Abstract
We present a novel G1091 to A mutation in the human liver and red blood cell (RBC) pyruvate kinase (PK) gene causing severe hemolytic anemia. In two families, three children were severely PK-deficient compound heterozygotes exhibiting the G1091 to A mutation and a common G1529 to A mutation on the other allele. In one family, the mother, a G1091 to A heterozygote, later had a second baby with a new husband, also a G1091 to A carrier. The baby was homozygous for the G1091 to A mutation and died 6 weeks after birth from severe hemolysis. Both mutant alleles were expressed at the RNA level. The G1091 to A mutation results in the substitution of a conserved glycine by an aspartate in domain A of RBC PK, whereas the G1529 to A mutation leads to the substitution of a conserved arginine residue with glutamine in the C-domain. Molecular modelling of human RBC PK, based on the crystal structure of cat muscle PK, shows that both mutations are located outside the catalytic site at the interface of domains A and C. The mutations are likely to disrupt the critical conformation of the interface by introducing alternative salt bridges. In this way the Gly364 to Asp and Arg510 to Gln substitutions may cause PK deficiency by influencing the allosteric properties of the enzyme.
PYRUVATE KINASE (PK) catalyzes the conversion of phosphoenol pyruvate to pyruvate and is an important regulator of glycolysis. Two different genes have been characterized in mammals. One encodes the liver and red blood cell (RBC) isoenzymes (PKLR gene), whereas the other generates the muscle isoenzymes M1 and M2 .1-3 Mutations in the PKLR gene are associated with PK deficiency, an important cause of nonspherocytic hemolytic anemia. The clinical symptoms are variable, ranging from a mild, compensated hemolytic anemia to death in early childhood.4
In the last few years a variety of different mutations in the PKLR gene have been identified, facilitating the molecular diagnosis of PK deficiency.5,6 Because of the lack of structural and functional information, it has been difficult to predict the phenotypic consequences of specific mutations. PK forms tetramers and its catalytic activity is allosterically regulated by phosphorylation and allosteric effectors. The tertiary structure of human R-type PK (HRPK) has not been determined, but the primary sequence is homologous to cat and rabbit muscle PK isoform M1 (M1PK),7-9 whose structures have been solved at 2.6 Å and 2.9 Å resolution, respectively (Larsen et al9; Brookhaven Data Bank, accession code 1pkm [Allen and Muirhead]). M1PK is composed of four domains: first, the N-terminal domain N, which is involved in subunit contacts in the tetrameric molecule; second, domain A, a classic α/β-barrel, that comprises the catalytic site; third, domain B, which loops out from the end of the third β-strand in domain A (the functional role of domain B is unclear); and, finally, the C-terminal domain C, which forms an open twisted αβ-type domain that may play an important role for tetramerization. However, the M1 type PK is the only PK enzyme that is not allosterically regulated and hence functionally different from the RBC type PK. Reflections on the effect of mutations in the human RBC PK gene have so far been based on the three-dimensional model of cat muscle, assuming that these conclusions were valid for the human RBC gene.
In this study, we report the finding of a novel PK mutation in two Danish families that causes severe nonspherocytic hemolytic anemia. We have examined the expression of the mutant alleles at the RNA and protein level. Simulation of the effect of the mutations in a human RBC PK model, based on the crystal structure of muscle PK, shows that none of the mutations involves the catalytic site, but both are likely to disrupt the structure of the critical interface between domains A and C. We postulate that the G1091 to A and common G1529 to A mutation cause PK deficiency by disrupting the allosteric properties of PK.
MATERIALS AND METHODS
Nomenclature. Nucleotides and amino acids are numbered according to Kanno et al7 (GenBank D13232-D13243).
Patients. The proband was an 11-year-old Danish boy. At birth, he suffered from a severe hemolytic crisis, with a hemoglobin concentration of 10.3 g/dL, reticulocytosis, and hepatosplenomegaly. PK activity was undetectable, and other possible causes of hemolysis were excluded. At present, he requires blood transfusions every 3 months. His parents do not suffer from any clinically noticeable hemolysis. After the boy's mother was divorced and remarried, she and her new husband had a baby girl. At birth, she was severely anemic and died after 6 weeks, despite blood transfusions. The PK activity of the baby was undetectable.
During the molecular investigations a second Danish family was referred to our laboratories for molecular analysis of the PKLR genes. Both parents were healthy adults of Danish origin with no known relation to the first family. The clinical course of their first child (girl) at birth was identical as described above for the proband. At present, she is 3 years old and requires blood transfusions every 3 months. The second child in this family, a boy, was also born with a hemoglobin concentration of 11.3 g/dL and reticulocytosis. His liver and spleen were normal. PK activity was low. Presently, he is 1 year old and receives transfusions every 3 months. Informed consent was obtained from all subjects.
DNA and RNA isolation. DNA from EDTA-stabilized blood and paraffin-embedded liver coupes was isolated as described.10,11 Total RNA was isolated from isolated blood reticulocytes as described.12
Polymerase chain reaction (PCR) procedures. To have high specificity and yield for sequencing purposes, the human PKLR gene was first amplified with primers PKr-1 (exon 1, 5′-CCC AGG CCC ACA CTG AAA GC-3′) and PKr-6 (exon 12, 5′-GTG TGG GCT GGA GAA CGT AGA-3′) using the XL-PCR kit from Perkin-Elmer (Norwalk, CT) according to the manufacturer's instructions, with modifications.13 Briefly, components were mixed using 0.2 mmol/L of each dNTP, 50 pmol of each primer, and 1.1 mmol/L Mg(OAc)2 . After 4 minutes of denaturation at 95°C, 4 U of rTth DNA polymerase XL was added. Cycles were as follows: 1 minute at 94°C, 10 minutes at 64°C, and 10 minutes at 72°C. After cycle no. 19, the extension time was increased with 15 seconds per cycle for a total of 30 cycles.
The amplified XL-PCR product was used as template in nested PCRs in which each exon and introns 1, 5, and 8, including the intron-exon boundaries, were amplified. Exon 1 was amplified directly from genomic DNA using primers PK-1F and PK-1R (Table 1). The reaction mixture in 100 μL volume contained 10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2 , 0.01% (wt/vol) gelatine, 0.2 mmol/L of each dNTP, 1 mmol/L of each primer, 1 μL XL-PCR product, and 2.5 U AmpliTaq DNA polymerase. All reagents were obtained from Perkin-Elmer. The samples were subjected to 30 cycles of amplification with denaturation at 94°C for 45 seconds (5 minutes at 95°C in the first cycle), annealing at 64°C for 45 seconds, and extension at 72°C for 45 seconds, followed by an elongated extension time of 7 minutes after the last cycle. Negative controls, without added DNA, were included in each run to exclude amplification of contaminating DNA.
Reverse transcription-PCR (RT-PCR) was performed as described.12 cDNA synthesis was initiated with random hexamers. cDNA was amplified with primers PKr-5 (exon 8, 5′-GGT GAG CGA CGG CAT CAT GG-3′) and CDPK-9 (exon 9, 5′-CGC ATG CTG CAT CTT CAC CGC-3′) for detection of the nt 1091 mutation. For the detection of the nt 1529 mutation, primers CDPK-11 (exon 11, 5′-CTC AGC CCA GCT TCT GTC TCG-3′) and PKr-6 were used. PCR conditions were as described under nested PCR. Normal human liver RNA was included as a positive control.12 These products were used for direct DNA sequencing. For all RNA samples amplified in an RT-PCR, we routinely included a control in which the reverse transcription step was omitted.
Sequencing of PCR products. Automated sequencing was performed with the ABI Prism Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer/Applied Biosystems, Foster City, CA), according to the instructions, and sequence reactions were analyzed on a Perkin-Elmer/Applied Biosystems ABI 310 Genetic Analyzer.
Restriction endonuclease digestion. All mutations in DNA and RNA and the polymorphisms in DNA were confirmed and characterized using restriction endonuclease digestion when applicable. To confirm the 1091 mutation detected by sequencing, we amplified a 264-bp fragment containing exon 8 from genomic DNA using primers PK-8F and PK-8R (Table 1). The fragment contains three restriction sites for Cac 8I (GCN/NGC), yielding fragments of 4, 22, 83, and 155 bps. The 1091 G/A mutation removes two sites simultaneously (fragments of 22 and 242 bp). For confirmation of the nt 1529 mutation, we amplified exon 11 with primers PK-11F and PK-11R (fragment of 330 bp). The mutation creates a restriction site for Sty I (C/CWWGG), producing fragments of 190 and 140 bp. The same enzymes were used for confirmation of the mutations in RNA. Primers used for RT-PCR were as mentioned above in “Polymerase chain reaction (PCR) procedures.”
To detect the polymorphism at nt 1705 (A/C),14 we amplified a fragment using primers PK-12F and PKr-6 (235 bp). BspHI (T/CATGA) will only cleave this product when an adenine is present (132 and 103 bp).
The nt 1738 (C/T) (this study, Van Solinge et al,15 and Bianchi et al16 ) polymorphism was detected using BseRI [GAGGAG(N)10 / and (N)8CTCCTC], only cleaving a fragment amplified with primers PK-12F and PKr-6 if a thymine is present at this position (182 and 53 bp).
The polymorphism at nt +51 in intron 5 (C/T) (this study, Fujii et al,17 and Baronciani et al18 ) was detected by sequencing intron 5, because no useful restriction enzyme recognition sites are present.
The trinucleotide ATT repeat in intron 1119 was amplified with 4,7,2′,7′-tetrachloro-6-carboxy-fluorescein (TET)-labeled primers JHATT19 and PK-TNR-R4 and examined with the GeneScan short denatured module on the ABI 310 Genetic Analyzer and by sequencing the fragments.
Protein studies. Enzyme assays and the heat stability assay of PK were performed according to the methods of Beutler.20 The antibody consumption assay was performed essentially as described earlier.21 Oligomerization in the presence and absence of 10 mmol/L fructose-1,6-diphosphate (FDP) was studied by gel filtration experiments on a Sephacryl S-200 superfine column (Pharmacia, Uppsala, Sweden) using lactate dehydrogenase (130 kD) as an internal standard.22
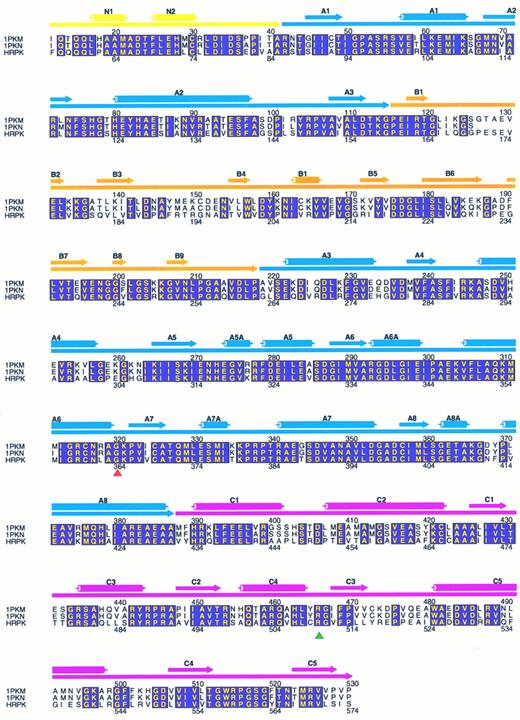
Molecular modelling. Coordinates of the x-ray crystal structure of the M1 muscle type PK from felis domesticus, obtained from the Brookhaven Data Bank as 1PKM (submitted by Allen and Muirhead), were used as a scaffold for the construction of a model of HRPK. Initially, the corrected cDNA sequence of the human PKLR gene7,23 was aligned with the sequences derived from both 1PKM (Allen and Muirhead) and 1PKN9 structures, using the Alignment of Multiple Protein Sequences (AMPS) program, as described.24 This resulted in the alignment as shown in Fig 1.
Alignment of muscle-type PK from felis domesticus (1PKM) and oryctolagus cuniculus (1PKN) with R-type PK from human (HRPK). The alignment was generated with AMPS, as described in the Materials and Methods and drawn with the program ALSCRIPT.35 Amino acids that are completely conserved among the three PKs are shown with yellow letters in boxes shaded with magenta. Only residues of human R-type PK (56-574) corresponding to 1PKM (12-530) are shown. The secondary structure elements, α-helixes and β-strands, assigned to 1PKM are shown above the sequences as cylinders and filled arrow, respectively. The domain organization of PK is represented by filled arrows right under the secondary structure elements. Color code: the N-terminal domain N is yellow, the catalytic domain A is blue, domain B is orange, and the C-terminal twisted αβ-type domain C is pink. The numbers above the sequences are in accordance with the numbering in 1PKM, whereas the numbers beneath the sequences refer to the codon numbering of HRPK. The red-filled triangle indicates the position of residue 364 (glycine to aspartic acid mutation), and the green filled triangle indicates the position of residue 510 (arginine to glutamine mutation).
Alignment of muscle-type PK from felis domesticus (1PKM) and oryctolagus cuniculus (1PKN) with R-type PK from human (HRPK). The alignment was generated with AMPS, as described in the Materials and Methods and drawn with the program ALSCRIPT.35 Amino acids that are completely conserved among the three PKs are shown with yellow letters in boxes shaded with magenta. Only residues of human R-type PK (56-574) corresponding to 1PKM (12-530) are shown. The secondary structure elements, α-helixes and β-strands, assigned to 1PKM are shown above the sequences as cylinders and filled arrow, respectively. The domain organization of PK is represented by filled arrows right under the secondary structure elements. Color code: the N-terminal domain N is yellow, the catalytic domain A is blue, domain B is orange, and the C-terminal twisted αβ-type domain C is pink. The numbers above the sequences are in accordance with the numbering in 1PKM, whereas the numbers beneath the sequences refer to the codon numbering of HRPK. The red-filled triangle indicates the position of residue 364 (glycine to aspartic acid mutation), and the green filled triangle indicates the position of residue 510 (arginine to glutamine mutation).
After alignment of the sequences, the HRPK was constructed from 1PKM with the program package from Biosym Technologies (San Diego, CA) in the following way: (1) the coordinates of 1PKM were read into the program INSIGHTII, (2) followed by alignment of the sequence of 1PKM with the HRPK sequence according to Fig 1 using the program HOMOLOGY and (3) transferring of 1PKM coordinates to HRPK. (4) The raw model of HRPK was modified to eliminate close Van der Waals contacts. (5) The adjusted model of HRPK was then subjected for minimization in the program DISCOVER for 6,000 iterations with the steepest descendants algorithm assuming a pH of 7.0, essentially as described.25 The minimized model of HRPK was used for inspection of the mutations described in this study.
RESULTS
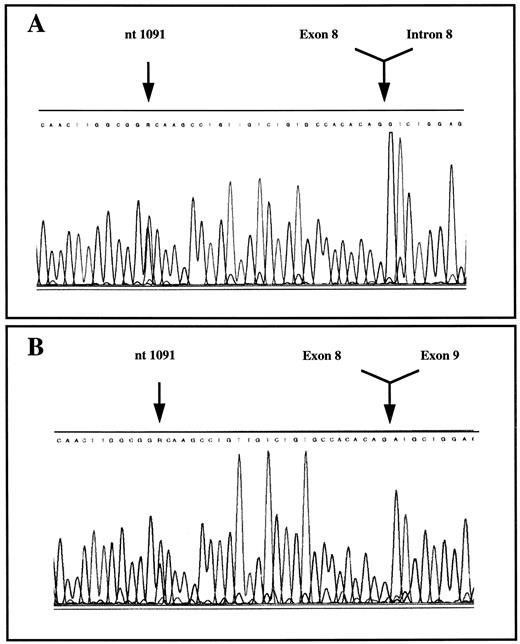
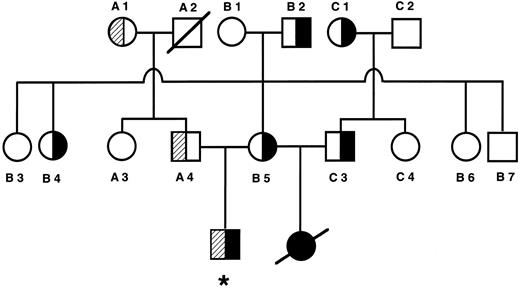
PK mutations. The proband was a compound heterozygote with a novel G1091 to A mutation in exon 8 and a second mutation (G1529 to A) in exon 11 on the other allele of the PKLR gene (Fig 2A, only G1091 to A shown). The G1091 to A mutation results in the substitution of a conserved glycine by an aspartate at codon 364, whereas the G1529 to A mutation leads to the substitution of a conserved arginine residue with glutamine at codon 510. The latter is a well-known and common mutation among non-gypsy whites.4 26 The G1091 to A mutation was also present in the mother, whereas the G1529 to A mutation was detected in his father. Analysis of the PKLR gene of the second husband showed that he was a heterozygote carrier of the G1091 to A mutation. Analysis of DNA obtained from liver coupes showed that the deceased baby was a G1091 to A homozygote (data not shown). The pedigree and mutation analysis of the family are shown in Fig 3. The second Danish family also exhibited the G1091 to A and G1529 to A mutations. The daughter and son were both compound heterozygotes and the 1091 mutation was inherited from the father, whereas the mother was carrier of the 1529 mutation. RT-PCR analysis of RNA followed by sequencing and restriction enzyme analysis indicated that both mutated alleles were expressed (Fig 2B, only G1091 to A shown).
Detection of the nucleotide G1091 to A mutation in exon 8 of the human PKLR gene and RNA of the proband by sequencing. (A) DNA; (B) RNA.
Detection of the nucleotide G1091 to A mutation in exon 8 of the human PKLR gene and RNA of the proband by sequencing. (A) DNA; (B) RNA.
Pedigree of family 1. Filled areas denote the nt 1091 mutation. Shaded areas denote the nt 1529 mutation. *Proband.
Pedigree of family 1. Filled areas denote the nt 1091 mutation. Shaded areas denote the nt 1529 mutation. *Proband.
The prevalence of the two mutations was determined by screening 100 normal controls for the G1091 to A and G1529 to A mutations. None was detected, indicating that the G1091 to A and G1529 to A allele frequency is less than 1:200.
Polymorphisms. A C/T polymorphic site was identified in exon 12, at position 1738, 13 nucleotides downstream of the translation termination codon.15 16 Estimated from 100 chromosomes of unrelated individuals, the allelic frequency was as follows: C-alleles, 0.26; T-alleles, 0.74. Observed homozygosity for C/C was 0.04, for C/T was 0.43, and for T/T was 0.53. In addition, the polymorphism in intron 5 at nt +5117,18 was used to determine the haplotypes of our patients.
Using four polymorphisms, we performed DNA haplotype analysis. The 1529 mutation was linked to IVS-5-T, 14 ATT repeats in intron 11, nt 1705-C, and nt 1738-T. The 1091 mutation was linked to IVS-5-C, 12 ATT repeats in intron 11, nt 1705-A, and nt 1738-C in all patients (Table 2).
Enzyme activities. RBC PK activity of the proband in family I as well as the affected children in family II was low, but detectable (Table 2). However, although blood samples were taken shortly before blood transfusion, it cannot be excluded that the activity in the patients was, at least partly, originating from the presence of residual donor cells. To evaluate the mean cell age of the RBC population, the cell age-dependent enzymes hexokinase and glucose-6-P-dehydrogenase were measured too. The largely increased activities of these enzymes indicate a very young mean cell population and emphasize the extent of the PK deficiency. Unfortunately, no blood samples were available from the deceased daughter in family I or from the affected son in family II. As expected, the proband's heterozygous parents have low PK activities. The second husband as well as the father in family II are heterozygous too. Although the mother in family II is a carrier of the G1529 to A mutation, her PK activity was not significantly lowered.
Protein studies. Biochemical studies at the protein level were hampered by the low PK activity and the potential presence of donor RBC in the patients. It could not be excluded that the residual enzyme activity in the proband was originating mainly from donor cells. Yet, we decided to characterize the enzyme from patient and heterozygotes. However, we were unable to detect any significant aberrations. First, the expression of inactive enzyme protein was assayed by performing an antibody comsumption assay. The amount of immuno-reactive material consumed by a fixed enzyme activity appeared to be normal in all cases. However, in the patient, a minor part of the activity could not be precipitated with the antibody for R-type PK, suggesting the presence of PK isoforms that are not recognized by the antibody (data not shown). In severe PK deficiency, the compensatory presence of M2 -type PK has been shown in some cases.27 However, no enzyme activity could be precipitated with antibody against M2 -type PK. In another set of experiments, the heat stability of the enzyme was investigated, but no labile enzyme could be detected. The allosteric properties of the enzyme were investigated by studying the effects of FDP on the activity at low phospho-enol-pyruvate concentrations. Again, the enzyme from patient and heterozygotes behaved completely normal. Also, the extent of tetramerization in presence and absence of FDP was studied by gel filtration experiments, but no signs of altered subunit interactions were observed (results not shown).
Because of the limitations as described above, these results did not allow any conclusions on the expression of mutant enzyme at the protein level.
Molecular modelling. Alignment of cat M1PK, rabbit M1PK, and human RBC PK (HRPK) shows that HRPK is approximately 70% similar to the two enzymes (Fig 1). Like cat and rabbit M1PK, HRPK may therefore be divided into four domains. Residues 42-85 form the N-terminal domain. Residues 86-159 and 263-431 form domain A, a classic α/β-barrel, that comprises the catalytic site. The residues that comprise domain B span from codons 160 to 262 and loop out from the end of the third β-strand in domain A. Finally, the C-terminal C-domain from residues 432 to 574 forms an open twisted αβ-type domain. The mutated Gly 364 is located in the A-domain, whereas Arg 510 is situated in the C-domain. Both aminoacids are conserved in cat and rabbit M1PK (Fig 1), yeast, rat LPK, chicken MPK,8 mouse RPK,28 and dog RPK.29 In Bacillus stearothermophilus the glycine 364 is conserved, whereas arginine 510 is not.30
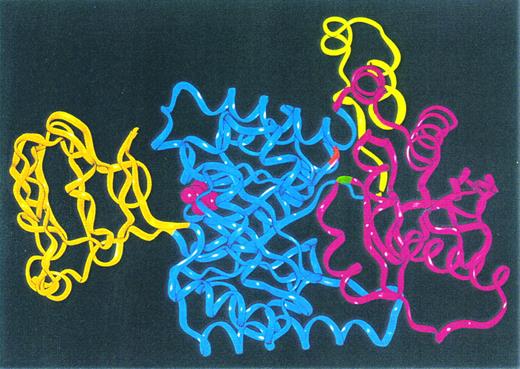
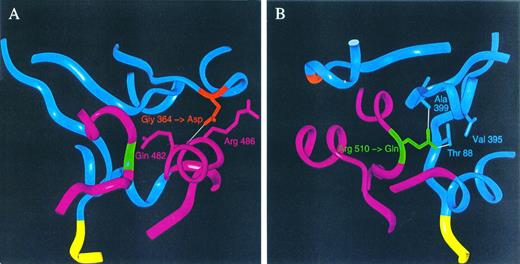
Figure 4 shows the Cα-ribbon trace of the model of human RBC PK. Placement of the mutations in this model shows that the mutations are both located at the interface of domains A and C. Glycine 364 is situated in the loop connecting Aα6 and Aβ7, whereas Arg 510 connects Cα4 and Cβ3. Please note that the substituted amino acids in the HRPK are not located in cis in vivo because mutations are present on separate alleles. Therefore, Fig 4 only indicates the position of the mutated codons. We then configured the mutated codons into the model and made a close-up view of areas around the respective parts of the PK monomer, as shown in Fig 5. The Gly 364 to Asp mutation in domain A is situated close to glutamine at position 482 and to Arg 486 in domain C of the monomer. Asp 364 is not involved in any salt bridge formation to Arg 486, but it forms a hydrogen bond to the backbone carbonyl of Gln 482. The glutamine for arginine substitution at position 510 leads to the formation of two hydrogen bonds (Thr 88 and Ala 399), whereas the wild-type has only one hydrogen bond to Ala 399.
Cα-ribbon trace of the model of human R-type PK monomer. The color code is as for Fig 1: the N-terminal domain N is yellow, catalytic domain A is blue, domain B is orange, and the C-terminal twisted αβ-type domain C is pink. The positions of the amino acids 364 (G1091 to A mutation) and 510 (G1529 to A mutation) are indicated by red and green color, respectively. The active site bound pyruvate molecule taken from 1PKN9 is represented by pink space filling.
Cα-ribbon trace of the model of human R-type PK monomer. The color code is as for Fig 1: the N-terminal domain N is yellow, catalytic domain A is blue, domain B is orange, and the C-terminal twisted αβ-type domain C is pink. The positions of the amino acids 364 (G1091 to A mutation) and 510 (G1529 to A mutation) are indicated by red and green color, respectively. The active site bound pyruvate molecule taken from 1PKN9 is represented by pink space filling.
Close-up view of areas around the amino acids 364 (A) and 510 (B). Residues in close contact with the mutated residues, translated from codons 364 and 510, are shown with side chains, whereas the residues further apart are shown in Cα-ribbon trace representation as in Fig 4. The color code is as for Fig 1. (A) and (B) are shown with the mutated aspartic acid and glutamine replacing the wild-type glycine and arginine, respectively. Hydrogen bonds less than 3.0 Å are shown as white lines.
Close-up view of areas around the amino acids 364 (A) and 510 (B). Residues in close contact with the mutated residues, translated from codons 364 and 510, are shown with side chains, whereas the residues further apart are shown in Cα-ribbon trace representation as in Fig 4. The color code is as for Fig 1. (A) and (B) are shown with the mutated aspartic acid and glutamine replacing the wild-type glycine and arginine, respectively. Hydrogen bonds less than 3.0 Å are shown as white lines.
DISCUSSION
Patients with clinically apparent PK deficiency are rare in the Western-European population. Although calculating the probability of the events as they happened to the first Danish family will be difficult, we think this is a very rare and unlucky event indeed. Not only did the son from the first marriage inherit both affected alleles, but in addition the mother remarried with a carrier of PK deficiency and the daughter born from this marriage again inherited two affected alleles, which proved fatal. The appearance of a second unrelated Danish family at the same time, again with two affected siblings and identical mutations, is coincidental, but raises questions concerning the prevalence of these mutations in Denmark. Although the nt 1529 mutation represents 45% of the diseased alleles in non-gypsy whites4,26 and probably is the most prevalent mutation in Western Europe,31 32 both mutations were not detected in a control group of 100 healthy individuals.
Within the two families, the G1091 to A mutation was present in the heterozygous, homozygous, and compound heterozygous form. No other sequence variants were found in the patients heterozygous for the G1091 to A mutation other than the polymorphisms (vide infra). Patients in the two families who are heterozygous for the G1091 to A mutation all have decreased PK activity. When the nt 1091 mutation is inherited with the nt 1529 mutation on the other allele, severe PK deficiency and clinical symptoms occur (proband and two siblings in family 2). The patient with the nt 1091 mutation present on both alleles was clinically severely affected, PK-deficient, and died. Thus, the G-to-A change at position nt 1091 correlates with the disease. In addition, the substituted amino acids are phylogenetically conserved, indicating that they are important for the function of the enzyme. Taking all these findings into consideration, we conclude that the G-to-A change at position nt 1091 causes PK deficiency.
We used four polymorphisms to study the haplotype of the described mutations.15-18 In accordance with previous studies, the nt 1529 mutation is linked to nt 1705-C and 14 ATT repeats in intron 11, supporting the theory of a common ancestor gene for this mutation. We find that the nt 1529 is linked to nt 1738-T and a thymine at nt 51 in intron 5, substantiating this hypothesis. The nt 1091 mutation was linked in all affected individuals to nt 1705-A, nt 1738-C, 12 ATT repeats, and a cytosine at nt 51 in intron 5.
Enzymatic studies and gel filtration experiments of PK from our patients did not demonstrate any abnormality other than the reduced activity. There may be several reasons for this. First, it is possible that the mutant enzymes do not differ in their kinetic properties. Many mutations present in compound heterozygous or homozygous form have reduced activity, but normal responses in kinetic studies.4 Interestingly, even identical mutations can exhibit different kinetic profiles. Moreover, the tetrameric structure of PK makes interpretation of gel filtration data difficult, because several hybrid proteins can be present. Secondly, interference of donor cells in the compound heterozygous patients, differences in enzymatic behavior in vivo and in vitro, unstability of mutant enzymes, and overexpression of M2PK might influence results.27 33
To obtain more insight in the pathogenesis of the nt 1091 and nt 1529 mutations, we constructed the three-dimensional model of human RBC PK, using the known crystal structure of cat PKM as a scaffold. Previous studies in other species have indicated that the precise conformation of the area of the molecule that is mutated in this study may be critical, not only in respect to proper alignment of residues in the active site, but also for allosteric transitions and binding of ADP and FDP.8,30 Walker et al30 have proposed the existence of two pockets at the interface of domains A and C: the Arg 42-pocket (in humans Arg 86) that is important for ADP binding and the residue 466-pocket (in humans Arg 510), which is possibly important for binding of a second allosteric activator. These pockets are divided by the fourth α helix of domain C (Cα4), which is bound by hydrogen bonds and salt bridges to domain A by connection from Ala 399 (in domain A) to Arg 510 (in domain C), Asp 400 (domain A) to Arg 488 (domain C), and Asn 113 (domain A) to Val 506 in domain C (human numbering). In this way, a critical conformation of the interface between domains A and C is specified. The area around Gly 364 is spacious and the insertion of the aspartate is not likely to disrupt the structure of PK by a simple sterical effect. However, aspartate comes in close contact with glutamine 482, and a salt bridge that would disrupt the crucial interaction between Arg 488 and Asp 400 is likely to be established (Fig 5A). In a similar manner, substitution of Arg 510 with glutamine reduces the conformational freedom between domains A and C, because an extra connection between these domains is created by formation of a salt bridge to Thr 88. Probably the salt bridge from Gln 510 to Ala 399 will be intact, but structural changes caused by the hydrogen bond to Thr 88 will affect the energy balance in this area and influence allosteric transitions (Fig 5B). Moreover, Thr 88 is close to Arg 86, which is the proposed ADP binding residue.
We propose that the Gly 364 to Asp and Arg 510 to Gln mutations affect the interdomain structure between domains A and C and that the function of both the Arg 86 and Arg 510 pockets might be affected. Because transition from the T state to the high-affinity R state of PK requires rotation of all subunits in the tetramer by flexible hinges,34 the formation or breakage of intersubunit contacts could restrict the conformation of the active site as well.
ACKNOWLEDGMENT
The authors thank Dr A. Kahn (Paris, France) for the kind gift of a rabbit anti–L-type antibody specific for L- and R-type PK.
Address reprint requests to Wouter W. van Solinge, PhD, Eemland Hospital, Department of Clinical Chemistry, Utrechtseweg 160, 3818 ES Amersfoort, The Netherlands.