Abstract
A 58-year-old man experienced episodes of fever, vomiting, and diarrhea over a 2-year period. The laboratory evaluation during these attacks consistently disclosed thrombocytopenia, leukopenia, and elevated liver enzymes. A liver biopsy performed at one of these attacks showed a typical picture of granulomatous hepatitis. In retrospect, all episodes seemed to be associated with the ingestion of quinine. Indeed, such a correlation was established by a challenge with quinine. By using flow cytometry, quinine-dependent IgG antibodies to platelets were detected in the patient serum. By a two-color flow cytometric assay, the patient serum was also found to hold quinine-dependent antibodies specific for neutrophils, T lymphocytes, and B lymphocytes. Moreover, serum absorbed with neutrophils in the presence of quinine continued to react with platelets, T lymphocytes, and B lymphocytes; serum that was absorbed with mononuclear cells continued to react with neutrophils and platelets. These experiments indicated that the antigen targets were different on platelets, neutrophils, and lymphocytes. Further, the patient serum in the presence of quinine immunoprecipitated surface-labeled platelet proteins with electrophoretic mobilities closely resembling those of glycoprotein (GP) Ib/IX and GPIIb/IIIa. By a modified monoclonal antibody–specific immobilization of platelet antigens assay, the patient serum in the presence of quinine reacted with platelet GPIb/IX and GPIIb/IIIa. Also, the patient serum in the presence of quinine immunoprecipitated an uncharacterized 15-kD double-band from surface-labeled granulocyte proteins. We conclude that our patient's thrombocytopenia, neutropenia, and lymphocytopenia were caused by quinine-dependent antibodies and that these antibodies recognized cell lineage-specific epitopes.
QUININE WAS FIRST implicated as a cause of purpura in the late 19th century,1 and has been regarded as one of the most common causative agents for drug-induced immune thrombocytopenia.2 The effect of quinine on other cells, beside platelets, might have been underestimated. Indeed, recent reports of quinine-induced pancytopenia,3,4 granulomatous hepatitis,5,6 disseminated intravascular coagulation,7 and hemolytic uremic syndrome (HUS)8,9 suggested the existence of drug-dependent immune-mediated effects on several cell lineages. Particularly, multiple quinine-dependent antibodies were found to target at platelets, red blood cells (RBCs), neutrophils, and T lymphocytes in patients with HUS and agranulocytosis.10 In the present study we report a patient having repeated episodes of thrombocytopenia, neutropenia, lymphocytopenia, and a typical picture of granulomatous hepatitis after the ingestion of quinine. Multiple quinine-dependent antibodies to platelets, neutrophils, T lymphocytes, and B lymphocytes were detected, and their corresponding targets on platelets and neutrophils were partially characterized.
MATERIALS AND METHODS
Case Report
A 58-year-old man suffered from episodes of fever, vomiting, and diarrhea over a 2-year period. These attacks had an acute onset in the late evenings, a spontaneous recovery within 24 hours, and a frequency of 1 to 2 times per month. The laboratory evaluation during these attacks consistently disclosed mild thrombocytopenia, leukopenia, and elevated liver enzymes with glutamic pyruvic transaminase (GPT) and glutamic oxaloacetic transaminase (GOT) being 4 to 6 times the normal values. A liver biopsy was performed at one of these attacks and displayed a typical picture of granulomatous hepatitis. In retrospect, all episodes seemed to be associated with the ingestion of quinine. In September 1994, the patient was, as part of the clinical work-up and after giving his informed consent, challenged with quinine, and a complete mirror of the previous episodes with fever, vomiting, and diarrhea was observed. Also, a profound diminution in platelet number and reduction of leukocyte count were seen within hours after the challenge. The laboratory evaluation during the quinine challenge is summarized in Table 1. The following serological studies were performed on plasma obtained 5 months after the quinine challenge and shipped to our laboratory.
Flow Cytometry
Detection of drug-dependent platelet antibodies. This technique closely resembles that of Visentin et al.11 Briefly, EDTA-anticoagulated and washed blood group O platelets (5 × 107 platelets in 100 μL) were sensitized with the patient serum in the presence of quinine (final concentration [f.c.] 300 μmol/L). Thereafter, the platelets were washed and labeled with fluorescein conjugated rabbit antihuman IgG (Dakopatts, Glostrup, Denmark) and analyzed on a flow cytometer (FACScan, Becton Dickinson, San Jose, CA). Control experiments, consisting of patient serum plus buffer and normal sera with and without quinine, were routinely performed in parallel. A total of 10,000 ungated events were collected in list mode, and a logarithmic amplifier was used for the fluorescence signal and the light scatter. The data were analyzed using Lysys II software (Becton Dickinson). The platelets were identified using forward and side scatter, and the median fluorescence intensity (MFI) of fluorescein isothiocyanate conjugated antibody binding was recorded.
Detection of drug-dependent lymphocyte-specific antibodies. Mononuclear cells were isolated from blood group O donors using a Ficoll-Hypaque gradient (Pharmacia AB, Uppsala, Sweden). The harvested cells were washed twice with 0.01 mol/L phosphate-buffered saline (PBS) and the cell count was adjusted. One hundred μL of the cell suspension (1 × 106 mononuclear cells) were mixed with 100 μL of patient sera in the presence of quinine (f.c. 300 μmol/L) and incubated for 45 minutes. Control experiments consisting of patient serum plus buffer and normal sera with and without quinine were routinely performed in parallel. Thereafter, the cells were washed twice and incubated for 30 minutes with fluorescein conjugated F(ab′)2 rabbit antihuman IgG (Dakopatts) to label lymphocyte-bound IgG. After an additional wash, T and B lymphocytes were labeled with the phycoerythrin-conjugated murine monoclonal antibodies (MoAbs), anti-CD3 and anti-CD19 (Dakopatts), respectively, for 15 minutes and finally diluted with 0.5 mL PBS containing 1% paraformaldehyde. Analysis was performed on the flow cytometer. The lymphocyte population was identified by the back gating method according to Loken et al,12 and a gate was constructed to include only CD19+ or CD3+ lymphocytes. The fluorescein fluorescence was determined within these gates and expressed as MFI.
Detection of drug - dependent neutrophil - specific antibodies. Heparin anticoagulated whole blood from blood group O donors was obtained, and neutrophils were isolated using a standard technique with Ficoll-Hypaque gradient separation followed by hydroxyethylstarch sedimentation.13 The isolated cells (>90% neutrophils) were then treated with 1% paraformaldehyde for 5 minutes to minimize the unspecific uptake of plasma proteins onto the cells, were washed twice, and were resuspended in 0.01 mol/L PBS. One hundred μL of the cell suspension (2 × 106 neutrophils) was incubated with 100 μL patient serum in the presence of quinine (f.c. 300 μmol/L) for 30 minutes. Control experiments consisting of patient serum plus buffer and normal sera with and without quinine were routinely performed in parallel. Thereafter, the cells were washed twice and incubated for 30 minutes with fluorescein-conjugated F(ab′)2 rabbit antihuman IgG (Dakopatts) to label neutrophil-bound IgG. After an additional wash the cells were labeled with the phycoerythrin-conjugated MoAbs, anti-CD13 or anti-CD33 (Becton Dickinson) for 15 minutes, and finally diluted with 0.5 mL 0.01 mol/L PBS. These MoAbs are routinely used in our laboratory to identify neutrophils. The cell-associated immunofluorescence was measured by flow cytometry. A threshold was set at the nadir between CD13+ and CD13− cells or between CD33+ and CD33− cells in the Fl1 vs Fl2 analysis. The fluorescein fluorescence was determined on distinctly CD13+ or CD33+ cells and expressed as MFI.
RBC Agglutination Assay
The standard indirect antiglobulin (Coombs) test was performed. Donor RBCs were sensitized with patient serum in the presence and absence of 300 μmol/L quinine, and bound Igs were detected using univalent and polyvalent antisera. The reactions were read visually for agglutination.
Modified Monoclonal Antibody–Specific Immobilization of Platelet Antigens (MAIPA)
The MAIPA assay has been described in detail elsewhere.14 Briefly, platelets from healthy blood donors (blood group O) were sensitized with patient or control sera in the presence or absence of quinine (f.c. 300 μmol/L) and washed and solubilized in Tris-buffered saline (TBS) containing 1% Triton X-100 (Pierce, Rockford, IL). Diluted sensitized platelet lysate was added in duplicate into the wells of a microtiter plate (Maxisorp; NUNC, Roskilde, Denmark) coated with the MoAbs SZ2 (Immunotech, Marseille, France), specific for glycoprotein (GP) Ib, or P2 (Immunotech), specific for GPIIb/IIIa. IgG bound to the captured GP was detected using alkaline phosphatase conjugated goat antihuman IgG (Fc-specific; Sigma Chemical Co, St Louis, MO). P-nitrophenyl-phosphate was used as substrate and the absorbance was recorded at 405 nm. All washing and incubation steps were performed in the presence or absence of 300 μmol/L quinine where appropriate. Sixty normal sera were analyzed as a control material for the modified MAIPA assay. Four of these normal sera were reanalyzed along with each plate to serve as an internal standard, and an absorbance above the mean absorbance +3 standard deviation (SD) recorded for the four normal sera was considered a positive reaction.
Electrophoresis (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) and Immunoblotting
One-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli,15 and the gel was subsequently transblotted according to the method of Towbin et al.16 Details of platelet immunoblotting have been described elsewhere.17 All washing and incubation steps were performed in the presence or absence of 300 μmol/L quinine where appropriate.
Immunoprecipitation of Surface-Labeled Target Proteins
Details of immunoprecipitation have been described elsewhere.17 Washed platelets, at a concentration of 1 × 109/mL, were incubated with 5 mmol/L N-hydroxysuccinimide-long chain biotin (NHS-LC-Biotin) in 0.01 mol/L PBS/EDTA for 30 minutes at ambient temperature. The platelets were then washed three times with PBS/EDTA and resuspended in the same buffer. One hundred μL biotinylated platelet suspension, at a concentration of 1 × 109/mL, were incubated with 100 μL of the patient serum in the presence of quinine for 60 minutes. Lymphocytes and neutrophils were biotinylated and fixed in 1% paraformaldehyde before sensitization. Control experiments consisting of patient serum plus buffer and normal sera with and without quinine were routinely performed in parallel. The cells were then washed four times with PBS/EDTA. The antibody-sensitized cells were solubilized in 100 μL 20 mmol/L TBS, containing 1% Triton X-100, Leupeptin 100 μg/mL, and 0.4 mmol/L phenylmethylsulfonyl fluoride. The IgG with target antigen was precipitated using Protein A beads (Protein A Sepharose CL-4B; Pharmacia LKB Biotechnology AB, Sollentuna, Sweden). All washing and incubation steps were performed in the presence or absence of 300 μmol/L quinine where appropriate. Bound biotin-labeled proteins were eluted by resuspending the final bead pellet in SDS-PAGE sample buffer (2% SDS with or without 5% 2-mercaptoethanol) and incubating the mixture at 100°C for 5 minutes. The mixture was then centrifuged, and the supernatants were electrophoresed on a 5% to 15% gradient SDS-PAGE gel using the discontinuous buffer system of Laemmli15 and both reducing and nonreducing conditions. Broad range biotinylated weight standards (Bio-Rad Laboratories, Richmond, VA) were included to allow localization of the major labeled proteins. After completion of the run, the gel was equilibrated in Towbin buffer,16 and then transferred to a nitrocellulose membrane (Bio-Rad Laboratories). The membrane was blocked with TBS, pH 7.5, containing 0.05% Tween-20 and 3% bovine serum albumin, and then incubated with avidin-biotin-alkaline phosphatase complex (Vectastain ABC-AP kit; Vector Laboratories Inc, Burlingame, CA). After additional washings in TBS-Tween, the bands were developed with the NBT/BCIP substrate.
Statistical Methods
Standard statistical methods were used for calculation of mean value and SD. The difference between mean values was evaluated with the Student's t-test for unpaired data and a P value <.05 was considered statistically significant.
RESULTS
Detection of Multiple Quinine-Dependent Antibodies
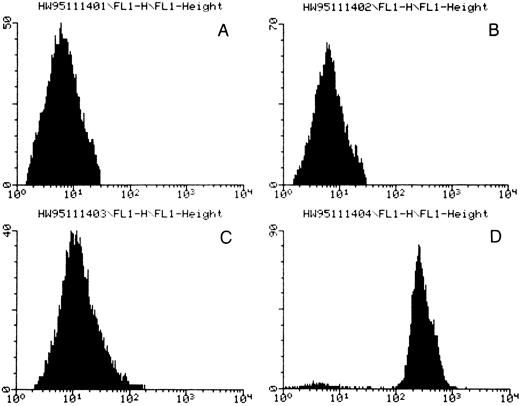
In flow cytometric studies, the patient serum plus quinine resulted in strong antibody binding to platelets (Fig 1 and Table 2). More interestingly, the patient serum in the presence of quinine also reacted with CD3+ T lymphocytes, CD19+ B lymphocytes, CD13+ neutrophils, and CD33+ neutrophils (Table 2). Similar reaction patterns were unequivocally observed on reacting the patient serum plus quinine with platelets, neutrophils, and lymphocytes from six different donors. Neither the patient serum alone nor normal human sera with or without quinine reacted with any of these cell types.
Flow cytometric analysis for quinine-dependent platelet antibodies. (A) normal serum, (B) normal serum plus quinine, (C) patient serum, (D) patient serum plus quinine. Fluorescence intensity is given by the horizontal axis and number of events by the vertical axis. A positive reaction was observed only in the presence of the patient's serum and quinine.
Flow cytometric analysis for quinine-dependent platelet antibodies. (A) normal serum, (B) normal serum plus quinine, (C) patient serum, (D) patient serum plus quinine. Fluorescence intensity is given by the horizontal axis and number of events by the vertical axis. A positive reaction was observed only in the presence of the patient's serum and quinine.
By using an RBC agglutination assay we were unable to detect any quinine-dependent RBC antibody in the patient serum (data not shown).
Absorption Studies
To determine the heterogeneity of these multiple drug dependent antibodies we absorbed the patient serum with neutrophils or mononuclear cells in the presence of quinine. The reactivity of the absorbed patient serum with platelets, neutrophils, T lymphocytes, and B lymphocytes was then reanalyzed using the flow cytometric assays. The results are summarized in Table 3. The patient serum that had been absorbed with neutrophils, in the presence of quinine, no longer reacted with neutrophils, but continued to react with platelets, T lymphocytes, and B lymphocytes. The patient serum that had been absorbed with mononuclear cells in the presence of quinine lost its reactivity with T lymphocytes and B lymphocytes while still reacting with platelets and neutrophils.
Biochemical Analysis
The patient serum was further tested for its reactivity with platelet GP complexes in the modified MAIPA assay. When quinine was added to the patient serum, IgG antibodies bound to both GPIb/IX and GPIIb/IIIa (Fig 2); no reactivity was discernible in the absence of the drug. Normal sera with and without quinine did not react with any of these GP complexes.
Analysis of quinine-dependent antibodies to platelet GPIb/IX and GPIIb/IIIa by a modified MAIPA assay. The results are the mean ± SD of six experiments and are given as absorbance at 405 nm.
Analysis of quinine-dependent antibodies to platelet GPIb/IX and GPIIb/IIIa by a modified MAIPA assay. The results are the mean ± SD of six experiments and are given as absorbance at 405 nm.
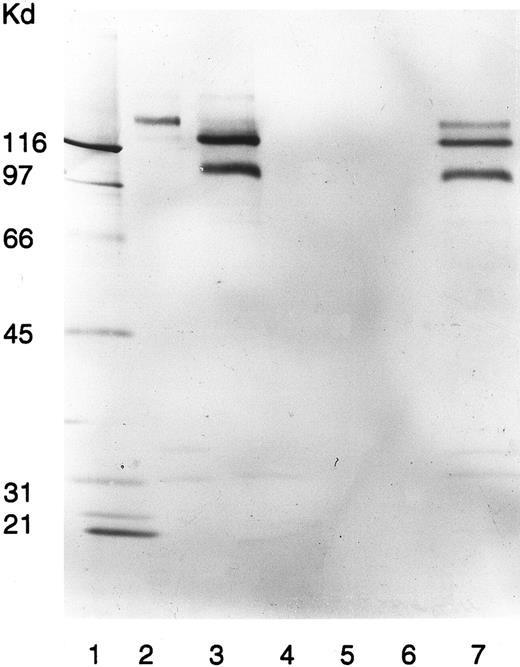
Neither in the presence nor in the absence of quinine did the patient serum immunoblot any platelet protein (data not shown). However, in the presence of quinine, the patient serum immunoprecipitated surface biotinylated platelet proteins with electrophoretic mobilities closely resembling those of GPIb, GPIIb, and GPIIIa (Fig 3). Neither the patient serum alone nor normal sera with or without quinine reacted with any of these platelet proteins.
Immunoprecipitation of platelet membranes by the patient's quinine-dependent antibodies. Donor platelets were surface labeled with NHS-LC-Biotin and immunoprecipitated with MoAb AN51, specific for GPIb (lane 2); MoAb P2, specific for GPIIb/IIIa (lane 3); normal serum (lane 4); normal serum plus quinine (lane 5); patient serum (lane 6); and patient serum plus quinine (lane 7). Immunoprecipitated GPs were analyzed by SDS-PAGE and visualized by ABC-AP Kits plus NBT/BCIP as substrate. Molecular weight standards were included in lane 1.
Immunoprecipitation of platelet membranes by the patient's quinine-dependent antibodies. Donor platelets were surface labeled with NHS-LC-Biotin and immunoprecipitated with MoAb AN51, specific for GPIb (lane 2); MoAb P2, specific for GPIIb/IIIa (lane 3); normal serum (lane 4); normal serum plus quinine (lane 5); patient serum (lane 6); and patient serum plus quinine (lane 7). Immunoprecipitated GPs were analyzed by SDS-PAGE and visualized by ABC-AP Kits plus NBT/BCIP as substrate. Molecular weight standards were included in lane 1.
To elucidate which neutrophil membrane components the quinine-dependent antibodies recognized, an immunoprecipitation assay was performed with surface-biotinylated neutrophils. In the presence of quinine, the patient serum precipitated a 15-kD double-band protein (Fig 4). The same protein was precipitated using neutrophils from six different donors. Neither the patient serum alone nor normal sera with or without quinine reacted with this neutrophil protein.
Immunoprecipitation of neutrophil membranes by the patient's quinine-dependent antibodies. Donor neutrophils were surface labeled with NHS-LC-Biotin, fixed in 1% paraformaldehyde, and immunoprecipitated with normal serum (lane 2), normal serum plus quinine (lane 3), patient serum (lane 4), and patient serum plus quinine (lane 5). Immunoprecipitated neutrophil membrane proteins were analyzed by SDS-PAGE and visualized by ABC-AP Kits plus NBT/BCIP substrate. Molecular weight standards were included in lane 1.
Immunoprecipitation of neutrophil membranes by the patient's quinine-dependent antibodies. Donor neutrophils were surface labeled with NHS-LC-Biotin, fixed in 1% paraformaldehyde, and immunoprecipitated with normal serum (lane 2), normal serum plus quinine (lane 3), patient serum (lane 4), and patient serum plus quinine (lane 5). Immunoprecipitated neutrophil membrane proteins were analyzed by SDS-PAGE and visualized by ABC-AP Kits plus NBT/BCIP substrate. Molecular weight standards were included in lane 1.
Using the patient serum with and without quinine, we could not immunoprecipitate any surface biotinylated lymphocyte protein.
DISCUSSION
In the present study, we reported a rare case of simultaneous occurrence of quinine-induced thrombocytopenia, neutropenia, lymphocytopenia, and granulomatous hepatitis. The notion that quinine was the causative agent was based on the following findings: (1) in retrospect all episodes seemed to be associated with ingestion of quinine, (2) a quinine challenge elicited the symptoms, and (3) serological studies disclosed quinine-dependent antibodies to platelets, neutrophils, T lymphocytes, and B lymphocytes. Our patient seemed unique in that even lymphocytopenia was elicited by the drug, and quinine-dependent antibodies binding to both T lymphocytes and B lymphocytes were disclosed by a two-color flow cytometric assay.
Hundreds of drugs that cause immune hemolysis and/or thrombocytopenia and others that predominantly affect leukocytes have been described.18 A few reports have suggested that even hematopoietic precursors can be involved in the drug-induced immune reaction.19 The available data on leukopenia are, in general, less informative than those of hemolysis and thrombocytopenia. This lack of information is presumably because of insufficient serological assays advocated for the detection of antileukocyte antibodies.20,21 We used a two-color flow cytometric technique in which the cells of interest were phenotypically identified by phycoerythrin-conjugated MoAbs, and bound drug-dependent antibodies (DDAbs) were determined on the selected cell population by the use of a fluorescein labeled antihuman IgG. Using this assay we were able to identify quinine-dependent antibodies specific for neutrophils (CD13+ and CD33+), B lymphocytes (CD19+), and T lymphocytes (CD3+). Quinine-dependent antibodies to platelets, RBCs, neutrophils, and T lymphocytes but not B lymphocytes have previously been described in three patients by Stroncek et al.10 Our method differs from the one used by these investigators. In the studies by Stroncek et al the lymphocytes were isolated according to their physiological properties and a lymphocytotoxicity assay was used for detection of antibodies against B lymphocytes. However, it seems reasonable to believe that a two-color flow cytometric technique is more suitable for detection of low titer DDAbs against lymphocytes. Indeed, flow cytometry is considered to be highly sensitive in the detection of DDAbs and allows their detection at pharmacological concentrations of the drug.11 22
Using flow cytometry we detected platelet-specific and quinine-dependent antibodies in our patient. By the immunoprecipitation assay and the MAIPA assay, we further observed that the patient's quinine-dependent platelet antibodies targeted at both GPIb/IX and GPIIb/IIIa, a finding in accordance with most previous reports.3,8-10 23-31
However, data concerning target characterization on other cell types are extremely few. Stroncek et al showed that the antigens recognized by quinine-dependent neutrophil antibodies were located on molecules of 80, 60, and 32 kD.32-34 Further, the 60-kD protein recognized by quinine-dependent antibodies was shown to carry the neutrophil-specific antigen NB1. However, our findings are at variance. The patient's serum in the presence of quinine immunoprecipitated an uncharacterized 15-kD double band from surface-labeled neutrophil proteins. This 15-kD neutrophil protein appeared to be different from all the previously described targets for quinine-dependent neutrophil antibodies.32-34 The nature of this protein awaits further characterization.
The inability to immunoprecipitate lymphocyte epitopes for DDAbs may be explained by low titer or low affinity of the lymphocyte antibodies in our patient's serum. Alternatively, the biotin labeling of the surface antigenic proteins on lymphocytes may not be sufficient and may even interfere with the antibody binding.
DDAbs seem to be highly tissue specific. In rare instances in which more than one cell type was affected, eg, RBCs and platelets or platelets and neutrophils, distinct Igs each reactive with a different cell type have been identified.10,23 There are two possible mechanisms for the simultaneous occurrence of quinine-induced thrombocytopenia, neutropenia, and lymphocytopenia: One possibility is that there is an antibody that cross-reacts with platelets, neutrophils, and lymphocytes. An alternative mechanism is that there are at least three different antibodies with separate cell specificity. The hypothesis that multiple quinine-dependent antibodies reacted with heterogeneous epitopes on platelets, neutrophils, and lymphocytes was recently proposed by Stroncek et al.10 Our findings provide additional evidence in favor of this concept. The patient serum absorbed with neutrophils in the presence of quinine continued to react with only platelets and lymphocytes. Conversely, the patient serum absorbed with mononuclear cells remained reactive with only neutrophils and platelets.
Regarding solid tissue damage, evidence for involvement of DDAbs in tissue destruction is largely indirect. Hepatotoxicity, granulomatous hepatitis, and disseminated intravascular coagulation have previously been reported as rare adverse reactions to quinidine and quinine.3-7,35 Also, most recently quinine-induced hemolytic uremic syndrome has been identified as a distinct clinical entity.8-10 The reason for the development of acute renal failure in some patients with quinine-induced antibodies is unclear. Stroncek et al suggested that binding of Igs to granulocytes renders them to adhere to microvasculature of the kidneys to produce renal dysfunction.10 Indeed, in a study by Gottschall et al,9 eight of nine patients with quinine-induced HUS had granulocyte antibodies. Neahring et al36 identified IgG and IgM antibodies binding to endothelial cells in patients with quinine-induced HUS and suggested that antibody-mediated damage to endothelium may be responsible. Our patient had no sign of RBC destruction or renal affection but displayed a clinical picture of granulomatous hepatitis; the pathophysiology behind this hepatotoxicity remains unknown.
ACKNOWLEDGMENT
The authors thank Ms Iréne Andersson for expert technical assistance.
Supported in part by grants from the Swedish Medical Research Council (B96-19X-11630-01A); the Swedish Cancer Society (0432-B93-13XAA), Roche AB, Sweden; and by a World Health Organization fellowship (0334/93) to M.H.
Address reprint requests to Hans Wadenvik, MD, PhD, Hematology Section, Department of Medicine, Sahlgrenska University Hospital, S-413 45 Göteborg, Sweden.