBMNCs are CD68-positive and, therefore, represent monocytes.
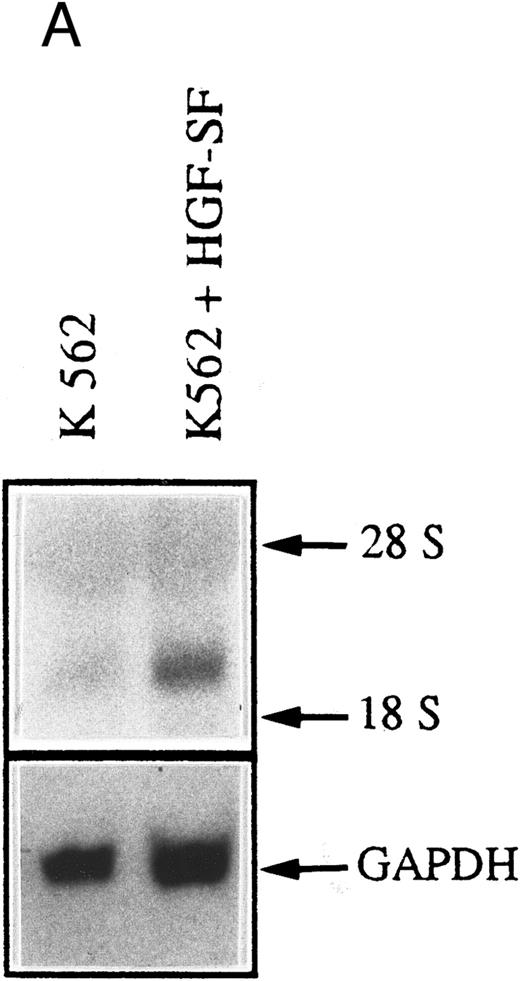
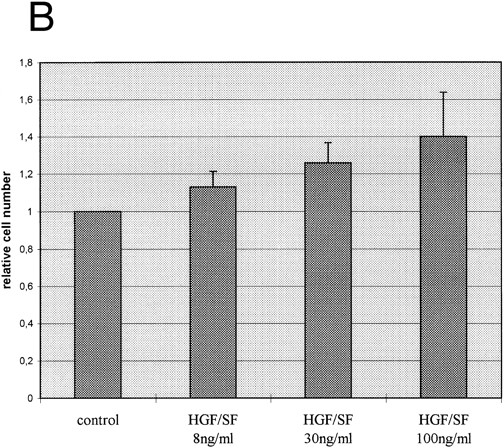
c-met Expressing myeloic cells respond to stimulation with HGF-SF. We also wanted to determine whether c-met-positive cells functionally respond to stimulation with HGF-SF. A marked increase in c-fos steady state transcript level, a known marker of early gene response after HGF-SF stimulation,5 40 was found in the constitutively c-met expressing myeloblastic cell line, K562, immediately after the addition of 30 ng/mL HGF-SF (Fig 4A). When purified monocytic cells isolated from healthy donors were costimulated with LPS/PHA for 17 hours and subsequently incubated with recombinant HGF-SF, a moderate increase in the cell number was observed compared with LPS/PHA-stimulated monocyte fractions not treated with exogenous HGF-SF (Fig 4B).
HGF-SF stimulation induces a functional response in c-met–expressing cells. (A) Northern hybridization analysis for c-fos expression in the myeloblastic cell line K562 after stimulation with 30 ng/mL recombinant HGF-SF. Hybridization for GAPDH is shown below. (B) Incubation of freshly isolated LPS/PHA prestimulated monocytes with recombinant HGF-SF. Bars represent mean of three independent experiments each measured in triplicate, with standard deviations and controls for each experiment indicated (PHA/LPS-stimulated monocytes adjusted to a relative value of 1). All values were determined by colorimetric assay (Biomedica; see Materials and Methods).
HGF-SF stimulation induces a functional response in c-met–expressing cells. (A) Northern hybridization analysis for c-fos expression in the myeloblastic cell line K562 after stimulation with 30 ng/mL recombinant HGF-SF. Hybridization for GAPDH is shown below. (B) Incubation of freshly isolated LPS/PHA prestimulated monocytes with recombinant HGF-SF. Bars represent mean of three independent experiments each measured in triplicate, with standard deviations and controls for each experiment indicated (PHA/LPS-stimulated monocytes adjusted to a relative value of 1). All values were determined by colorimetric assay (Biomedica; see Materials and Methods).
DISCUSSION
We analyzed the expression and significance of a potent pleiotropic growth factor, HGF-SF and its receptor c-met, in a representative panel of myeloid and lymphoid cell lines. HGF-SF or c-met expression is predominately detected in myeloid cell lines, while, with the exception of HGF-SF expressing B-cell line, JVM-2, expression in B- or T-lymphoid cell lines is absent or very low. This is supported by findings of other groups reporting HGF-SF expression in other myeloid cell lines, such as HL-6019 and THP-1.41 In contrast, only a single T-cell line has thus far been shown to express significant amounts of HGF-SF.42 Although a tendency towards higher expression of HGF-SF in less differentiated myeloid cell lines (ie, KG-1, HEL) was noted, a correlation between HGF-SF/c-met expression and differentiation or stages of maturation could not be established for the analyzed cell lines, despite a preference for myeloid differentiation and earlier stages of maturation. This absence of correlation was further supported by stimulation experiments using TPA, DMSO, and dbcAMP. Although these substances either activate (TPA and dbcAMP in some lines) or suppress (DMSO) HGF-SF expression, the resulting effects do not correlate with the differentiation-inducing activities of these substances on myeloid cells. In particular, DMSO and dbcAMP, both known to induce granulocytic differentiation of myeloid cells,39 43 did not correlate in their effect on HGF-SF expression. The observed stimulations thus represent specific effects of the substances used and are not coupled to differentiation induction in the myeloid cell lines.
It is important to note that all of the analyzed myeloid cell lines expressed either HGF-SF or c-met, and expression of one gene excluded expression of the other. There is no indication in myeloid cell lines of autoactivation of the HGF-SF/MET signaling pathway. Coexpression of HGF-SF and c-met was found only in two B-cell lines, OPM-2 and REH. However, the expression levels of both genes were very low in these lines.
Furthermore, coexpression of HGF-SF and c-met was not observed in primary cultures of normal PBMNCs. HGF-SF was present only in freshly isolated PBMNCs, while c-met was absent in freshly isolated cells, but neoexpressed after stimulation with PHA and/or LPS. Despite the frequency of c-met or HGF-SF expression, mechanisms inherent in cells of myeloid differentiation appear to prevent coexpression of both genes. Para- or endocrine-supplied HGF-SF is, therefore, required to stimulate c-met signaling in activated myeloic cells.
HGF-SF/MET signaling has been identified as one of the major regulators of epithelial organ regeneration, particularly in the liver and kidney. The HGF-SF/MET signaling system may play an additional role during necroinflammation. Stimulation of freshly isolated PBMNCs, eg, by PHA and/or LPS, activates c-met expression that is absent in freshly isolated PBMNCs from healthy donors. Further expression analyses show that c-met is activated in prestimulated monocytes, and in situ expression analysis confirmed that the vast majority of c-met positive cells is part of the monocytic compartment. We were unable to detect a significant c-met signal in CD68-negative cells. Neoexpression of c-met has been described for B lymphocytes after incubation with specific stimulatory substances.44 However, B lymphocytes do not represent a significant cell fraction among the analyzed preparations.
It can be hypothesized that activation of monocytes, eg, as a result of inflammation, may render these cells susceptible to HGF-SF signaling. This is supported by the fact that a functional response (c-fos activation) was activated by HGF-SF in the c-met expressing myeloblastic cell line K562. Furthermore, freshly isolated monocytes activated by PHA and LPS responded to exogenously added HGF-SF with a moderately elevated cell number compared with control populations not stimulated with HGF-SF. Interestingly, c-met expression is activated in the monocytoid cell line THP-1 by TPA, LPS, and cytokines like tumor necrosis factor-α (TNF-α), IL-6, and interferon-γ (IFN-γ) and mediates cell attachment and process formation.45 Furthermore, HGF-SF is known to induce motility of the monocytoid cell line, J-111.46 It is thus tempting to speculate as to whether HGF-SF may activate monocytes during inflammation and modulate specific monocytic/macrophage functions at the site of the inflammatory reaction. This hypothesis is supported by the fact that the addition of exogenous HGF-SF promotes viability of activated monocytes. In our experiments using PBMNCs, c-met expression in monocytes was not only stimulated by conventional monocyte activators, such as LPS, but also by PHA, which is known to activate T lymphocytes. It may be possible that not only direct stimulation of monocytes, but also activation of T-cell dependent cytokines may induce monocytic c-met expression.
Moderate stimulatory effects of HGF-SF have also been described for other peripheral blood cells. HGF-SF preparations prime a neutrophil oxidative response24 and moderately stimulate T-cell adhesion and migration,25 as well as murine B-cell–derived humoral response.23 Although these results suggest a broader function of HGF-SF in the stimulation of inflammatory cells, c-met expression has not been demonstrated in the responsive cell populations, in particular HGF-SF responsive T-cell fractions are negative for c-met. It has, therefore, been speculated that these effects may represent either cross-reactivity with different tyrosine kinase receptors, such as c-sea,25 or interaction with other HGF-SF receptors of lower affinity.47
HGF-SF has been proposed as a priming factor in early hematopoiesis, as it stimulates erythroid burst-forming units in the presence of erythropoietin, and CFU in synergy with stem cell factor, IL-3, and GM-CSF.20 22 This hypothesis can be extended with a view to the fact that a peripheral blood cell fraction (monocytes) expresses c-met following prestimulation and is thus primed to respond to HGF-SF. Future studies will have to further characterize the spectrum of specific functional effects of HGF-SF on activated c-met-positive monocytes and their significance in the inflammatory response.
ACKNOWLEDGMENT
This work represents part of the PhD thesis of M.B. from the Faculty of Biology at the University of Mainz. We thank P. Pulkowski and T. Böhm for photography and S. Hunger for typing the manuscript.
Supported by Grants No. Schi 273/3-2 (to P.S.) and SFB 311, project A5 (to H.P.D.) of the “Deutsche Forschungsgemeinschaft” and the “Stiftung Rheinland-Pfalz für Innovation” (to P.S. and H.P.D.). This research was sponsored in part by the National Cancer Institute, DHHS, under contract with ABL (G.F.V.W.).
Address reprint requests to Peter Schirmacher, MD, Institute of Pathology, University Hospital, Langenbeckstr. 1, D-55101 Mainz, Germany.

![Fig. 3. c-met Expression in activated CD68+ monocytes obtained from normal PBMNCs. Combined in situ hybridization/immunocytology was performed with a c-met [35S] RNA-probe together with T-cell and monocyte-specific antibodies. Cell surface staining was performed with a rabbit polyclonal antihuman CD3 antibody (A) and a mouse monoclonal antihuman CD68 antibody (B and C). Note the specific hybridization signal (accumulation of black grains) in the CD3-negative cell population (A) and the CD68-positive cell population (B, large arrows: markedly positive cells; arrowhead: slightly positive cell; small arrows: negative cells). (C) Shows negative control hybridization with a c-met sense probe in combination with immunocytology for CD68.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4450/3/m_bl_0026f3.jpeg?Expires=1766555903&Signature=OmD976460B1IgzDOSJiiP~aHPcZkC5q1GRgq1AfsvMYLEAKsfaha0SHuNTXTD6eMAIm-H-hcrxf4TlvVIt8gVFXFeQNPtx~TdXWcIqy-Yq6BXaYZnl-~CxFXAP0uYnSMAQwoWvmzn~xTgOq0YyuKUQgbKeYA-F3WOkk~4a~sTubvBNwc2gob2B603MEa35b9KZgoSKvENWaap0Xn~HClARYT8PSc39jgmC4IjIzT1yC8xRgAzPw5HmXG7Ux74bztlRsEzQx2IDIWLhH4~-3BusRZptfsFDc8200keUX9vlPiw21HxSk4Qu8zOa0DgMA9JtdpeH~B40o97Tnpk8CWkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. HGF-SF and c-met expression in primary cell cultures derived from peripheral blood cells. Northern hybridization analysis of total RNA (20 μg) from PBMNCs isolated from peripheral blood of healthy individuals for HGF-SF (A) and c-met (B) (lanes: respective positive controls (K562 or JVM-2), primary isolated PBMNCs from two healthy individuals (PBMNC [1] and [2]), primary culture of PBMNCs after 5 days (PBMNC [5D]), PBMNCs stimulated with PHA (PHA), PWM (PWM), or PHA, PWM, and LPS (PHA + PWM + LPS). Rehybridization for GAPDH is shown below. (C) Kinetic analysis of c-met expression under different conditions of stimulation. (), 1 and 2 hours; (▪), 6 hours; (□), 20 hours. Data represent evaluation of two independent Northern hybridizations with highest expression arbitrarily set to 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4450/3/m_bl_0026f2a.jpeg?Expires=1766555903&Signature=AAXQ-YhwXA2OMPh1hea~ty-IGsI2bYbfcLqzHhzomqaJ0CTrh7-NP6iSPATOM5wJ4CwSQasLEkEDKKxGL17FWfacGy65mDPzlBNijz51HRiWu5U7WwDkHUdS~1VeqerQkN-~rlsfvIm~qZimjWfprnQ58sDsQ86vQg-kQRUKlwsn3u-hKPgVOkl-MgSSVKqOO1ZwHpiN0Khk4RqFN-BmnGKDRzFxQibG6fGbG7f71IV5s-ZwxtRZJlaKFl6JJNlYgKpmS1DbxjuWbsnyyCfNgnxjFFJokNmOu3Tg6NpkxkmmBexlMrw7fhCs7Qt5KzeqHueBeEEPB9B5V2621s8eKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. HGF-SF and c-met expression in primary cell cultures derived from peripheral blood cells. Northern hybridization analysis of total RNA (20 μg) from PBMNCs isolated from peripheral blood of healthy individuals for HGF-SF (A) and c-met (B) (lanes: respective positive controls (K562 or JVM-2), primary isolated PBMNCs from two healthy individuals (PBMNC [1] and [2]), primary culture of PBMNCs after 5 days (PBMNC [5D]), PBMNCs stimulated with PHA (PHA), PWM (PWM), or PHA, PWM, and LPS (PHA + PWM + LPS). Rehybridization for GAPDH is shown below. (C) Kinetic analysis of c-met expression under different conditions of stimulation. (), 1 and 2 hours; (▪), 6 hours; (□), 20 hours. Data represent evaluation of two independent Northern hybridizations with highest expression arbitrarily set to 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4450/3/m_bl_0026f2b.jpeg?Expires=1766555903&Signature=CrDQQGXV87b1NWopjg4dXcH8yRPngDLVlkdsomev4ne3l~AcA3XmLKmFAl04BrvFrtL4JmO4etEPrxcYNzJusv-KARo4otqYF7wiFMoR7qtGW2-ozrRyPVSgocWDZPL72~wyCHwni56y69q13uxCllbFLYN1PIXXbreVEqsd4syxqivVRm3Anhh2mSz~PF1IaC0v~58JmSTPfLmgorCzLJCmmGvpoL8YzrsE3AafJwFtvZupalPjINgM5vYzDS4p4Rak1r5yQ-2djZ3TrDn0iDmjP-hBn0ACbcL2c3UG-wmWA-6f1-mijH7tMkKvPnPmXYjwAH0SaIFjqWMzt~8GNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. HGF-SF and c-met expression in primary cell cultures derived from peripheral blood cells. Northern hybridization analysis of total RNA (20 μg) from PBMNCs isolated from peripheral blood of healthy individuals for HGF-SF (A) and c-met (B) (lanes: respective positive controls (K562 or JVM-2), primary isolated PBMNCs from two healthy individuals (PBMNC [1] and [2]), primary culture of PBMNCs after 5 days (PBMNC [5D]), PBMNCs stimulated with PHA (PHA), PWM (PWM), or PHA, PWM, and LPS (PHA + PWM + LPS). Rehybridization for GAPDH is shown below. (C) Kinetic analysis of c-met expression under different conditions of stimulation. (), 1 and 2 hours; (▪), 6 hours; (□), 20 hours. Data represent evaluation of two independent Northern hybridizations with highest expression arbitrarily set to 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4450/3/m_bl_0026f2c.jpeg?Expires=1766555903&Signature=xVwkqp07HTn0y8kb6UxumuFDG-YZGjESOPQ5xyT59pSrBbQauT2SVOct1GhjgKVOr5e6bcAsa~fRI31GmMmDdGVnZMnHEhC-X6x9kIS0q9SYx8u55UDUu-wa-SOLF9PjAIZ9pl48XjTcWoJpJBr5QWeJgSlE6-apuNNe8nWu8BuoeHi50Vth9xqDMRWxSbSqd9PUkggYsHF84zE7l1aiJkMyNXsJfYqoXh-LQsLHAiz0Eq7XQaGpjtlJU-ot6Wok0hOHvTPl2qgIGJKHfyXkKDYpRzWI58~D~QaWuvbLRzqIXD9rkrBC7t2DTsONnaAOCky1RG3ge7rtshJDH7OE5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)