Abstract
To elucidate the capacity of murine early hematopoietic progenitor cells (HPCs) to differentiate into dendritic cells (DCs), lineage phenotypes (Lin)−c-kit+ HPCs were highly purified from either wild-type or tumor necrosis factor (TNF) receptor p55 (TNF-Rp55)-deficient mice. Upon culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) and stem cell factor (SCF) for 14 days, wild-type mouse Lin−c-kit+ HPCs did not exhibit characteristic features of DC such as sheet-like projections and veil processes. Moreover, these cells expressed a marginal level of DC markers such as DEC-205, CD86, and barely supported allogenic MLR. However, the addition of mouse TNFα generated a large number of cells with typical DC morphology, expression of high levels of Ia, DEC-205, CD86, and function of stimulating allogenic MLR. Moreover, a proportion of these mature DCs and thymic DCs expressed Thy-1 mRNA as well as Thy-1 antigen, whereas freshly isolated splenic DCs did not. These results suggested that DCs generated in our culture system phenotypically resemble thymic ones. In contrast, mouse TNFα failed to induce TNF-Rp55-deficient mice-derived Lin−c-kit+ HPCs to generate DCs with characteristic morphology, immunophenotype, and accessory function for T cells under the same culture conditions, suggesting a crucial role of TNF-Rp55 in TNFα-mediated DC differentiation from HPCs. Interestingly, human TNFα, which can bind to mouse TNF-Rp55 but not TNF-Rp75, was incapable to augment DC generation from wild-type mouse Lin−c-kit+ HPCs. Collectively, these results suggest that TNFα has a pivotal role in DC generation from murine early HPCs in collaboration with GM-CSF and SCF through the interaction of TNF-Rp55 and TNF-Rp75.
DENDRITIC CELLS (DCs) are characterized by an irregular shape with numerous projections in their surface and expression of high levels of major histocompatibility complex (MHC) class II antigens and other DC markers.1-6 These cells are specialized to present antigens in an MHC-restricted manner and initiate a primary immune response more efficiently than other types of antigen-presenting cells such as B cells and macrophages.1,2 DCs comprise heterogeneous populations and are classified as follows depending on their location: thymic DC in thymus; interdigitating DC (IDC) in lymph node, thymus, and spleen; veiled cells in afferent lymph; interstitial DC in heart, lung, kidney, and intestine; Langerhans' cells (LC) in skin; and blood DC in peripheral blood.1-8 The various tissue distributions of DC suggest the presence of different DC precursors in vivo with differential requirements for their differentiation into mature DC.1-3
The ontogeny of DCs has been widely investigated using various DC precursors. DCs can be generated in a large number from human peripheral blood mononuclear cells in responding to the stimulation of several combined cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and tumor necrosis factor α (TNFα).3,9-11 DCs, which can differentiate from the common precursors shared by granulocyte- and macrophage-lineage cells,12 were also generated in vitro from murine MHC class II-negative bone marrow hematopoietic cells in a suspension culture in response to GM-CSF.13 However, a relative high cell concentration was required for the induction of DCs in the system, suggesting that an additional factor(s) may be necessary for the generation of DCs. This notion is supported by the observation that, in collaboration with GM-CSF, human TNFα is essentially required for DC differentiation and maturation from human CD34+ hematopoietic progenitor cells.14-21 Moreover, TNFα can also induce the terminal differentiation of blood monocyte-derived DCs that are generated by the stimulation with GM-CSF and IL-4.10 11 These results implicate TNFα as an important regulator of human DC differentiation and maturation. However, until now, it remains to be established whether highly purified murine early hematopoietic progenitor cells (HPCs) could generate DC in vitro in response to TNFα.
Mice deficient in TNFα, lymphotoxin α (LTα), or TNF-receptor p55 (TNF-Rp55) gene lack germinal centers and exhibit dysregulated antibody generation.22,23 Moreover, these mice lack one type of DCs, follicular dendritic cells (FDCs) within germinal centers, suggesting that the TNFα-TNF-Rp55 system is involved in the development of FDC, thereby regulating antibody response.22,23 Although FDC is one type of DC that presents native antigens as immune complexes to B cells,2 24 we addressed whether the TNFα-TNF-Rp55 system might be involved in regulating the differentiation of other types of DC in vitro.
Accumulating evidence indicates that murine bone marrow Lin−c-kit+ cells represent uncommitted HPCs with the capability of reconstitution of lymphoid and myeloid hematopoiesis in vivo.25 26 However, it is not clarified whether this cell population can differentiate in vitro into DC. We provide evidence here that TNFα, in concert with GM-CSF and stem cell factor (SCF), has a crucial role in inducing highly purified murine Lin−c-kit+ HPCs to differentiate into functionally competent DCs and that the interaction of TNFα with both TNF-Rp55 and TNF-Rp75 is indispensable for this process.
MATERIALS AND METHODS
Cytokines and antibodies. Recombinant murine GM-CSF and murine SCF were generous gifts from Kirin Brewery Co (Tokyo, Japan). Human TNFα was a kind gift from Dainippon Pharmaceutical Co (Osaka, Japan). Mouse TNFα was produced as described previously.27 Endotoxin was not detected in these cytokine preparations using a Toxicolor assay kit (Seikagaku-Kogyo, Tokyo, Japan). These cytokines were used at the optimal concentrations determined in the preliminary experiments as follows: GM-CSF, 4 ng/mL; SCF, 20 ng/mL; mouse TNFα, 25 ng/mL; and human TNFα, 50 ng/mL. An anti-c-kit antibody (ACK-2) was kindly provided by Dr T. Sudo (Toray, Kamakura, Japan)28 and conjugated with biotin by using a NHS-Biotin kit (Pharmacia-Biotech, Uppsala, Sweden) according to the manufacturer's instructions. A rat monoclonal antibody (MoAb) to murine dendritic cell marker, DEC-205 (NLDC145),5 29 was a generous gift by Dr R.M. Steinman (Rockefeller University, New York, NY). Other MoAbs and reagents used for immunostaining were obtained from PharMingen (San Diego, CA), unless otherwise indicated.
Mice. TNF-Rp55-deficient mice (designated thereafter as TNF-Rp55−/− mice) were obtained from Dr H. Bluethmann (Hofmann-La Roche Ltd, Basel, Switzerland)30 and maintained by mating with C57BL/6 mice under pathogen-free conditions in the Animal Research Center of Kanazawa University (Kanazawa, Japan). F1 mice between C57BL/6 and 129 (Japan SLC, Hamamatsu, Japan) were used as wild-type mice (designated as TNF-Rp55+/+ ). All animal experiments complied with the standards set out in the Guideline for Care and Use of Laboratory Animals of Takara-machi Campus of Kanazawa University.
Suspension culture of Lin−c-kit+ HPCs. Bone marrow cells were obtained by aspirating femurs and tibiae of 8- to 10-week-old female TNF-Rp55+/+ or TNF-Rp55−/− mice. Lin−c-kit+ HPCs were isolated from nonadherent bone marrow mononuclear cells with the use of an EPICS ELITE cell sorter (Coulter Electronics, Hialeah, FL) as previously described, with some modifications.31,32 In brief, nonadherent cells were stained with an indirect staining composed of biotin-labeled anti-c-kit MoAb and phycoerythrin (PE)-conjugated streptavidin followed by a set of fluorescein isothiocyanate (FITC)-labeled MoAbs to CD3 (145-2C11), CD4 (H129.19), CD8 (53-6.7), B220 (RA3-6B2), Gr-1 (Ly-6G), CD11a (2D7), and CD11b (M1/70). The contamination of other types of cells in this preparation was consistently less than 0.5%, as shown by an immunofluorescence analysis. Purified Lin−c-kit+ HPCs were incubated at a cell concentration of 1 × 104 cells/mL in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum (FBS), 5 × 10−5 mol/L 2-mercaptoethanol, penicillin G (100 U/mL), and streptomycin (100 μg/mL) in the presence of GM-CSF, SCF, and TNFα until 7 days after the initiation of culture (Fig 1). The cells were thereafter cultured in the presence of TNFα and GM-CSF. Because this condition increased cell yields without changing the morphology and phenotype of the obtained cells,16 18 it was used in the present study unless otherwise stated. The cells were split at day 5 or 7 after the initiation of the culture and half of the medium was exchanged every 3 to 4 days. At the indicated time intervals, the cells were collected for immunofluorescence staining and allogenic MLR analysis. Morphologic analyses were performed directly on cultured cells with an inverted microscope (Nikon, Tokyo, Japan) and on cytocentrifuged cells that were stained with Giemsa solution.
Schematic representation of culture conditions of highly purified murine Lin−c-kit+ HPCs.
Schematic representation of culture conditions of highly purified murine Lin−c-kit+ HPCs.
Immunofluorescence analysis. Immunofluorescence analyses were performed as previously described.31 32 In two-color immunofluorescence analysis, 2 to 4 × 105 cells were incubated with the optimal concentrations of anti-CD86 (GL1) or anti-DEC-205 MoAb, followed by FITC-labeled goat antirat IgG(Fab′ )2 antibody (Caltag, Camarillo, CA). The cells were then stained with PE-conjugated mouse antimouse Ia MoAb (AF6-120.1). In three-color immunofluorescence analysis, the cells were sequentially incubated with an anti-DEC-205 MoAb and FITC-labeled goat antirat IgG(Fab′ )2 antibody, followed by the incubation with the optimal concentrations of PE-labeled anti-Ia and biotinylated anti-CD86 MoAbs. Then the cells were finally stained with CY-conjugated streptavidin. In another series of three-color immunofluorescence analyses, the cells were sequentially incubated with either a rat anti-DEC-205 or anti-CD86 MoAb and PE-labeled goat antirat IgG(Fab′ )2 antibody, followed by the incubation with biotinylated anti-Ia MoAb (25-9-17). The cells were then finally incubated sequentially with CY-conjugated streptavidin and FITC-labeled MoAbs to CD8, Thy-1.2 (53-2.1), CD11a, CD11b, and CD11c (HL3). All incubations were performed for 30 minutes on ice, followed by two washes, and instrument compensation was set in each experiment using single-color and/or two-color stained samples. In some experiments, the corresponding cell populations were isolated by using an EPICS ELITE cell sorter (Coulter Electronics, Hialeah, FL) according to the manufacturer's instructions.
Separation of DCs from thymus and spleen. DCs were separated from thymuses and spleens of wild-type mice according to the method previously described by Vremec et al.6 Briefly, single-cell suspensions were prepared by digesting thymus and spleen with 1 mg/mL of collagenase type IV (Sigma Chemical Co, St Louis, MO) at 37°C for 30 minutes in Hank's buffer. Low-density cells were separated from a single-cell preparation suspended in phosphate-buffered saline (PBS) containing 5% FBS and 10 mmol/L EDTA by a sequential centrifugation on Histopaque 1077 and 14.5% metrizamid medium (Sigma Chemical Co). For allogenic MLR, nonadherent cells were further depleted from the resultant cell preparations according to the method as previously described.33 The purity of DCs prepared by this method was usually greater than 60%, as shown by two-color immunostaining with anti-Ia and CD11c MoAbs. In some experiments, Ia+CD11c+ CD3−CD4−B220−Gr-1−NK1.1−TER-119− cells were enriched from low-density cells by a cell sorter after three-color immunofluorescence, because these phenotypes are presumed to represent mature DCs.1,2,6-8 34 A highly homogenous DC population with a purity of greater than 99.6% was consistently obtained by repeatedly sorting three times and was used for extraction of total RNA.
Reverse transcription-polymerase chain reaction (RT-PCR). Total RNAs were extracted from 1 × 105 thymocytes, splenocytes, highly purified thymic and splenic DCs, or DCs generated from Lin−c-kit+ HPC culture by using RNAzol B (Biotex Laboratories Inc, Houston, TX), according to the manufacturer's instructions. Ia+CD11c+ cells were enriched from Lin−c-kit+ HPC-derived DCs and, in some experiments, they were further sorted into Thy-1+ and Thy-1− populations. First-strand cDNA was synthesized in a 25-μL reaction volume using an RT-PCR kit (Takara Shuzo, Kyoto, Japan) with random primers. Thereafter, cDNA was amplified for 35 cycles consisting of 94°C for 30 seconds, 60°C for 1 minute, and 72°C for 1.5 minutes with the Thy-1-specific oligonucleotide primers (5′-AACCCAGCCATCAGCGTCGC-3′ and 5′-CAGAGAAATGAAGTCCAGGG-3′), which will give rise specifically to a 480 bp-cDNA encoding Thy-1 glycoprotein.35 As a control, mouse β-actin transcript was amplified in parallel as previous described.32 The PCR products were fractionated on a 1.5% agarose gel and visualized by ethidium bromide staining.
MLR. T cells were obtained from spleens of allogenic mice (BALB/c) by depleting nylon wool column-retained cells from spleen-derived mononuclear cells.13 33 The T-cell preparations were further incubated with a mouse anti-Ia MoAb and followed by goat antimouse IgG-conjugated magnetic beads (Dynal, Oslo, Norway) to remove residual antigen-presenting cells. After being exposed to 30 Gy of x-ray irradiation, the indicated numbers of the cells from Lin−c-kit+ cell cultures, freshly isolated splenic DCs, or peritoneal macrophages were added to 3 × 105 allogeneic T cells per well in 96-well, round-bottom culture plates. In some experiments, the cells from Lin−c-kit+ HPC cultures were further sorted based on their immunofluorescence intensities of Ia, CD86, and DEC-205 and used for MLR. After the incubation at 37°C for 5 days, cell proliferation was determined using 3-(4,5-dimethyl thiazolyl-2-)-2,5-diphenyltetrazolium bromide (MTT; Sigma Chemical Co). In brief, 15 μL of MTT (5 mg/mL in PBS) was added into each well and the plates were incubated at 37°C for an additional 4 hours. After removing the supernatant, 150 μL of isopropanol containing 0.04 N HCl was added to each well and mixed thoroughly. The resultant absorbance at 550 nm was read by a microplate immunoreader.
Statistical analysis. Differences were evaluated using the Student's t-test. P values of less than .05 were considered to be statistically significant.
RESULTS
Enhanced DC-like cell generation from TNF-Rp55+/+ mice-derived Lin−c-kit+ HPCs by mouse TNFα. HPCs were extinguished by 3 days after the start of the culture in the absence of any cytokines or in the presence of mouse TNFα alone (data not shown). GM-CSF increased the number of nonadherent cells from highly purified murine bone marrow Lin−c-kit+ HPCs in the cultures (1 × 104 at day 0, 51.1 ± 7.7 × 104 cells on day 7, and 26.0 ± 2.5 × 104 cells at day 14, n = 7). On a phase contrast microscopic observation, at day 5 of culture, GM-CSF induced cell cluster formation without sheet-like cells in its periphery (Fig 2A), and thereafter many round cells appeared in the cultures. Giemsa staining on these cells demonstrated that they were mostly macrophages but not DCs after 14 days of culture (Fig 2E and G). Addition of SCF during the first week of the culture remarkably increased the number of nonadherent cells (268 ± 8.0 × 104 cells, n = 8) without affecting the phenotype of the recovered cells (data not shown), consistent with the previous reports.16,18 The addition of mouse TNFα to the cultures increased the yields of nonadherent cells to 78.1 ± 15.1 × 104 (n = 3) and 42.7 ± 7.5 × 104 cells at day 14 (n = 8) in the presence and the absence of SCF, respectively. After culturing Lin−c-kit+ HPCs for 5 days in the presence of mouse TNFα combined with GM-CSF, cell aggregates appeared with the profiles of typical sheet-like projections and veil processes on its periphery, irrespective of the presence of SCF (Fig 2B). The aggregates grew thereafter, adjoining the adherent cells that also progressively grew. When the cell density was kept relatively low by splitting the cells at day 7, the number of aggregates with apparent sheet-like cells in the periphery continued to increase with generating DC-like cells until 14 days of the culture (Fig 2C). The aggregates were easily dislodged from the adherent layers and the cells with veil- or sheet-like processes were clearly observed after replating into a new well (Fig 2D), consistent with the previous report.13 On Giemsa staining, these cells exhibited an irregular shape with an eccentric nuclear and polarized lamellipodia (Fig 2F and H), suggesting that these were DCs on morphologic criteria.
Generation of mature DCs by culturing murine Lin−c-kit+ HPCs in the presence of mouse TNFα, GM-CSF, and SCF. Morphologic analyses were performed on cultured cells (A through D) or Giemsa-stained cells after cytocentrifugation (E through H). (A through D) A phase contrast microscopic observation was performed on murine Lin−c-kit+ HPCs stimulated by the combination of GM-CSF + SCF (A) or that of GM-CSF + SCF + mouse TNFα (B through D) for 5 (A and B) or 14 days (C) as described in Fig 1. In (D), the aggregates shown in (C) were isolated and observed for 2 hours after replating into a new plate. Original magnifications: (A), (B), and (D), × 200; (C), × 100. (E through H) Giemsa staining was performed after cytocentrifuging nonadherent cells or aggregate cells (from D) from murine Lin−c-kit+ HPCs cultured for 14 days in the absence (E and G) or in the presence of mouse TNFα (F and H) combined with GM-CSF and SCF. Original magnifications: (E) and (F), × 160; (G) and (H), × 400.
Generation of mature DCs by culturing murine Lin−c-kit+ HPCs in the presence of mouse TNFα, GM-CSF, and SCF. Morphologic analyses were performed on cultured cells (A through D) or Giemsa-stained cells after cytocentrifugation (E through H). (A through D) A phase contrast microscopic observation was performed on murine Lin−c-kit+ HPCs stimulated by the combination of GM-CSF + SCF (A) or that of GM-CSF + SCF + mouse TNFα (B through D) for 5 (A and B) or 14 days (C) as described in Fig 1. In (D), the aggregates shown in (C) were isolated and observed for 2 hours after replating into a new plate. Original magnifications: (A), (B), and (D), × 200; (C), × 100. (E through H) Giemsa staining was performed after cytocentrifuging nonadherent cells or aggregate cells (from D) from murine Lin−c-kit+ HPCs cultured for 14 days in the absence (E and G) or in the presence of mouse TNFα (F and H) combined with GM-CSF and SCF. Original magnifications: (E) and (F), × 160; (G) and (H), × 400.
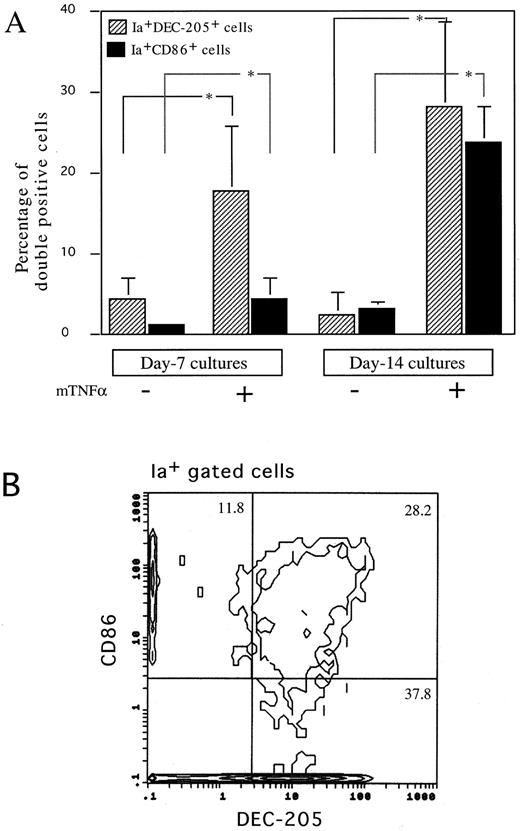
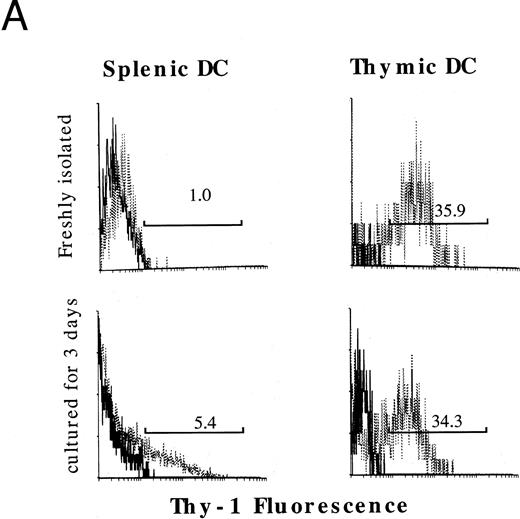
The effects of GM-CSF and TNFα on immunophenotypes of cultured HPCs. Because murine mature DCs express high levels of MHC class II antigen, DEC-205, and costimulatory molecule, CD86,1,2,4-8 we then performed immunofluorescence analyses on the cells using MoAbs to these antigens. GM-CSF and SCF marginally increased the proportion of both Ia+DEC-205+ and Ia+CD86+ cells at days 7 and 14 (Fig 3A). The addition of mouse TNFα synergistically increased the proportion of Ia+DEC-205+ and Ia+CD86+ cells at days 7 and 14, although Ia+CD86+ cells started to increase later than that of Ia+DEC-205+ cells (Fig 3A). Three-color immunofluorescence analyses detected a significant proportion of Ia+DEC-205+CD86+ cells in HPCs cultured for 14 days (Fig 3B). These cells expressed high levels of CD11b and DC marker, CD11c (Fig 4), but not CD4, B220, and Gr-1 (data not shown). The expression of CD8α was not detectable (Fig 4). Moreover, both Ia+DEC-205+ and Ia+CD86+ cells expressed Thy-1 at similar levels as CD11b (Fig 4). Thy-1 antigen was expressed by freshly isolated thymic DCs, but not those splenic ones (Fig 5A), although marginal upregulation of Thy-1 expression was observed in splenic DCs but not thymic DCs upon stimulation with GM-CSF and TNFα for 3 days. Furthermore, RT-PCR showed that Thy-1 mRNA was expressed by freshly isolated, purified thymic DCs and Thy-1+ DCs generated from Lin−c-kit+ HPCs, but neither splenic DCs nor Thy-1− DCs generated from Lin−c-kit+ HPCs (Fig 5B). These results suggest that Thy-1 on thymic DCs and HPC-derived DCs was, at least in part, endogenously synthesized from Thy-1 transcripts, although it has been shown that Thy-1 expression on the surface of thymic DCs may be partially due to passive acquisition from surrounding cells.34,36 37 Moreover, HPC-derived DCs shared several characteristic surface markers with freshly isolated thymic DCs.
Immunofluorescence analysis on nonadherent cells generated in Lin−c-kit+ HPCs cultures. (A) Using a two-color immunofluorescence analysis, the proportion of Ia+DEC-205+ (▨) or Ia+CD86+ cells (▪) was determined on nonadherent cells generated from Lin−c-kit+ HPCs cultured with or without mouse TNFα combined with GM-CSF and SCF for 7 and 14 days, respectively. The data are expressed as the mean ± SD of four independent experiments or more. Error bars indicate 1 SD. *P < .05 significance as compared with the cultures without mouse TNFα addition. (B) Three-color immunofluorescence analysis on nonadherent cells generated in Lin−c-kit+ HPCs stimulated with mouse TNFα combined with GM-CSF and SCF for 14 days. These cells were sequentially stained with DEC-205 MoAb, biotinylated CD86 MoAb, and PE-labeled Ia MoAb. DEC-205 and CD86 were then shown by antirat IgG(Fab′ )2-conjugated FITC and CY-streptavidin, respectively. The cell population that expressed high levels of Ia-antigen was gated and the coexpression of DEC-205 and CD86 was analyzed. More than 20,000 events were acquired. Three independent experiments were performed and representative results are shown here. The figures in each quadrant represent the percentage of the cells.
Immunofluorescence analysis on nonadherent cells generated in Lin−c-kit+ HPCs cultures. (A) Using a two-color immunofluorescence analysis, the proportion of Ia+DEC-205+ (▨) or Ia+CD86+ cells (▪) was determined on nonadherent cells generated from Lin−c-kit+ HPCs cultured with or without mouse TNFα combined with GM-CSF and SCF for 7 and 14 days, respectively. The data are expressed as the mean ± SD of four independent experiments or more. Error bars indicate 1 SD. *P < .05 significance as compared with the cultures without mouse TNFα addition. (B) Three-color immunofluorescence analysis on nonadherent cells generated in Lin−c-kit+ HPCs stimulated with mouse TNFα combined with GM-CSF and SCF for 14 days. These cells were sequentially stained with DEC-205 MoAb, biotinylated CD86 MoAb, and PE-labeled Ia MoAb. DEC-205 and CD86 were then shown by antirat IgG(Fab′ )2-conjugated FITC and CY-streptavidin, respectively. The cell population that expressed high levels of Ia-antigen was gated and the coexpression of DEC-205 and CD86 was analyzed. More than 20,000 events were acquired. Three independent experiments were performed and representative results are shown here. The figures in each quadrant represent the percentage of the cells.
Immunofluorescence analysis on murine Lin−c-kit+ HPCs cultured for 14 days in the presence of mouse TNFα, GM-CSF, and SCF. Three-color immunofluorescence analyses were performed as described in the Materials and Methods on the cultured HPCs at day 14. The indicated FITC-labeled MoAbs (CD8, Thy-1, CD11a, CD11b, and CD11c) were used to demonstrate the phenotype characteristics of the generated dendritic cells by gating on Ia+DEC-205+ or Ia+CD86+ cell population. Solid and dotted lines indicate the immunofluorescence intensity of cells stained with a control and the test antibodies, respectively. Representative results from three independent experiments are shown.
Immunofluorescence analysis on murine Lin−c-kit+ HPCs cultured for 14 days in the presence of mouse TNFα, GM-CSF, and SCF. Three-color immunofluorescence analyses were performed as described in the Materials and Methods on the cultured HPCs at day 14. The indicated FITC-labeled MoAbs (CD8, Thy-1, CD11a, CD11b, and CD11c) were used to demonstrate the phenotype characteristics of the generated dendritic cells by gating on Ia+DEC-205+ or Ia+CD86+ cell population. Solid and dotted lines indicate the immunofluorescence intensity of cells stained with a control and the test antibodies, respectively. Representative results from three independent experiments are shown.
The expression of Thy-1 antigen and its mRNA in thymic DCs, splenic DCs, and DCs generated from Lin−c-kit+ HPCs.Ia+CD11c+CD3−CDB220−Gr-1−NK1.1−TER-119− thymic and splenic DCs were highly purified by sorting them from low-density thymocytes and splenocytes after centrifuging on 14.5% metrizamid medium. The expression of Thy-1 antigen was reanalyzed on either freshly isolated or cultured thymic and splenic DCs upon stimulation with GM-CSF + mouse TNFα for 3 days. The percentage of Thy-1+ cells is indicated in the histograms (A). Thy-1 mRNA was examined in the indicated cells using RT-PCR (B). Total RNAs were extracted from 1 × 105 cells of splenocytes, thymocytes, splenic DCs, thymic DCs, and Lin−c-kit+ HPC-derived DCs. DCs generated from Lin−c-kit+ HPCs were further sorted into Thy-1+ and Thy-1− subpopulations. The β-actin transcripts were used as control (B). Similar experiments were performed three times and representative results are shown here.
The expression of Thy-1 antigen and its mRNA in thymic DCs, splenic DCs, and DCs generated from Lin−c-kit+ HPCs.Ia+CD11c+CD3−CDB220−Gr-1−NK1.1−TER-119− thymic and splenic DCs were highly purified by sorting them from low-density thymocytes and splenocytes after centrifuging on 14.5% metrizamid medium. The expression of Thy-1 antigen was reanalyzed on either freshly isolated or cultured thymic and splenic DCs upon stimulation with GM-CSF + mouse TNFα for 3 days. The percentage of Thy-1+ cells is indicated in the histograms (A). Thy-1 mRNA was examined in the indicated cells using RT-PCR (B). Total RNAs were extracted from 1 × 105 cells of splenocytes, thymocytes, splenic DCs, thymic DCs, and Lin−c-kit+ HPC-derived DCs. DCs generated from Lin−c-kit+ HPCs were further sorted into Thy-1+ and Thy-1− subpopulations. The β-actin transcripts were used as control (B). Similar experiments were performed three times and representative results are shown here.
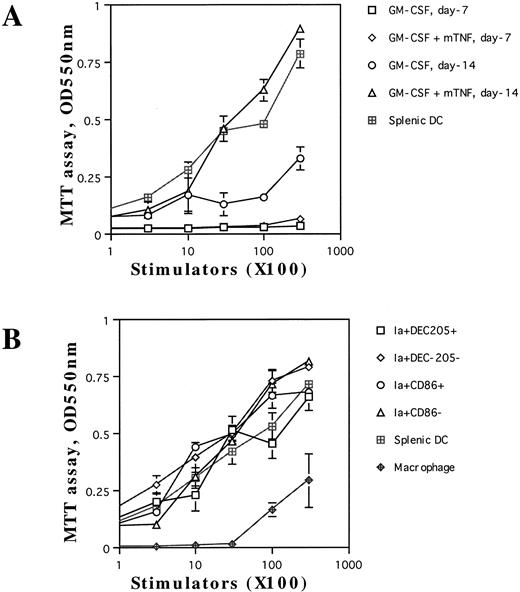
Enhancement of allogenic MLR by DCs generated from TNF-Rp55+/+ mice-derived Lin−c-kit+ HPC cultures. We next examined whether the nonadherent cells including aggregated cells could exhibit a characteristic activity of DCs1-4 to enhance allogenic MLR. When we cultured Lin−c-kit+ HPCs with either combination of cytokines only for 7 days, these HPC-derived cells failed to induce T-cell proliferation (Fig 6A). After incubation for 14 days with GM-CSF and SCF, a marginal level of T-cell proliferation was observed upon their addition. In contrast, the cells, which were incubated for 14 days with mouse TNFα and the combination of GM-CSF and SCF, enhanced T-cell proliferation at a comparable level to splenic DCs (Fig 6A), suggesting that mouse TNFα was indispensable for the generation of functionally competent DCs from purified HPCs. Because the cultured cells were heterogeneous with respect to CD86 and DEC-205 expression, they were further purified by a cell sorter to determine the capacity of each subpopulation to augment allogenic MLR. The four subpopulations obtained (Ia+CD86+, Ia+CD86−, Ia+DEC-205+, and Ia+DEC-205− cells) enhanced allogenic MLR as efficiently as splenic DC (Fig 6B). Under the same conditions, peritoneal macrophages supported MLR marginally even at the highest cell concentrations used (Fig 6B). Ia+CD86− and Ia+DEC-205− cells in these experiments should represent Ia+CD86−DEC-205+ and Ia+CD86+DEC-205− cells, respectively (Fig 3B). Thus, these results suggest that the obtained highly Ia-antigen-positive cells represented functionally competent DCs irrespective of expression of CD86 and DEC-205.
The capacity of the cultured cells to enhance allogenic MLR. Allogenic MLR was performed using purified T cells (3 × 105 cells per well in 96-round-well plates) as responder cells. The unfractionated nonadherent cells, which were derived from Lin−c-kit+ HPC cultures in the presence of mouse TNFα combined with GM-CSF and SCF at days 7 and 14, respectively, as described in the Materials and Methods, were irradiated and used as stimulators at the indicated cell numbers (A). In (B), the indicated cell populations were used as stimulator cells that were separated by using a cell sorter from Lin−c-kit+ HPC cultures stimulated with mouse TNFα in combination with GM-CSF and SCF for 14 days. The proliferation of T cells was measured by MTT assay after 5 days of culture. Results are expressed as the mean ± 1 SD of triplicate cultures. Results of each panel are representative of three independent experiments.
The capacity of the cultured cells to enhance allogenic MLR. Allogenic MLR was performed using purified T cells (3 × 105 cells per well in 96-round-well plates) as responder cells. The unfractionated nonadherent cells, which were derived from Lin−c-kit+ HPC cultures in the presence of mouse TNFα combined with GM-CSF and SCF at days 7 and 14, respectively, as described in the Materials and Methods, were irradiated and used as stimulators at the indicated cell numbers (A). In (B), the indicated cell populations were used as stimulator cells that were separated by using a cell sorter from Lin−c-kit+ HPC cultures stimulated with mouse TNFα in combination with GM-CSF and SCF for 14 days. The proliferation of T cells was measured by MTT assay after 5 days of culture. Results are expressed as the mean ± 1 SD of triplicate cultures. Results of each panel are representative of three independent experiments.
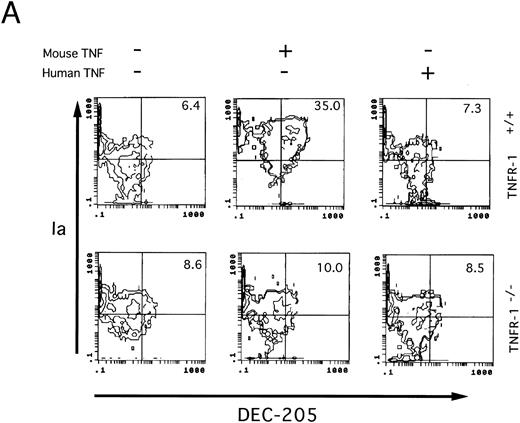
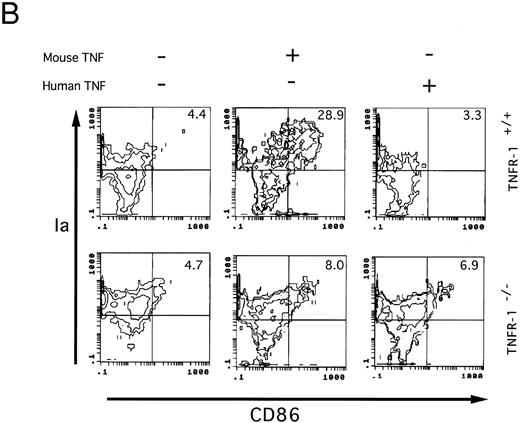
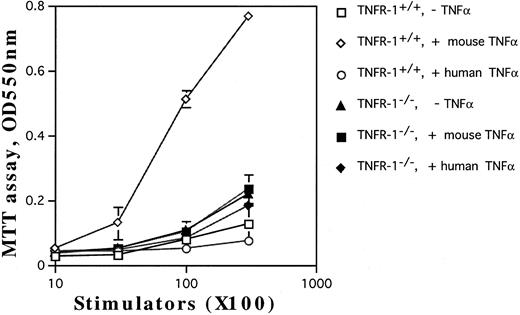
Involvement of TNF-Rp55 and TNF-Rp75 in generation of DC from HPCs. TNFα differentially uses two distinct receptors, TNF-Rp55 and TNF-Rp75, to mediate intracellular signals.38,39 We previously showed that TNF-Rp55 has a pivotal role to regulate the differentiation and proliferation of myeloid cells from HPCs in vivo and in vitro.31 Hence, using TNF-Rp55−/− mice, we examined the role of TNF-Rp55 in TNFα-induced enhancement of DC generation from Lin−c-kit+ HPCs. Combined with GM-CSF and SCF, mouse TNFα could neither induce the DC aggregate formation nor enhance the expression of DEC-205 and CD86 antigens on those cultured cells from TNF-Rp55−/− mice-derived Lin−c-kit+ HPCs during the whole culture periods (Figs 7 and 8A and B). Moreover, these cells were incapable to stimulate the allogenic MLR as compared with that of TNF-Rp55+/+ mice-derived Lin−c-kit+ HPCs (Fig 9). To further dissect the role of TNF-Rp55 and TNF-Rp75, the effects of human TNFα were investigated on DC generation from Lin−c-kit+ HPCs. Interestingly, under the same culture conditions, human TNFα could not induce DC differentiation and functional maturation from either TNF-Rp55+/+ or TNF-Rp55−/− mice-derived Lin−c-kit+ HPCs, even in the presence GM-CSF and SCF (Figs 7, 8, and 9). On the other hand, we observed that human TNFα was able to significantly inhibit the proliferation of Gr-1+ granulocytes from TNF-Rp55+/+ mice-derived Lin−c-kit+ HPCs, but not from TNF-Rp55−/− mice-derived ones, to the comparable levels as mouse TNFα did (data not shown), indicating that human TNFα used in our culture system retained biologic activities. Thus, these results strongly suggest that TNF-Rp75 may also be cooperatively involved in TNFα-induced DC differentiation and maturation from HPCs in vitro.
Development of dendritic cell aggregates in cultured Lin−c-kit+ HPCs-derived from TNF-Rp55+/+ mice bone marrow. Morphologic observation was performed under a phase contrast microscope on cultured Lin−c-kit+ HPCs. Lin−c-kit+ HPCs were isolated from TNF-Rp55+/+ or TNF-Rp55−/− mice bone marrow cells and incubated for 14 days (14 d) as described in the Materials and Methods. Similar experiments were repeated three times and representative results are shown here. Original magnification × 100.
Development of dendritic cell aggregates in cultured Lin−c-kit+ HPCs-derived from TNF-Rp55+/+ mice bone marrow. Morphologic observation was performed under a phase contrast microscope on cultured Lin−c-kit+ HPCs. Lin−c-kit+ HPCs were isolated from TNF-Rp55+/+ or TNF-Rp55−/− mice bone marrow cells and incubated for 14 days (14 d) as described in the Materials and Methods. Similar experiments were repeated three times and representative results are shown here. Original magnification × 100.
Two-color immunofluorescence analysis on the cultured Lin−c-kit+ HPCs. Lin−c-kit+ HPCs from TNF-Rp55+/+ wild-type or TNF-Rp55−/− mice bone marrow were cultured in the presence or absence of mouse or human TNFα combined with GM-CSF and SCF for 14 days, and two-color immunfluorescence analysis was performed to determine the expression of Ia with DEC-205 (A) or CD86 (B) as described in the Materials and Methods. Representative results from three independent experiments are shown here.
Two-color immunofluorescence analysis on the cultured Lin−c-kit+ HPCs. Lin−c-kit+ HPCs from TNF-Rp55+/+ wild-type or TNF-Rp55−/− mice bone marrow were cultured in the presence or absence of mouse or human TNFα combined with GM-CSF and SCF for 14 days, and two-color immunfluorescence analysis was performed to determine the expression of Ia with DEC-205 (A) or CD86 (B) as described in the Materials and Methods. Representative results from three independent experiments are shown here.
The capacity of the cultured HPCs from TNF-Rp55+/+ or TNF-Rp55−/− mice bone marrow to stimulate allogenic MLR. Lin−c-kit+ HPCs were obtained from TNF-Rp55+/+ or TNF-Rp55−/− mice bone marrow cells and incubated for 14 days in the presence of the indicated combinations of cytokines. Allogenic MLR was performed using purified T cells and cultured HPC progenies as responder and stimulator cells, respectively, as described in the Materials and Methods.
The capacity of the cultured HPCs from TNF-Rp55+/+ or TNF-Rp55−/− mice bone marrow to stimulate allogenic MLR. Lin−c-kit+ HPCs were obtained from TNF-Rp55+/+ or TNF-Rp55−/− mice bone marrow cells and incubated for 14 days in the presence of the indicated combinations of cytokines. Allogenic MLR was performed using purified T cells and cultured HPC progenies as responder and stimulator cells, respectively, as described in the Materials and Methods.
DISCUSSION
Several groups independently reported that functional DCs could be generated from human CD34+ and mouse MHC class II-negative bone marrow cells.40-43 However, because these cell populations consist of heterogeneous HPCs that are thought to be at different differentiation stages,40-43 it remains elusive whether purified early HPCs can differentiate in vitro into DC. We have provided evidence here that highly purified murine Lin−c-kit+ HPCs could differentiate into DCs with characteristic morphology and surface markers after culture for 14 days at a relatively low cell density in the presence of GM-CSF, SCF, and mouse TNFα. Moreover, the cells enhanced allogenic MLR as effectively as spleen-derived DCs, suggesting that these cells also function as an antigen-presenting cell. The results would imply that the generated cells are DCs, concordant with the proposed characteristics of DC.1-3
Murine DC exhibits heterogeneity in surface markers and intracytoplasmic structures, depending on their locations.1-8 DEC-205 is expressed by a majority of DCs in murine thymus, whereas only less than 20% of DCs in spleen express DEC-205.4,5,7,8,29,44 However, a major difference in splenic and thymic DC lies in the expression of T-cell markers.6,34,37,45 Thymic DCs express moderate levels of Thy-1 and CD2 with a low level of CD4, whereas splenic DCs express little, if any, of these antigens. Hence, these T-cell markers, especially Thy-1 and CD2, can be useful to distinguish between thymic and splenic DCs,6 although there is some controversy concerning the expression by thymic DCs of certain cell-surface markers.6,34,36,37,46 It has been reported that thymic DCs absorbed Thy-1 antigen from surrounding cells.36,37 However, the expression of Thy-1 mRNA was detected in this study in freshly isolated thymic DCs and HPC-derived Thy-1+ DCs but not HPC-derived Thy-1− ones, indicating that a subclass of freshly isolated thymic DCs and HPC-derived DCs could produce Thy-1 antigen, at least in part, from its specific transcript. In addition, Thy-1 expression was not detectable in freshly isolated splenic DCs, even though marginal upregulation of its expression was observed upon stimulating these DCs with GM-CSF and TNFα. These results would suggest that a subpopulation of HPC-derived DCs phenotypically resembled those freshly isolated thymic DCs. The latter notion may be corroborated by the previous observation that intrathymic injection of murine Lin−Sca-1+ primitive hematopoietic stem cells generated DCs with DEC-205 in thymus.7
Previously, Inaba et al13 reported that GM-CSF alone can induce murine MHC class II-negative bone marrow cells to differentiate into splenic-type DCs that expressed high levels of Ia-antigens but low levels of DEC-205 and CD11c without Thy-1 expression, in contrast to our present results. Moreover, functional DCs appeared at 6 to 7 days after the culture in their system,13 whereas Lin−c-kit+ cells could not enhance allogenic MLR at 7 days after the culture under our experimental conditions, even in the presence of mouse TNFα, GM-CSF, and SCF. These results suggest that the kinetics of DC maturation from Lin−c-kit+ HPCs also differs from that of MHC class II-negative DC precursors. Moreover, in their system, a relatively high cell concentration was used to induce DC differentiation.13 When we incubated these HPCs at a low cell concentration, only in the presence of GM-CSF and/or SCF, most of the shown colonies consisted of granulocytes, macrophages, and a few numbers of DC. Thus, GM-CSF may induce MHC class II-negative bone marrow cells to produce some unknown factor(s) that affects the differentiation of DCs. It has been reported that there exist at least two distinct types of DC precursors with independent differentiation pathways in human CD34+ hematopoietic progenitors.17 21 Thus, the discrepancy may be also explained by the presence of an additional type(s) of DC precursors in murine MHC class II-negative bone marrow cells, which possibly differ from those in Lin−c-kit+ HPCs. Nevertheless, these results would imply that different HPC subpopulations or those hematopoietic cells at different maturation stages may generate distinct DC subsets, depending on the added stimuli.
A growing number of experiments demonstrates that each step of hematopoietic cell differentiation is under the strict and cooperative control of transcription factors.47 Mature DCs contain abundant members of transcription factors, NF-κB, particularly p50, and relB.11,48 Moreover, targeted disruption of relB gene has resulted in an impairment of DC development in either spleen or thymus,49,50 suggesting that members of the NF-κB family have an essential role in regulating differentiation and maturation of DC in vivo. Accumulating evidence indicates that TNFα can induce the transcription of several genes by activating NF-κB.51 Thus, it is tempting to speculate that TNFα activates NF-κB, which may be involved in the expression of a gene(s) essentially required for DC differentiation. The identification of a target gene(s) might provide a novel insight into the molecular mechanisms of DC differentiation.
Two related but distinct receptors, p55 and p75, exist for TNF.38,39 Although these receptors show sequence similarity with each other in the extracellular domains, those of intracellular domains diverge, accounting for mediation of overlapping but distinct signals.38,39 We recently observed increased HPC numbers in TNF-Rp55−/− mice, implicating TNF-Rp55 as a physiologic regulator of HPC differentiation and proliferation.32 Moreover, TNF-Rp55−/−, TNFα−/−, or LTα−/− mice exhibit impaired FDC development, although no information has been available yet on other types of DCs.22,23,50 Hence, we first evaluated here the role of TNF-Rp55 in in vitro DC differentiation from HPCs. Our study using TNF-Rp55−/− mice-derived Lin−c-kit+ HPCs showed that these early HPCs cannot generate morphologic and functional mature DCs in response to the stimulation of mouse TNFα combined with GM-CSF and SCF, implying a critical role of TNF-Rp55 in mouse TNFα-induced in vitro DC differentiation. However, human TNFα, which can only bind to mouse TNF-Rp55 but not to mouse TNF-Rp75, could not induce DC generation even from TNF-Rp55+/+ mice-derived Lin−c-kit+ HPCs. Thus, these results suggested that both types of TNF-Rs p55 and p75 are coordinately involved in in vitro TNFα-induced DC differentiation from early HPCs. The recruitment of TNF by TNF-Rp75 for TNF-Rp55 has been documented.52 Thus, endogenously produced TNFα may interact initially with TNF-Rp75 and be recruited to TNF-Rp55. The interaction between TNFα and TNF-Rp55 may eventually prevent HPCs from proliferation, thereby inducing differentiation into more committed cells, including DCs.
Targeted disruption of TNF-Rp55 resulted in a lack of FDC network in spleen and impaired humoral immune response without apparent changes in splenic architecture, whereas that of TNF-Rp75 has few effects on FDC in spleen.22,53,54 Moreover, mice deficient in either TNFα or LTα lacked follicular structures in spleen and exhibited impaired humoral immune responses.23,54 These results suggest that the interaction of TNF-Rp55 but not p75 with its ligands is indispensable for FDC formation in spleen. The discrepancy on the roles of TNF-Rp75 between FDC formation and DC generation in our system may be explained by the difference of target cells. However, the phenotypic changes of TNF-Rp55−/− were much milder in spleen as compared with those of TNFα−/− and LTα−/− mice.22,23,53,54 Thus, TNF-Rp75, an additional receptor for TNFα and LTα, may play a role in FDC differentiation as well as in vitro DC differentiation. Additionally, many other cytokines have been shown to be able to induce DC's differentiation either in vivo or in vitro.2,3,34,46,55,56 These may explain the failure to detect aberrant distribution and phenotypic changes in DCs in thymus, where bone marrow-derived DCs have been recorded,7 from TNF-Rp55−/− mice under normal conditions (data not shown).
We show here that murine HPCs could differentiate into DC resembling freshly isolated thymic one with respect to surface marker expression. Thymic DCs have been proposed to have a pivotal role in negative and positive selection in thymus.57,58 Hence, the generation of a large number of thymic DC-like cells using this system may facilitate to elucidate the mechanisms of thymic selection in vitro. Moreover, DCs generated in our culture system may be a useful weapon to control autoimmune diseases in which disturbed thymic selection is frequently observed.24,57 58
ACKNOWLEDGMENT
The authors express their gratitude to Dr R.M. Steinman (The Rockefeller University) for his kind gifts of MoAbs to DEC-205 (NLDC-145) and 33D1. We also thank Dr H. Bluethmann for his generous present of TNF-Rp55 gene-targeted knockout mice. We highly appreciate the productive discussion with Dr K. Inaba (Department of Zoology, Faculty of Science, Kyoto University, Kyoto, Japan).
Supported in part by Grants-in-Aid from the Ministry of Education, Culture, Science, and Sports of the Japanese Government and from CREST, Japan Technology Corp.
Address reprint requests to Kouji Matsushima, MD, PhD, Department of Molecular Preventive Medicine, School of Medicine, University of Tokyo, 7-3-1, Hongo, Bunkyoku, Tokyo 113, Japan; email: koujim@m.u-tokyo.ac.jp.