Abstract
The pathophysiology of thrombocytopenia in the syndrome of thrombocytopenia with absent radii (TAR) is not yet understood. We examined thrombopoietin (TPO) serum levels and the in vitro reactivity of platelets to TPO in five patients affected with TAR syndrome. We found elevated TPO serum levels in all patients tested, excluding a TPO production defect as cause for thrombocytopenia in TAR syndrome. In addition, we found similar expression of the TPO receptor c-Mpl on the surface of platelets from TAR patients (5 of 5) and a similar molecular weight of the receptor as compared with healthy controls (4 of 4). Platelet response to adenosine diphosphate or thrombin receptor agonist peptide SFLLRN (TRAP) was normal in TAR patients. However, in contrast to results with healthy controls we could show absence of in vitro reactivity of platelets from TAR patients to recombinant TPO as measured by testing TPO synergism to adenine diphosphate and TRAP in platelet activation. TPO induced tyrosine phosphorylation of platelet proteins was completely absent (3 of 4) or markedly decreased (1 of 4). Our results indicate that defective megakaryocytopoiesis/thrombocytopoiesis in TAR syndrome is not caused by a defect in TPO production but a lack of response to TPO in the signal transduction pathway of c-Mpl.
THROMBOCYTOPENIA WITH absent radii (TAR) is a rare congenital defect with hypomegakaryocytic thrombocytopenia and bilateral radial aplasia. Although first described in 1929,1 it was defined as a syndrome by Hall et al in 1969.2 TAR seems to be inherited in an autosomal recessive manner.3 The pathogenesis of TAR is poorly understood. Bone marrow aspirates from TAR patients show either decreased or absent megakaryocytopoiesis, indicating that the thrombocytopenia is caused by a defective platelet production.
There are inconsistent reports about “thrombopoietin-like” activity and megakaryocyte colony-stimulating activity (Mega-CSA) in the serum of TAR patients.4-7
The presence of colony forming units for megakaryocytes (CFU-Mega) in the bone marrow of TAR patients is controversial, too. Whereas some investigators did not find any growth of megakaryocytic colonies from the bone marrow of TAR patients,4,6 de Alarcon et al7 showed normal CFU-Mega counts with an abnormal colony morphology. Cells in these colonies were smaller, and the number of cells per colony was much higher than in normal CFU-Mega-derived colonies.
Platelet function seems to be normal in TAR patients,3 but there are also some reports about abnormal platelet morphology and function.8 9
In 1994 several groups purified and cloned a new hematopoietic factor: thrombopoietin (TPO).10-14 Since then, this cytokine has been shown to be the major regulator of megakaryocytopoiesis and thrombocytopoiesis. Investigations of the in vitro and in vivo effects of TPO showed that it acts as a Mega-CSF as well as a megakaryocyte maturation factor and positive regulator of platelet production in vivo.15 The receptor for TPO, the product of the proto-oncogene c-mpl,16 is expressed in the megakaryocytic lineage from progenitor cells to platelets.17 c-mpl, a cellular homologue of the v-mpl oncogene,18 was found to be a member of the cytokine-receptor superfamily16,19 that share common signal transduction pathways.20 Binding of the ligand leads to tyrosine phosphorylation of some cellular proteins in cell lines and platelets.21-23
Our aim was to elucidate a possible relationship between the TAR-related thrombocytopenia and the TPO–c-Mpl system. Here we present the first report about TPO serum levels in TAR patients and about the in vitro reactivity of platelets from TAR patients to recombinant human (rh) TPO. Our results indicate that defective megakaryocytopoiesis/thrombocytopoiesis in TAR syndrome is not caused by a defect in TPO production but by a lack of response to TPO in the signal transduction of c-Mpl.
MATERIALS AND METHODS
Patients.We examined samples from five unrelated children with TAR syndrome. The patients' characteristics are described in detail in Table 1. It is important to note that four of the patients required platelet transfusions.
Patient 1 was a 3-year-old girl with bilateral absent radii and hypoplastic, curved ulna and with platelet counts usually about 50,000 /μL. She needed three platelet transfusions during the first year of life. Aside from this, a clinically irrelevant ventricle septum defect (VSD) was stated.
Patient 2 was a 3-year-old girl with bilateral absent radii and short ulna with platelet counts usually approximately 20,000 /μL. During infections she required platelet transfusions because of bleedings from nose and mouth.
Patient 3 was a 6-week-old boy at first examination. He showed bilateral absent radii, a short ulna right, an ulna aplasia left, and a bilateral hip translocation. Because of a severe thrombocytopenia he needed platelet transfusions twice a month for the first 3 months of life. Platelet counts were between 25,000 at the time of the first examination and 72,000 at the age of 10 months.
Patient 4 was a 6-month-old baby girl. Bilateral absent radii and short ulnae, as well as a severe thrombocytopenia, lead to the diagnosis of TAR. Megakaryocytes were completely absent in a bone marrow sample from the first week of life. This sample was used for the clonogenic assay presented here.
Patient 5, a 7-year old girl, has been described in an earlier publication by us.6 She showed absent radii and short ulnae, thumb hypoplasia and shoulder-girdle involvement. In addition, a VSD was stated. In contrast with other reports about TAR-syndrome, bleeding problems continued up to now. For instance, in the last year she had problems with petechiae and bleeding with platelet counts between 10,000 and 20,000 /μL.
Materials.rhTPO was provided by Dr A. Shimosaka, Kirin Brewery (Tokyo, Japan). rh granulocyte colony-stimulating factor (G-CSF ) and rh stem cell factor (SCF) were provided by Amgen (Thousand Oaks, CA). rh granulocyte-macrophage colony-stimulating factor (GM-CSF ) and rh interleukin-3 (IL-3) were a gift from Behringwerke (Marburg, Germany), rh erythropoietin (EPO) was obtained from Boehringer Mannheim (Mannheim, Germany). Adenosine diphosphate (ADP), prostaglandin E1 (PGE1), acetyl salicylic acid, apyrase (type VIII), and bovine serum albumine (BSA) were purchased from Sigma (Deisenhofen, Germany). The thrombin receptor agonist peptide (serine-phenylalanine-leucine-leucine-arginine-asparagine, TRAP) was purchased from Bachem (Heidelberg, Germany). Monoclonal antibodies against CD62 and CD41, as well as the IgG1 isotype control were purchased from Immunotech (Hamburg, Germany), the monoclonal antibody against human c-Mpl was obtained from Genzyme (Rüsselsheim, Germany), the horse-radish peroxidase (HRP)-conjugated recombinant antiphosphotyrosine antibody RC20 was purchased from Transduction Laboratories (Lexington, KY). The reagents for the enhanced chemiluminescence (ECL) and the 3H-thymidine were obtained from Amersham (Braunschweig, Germany). Cell culture media and fetal calf serum were purchased from Life Technologies (Eggenstein, Germany).
TPO serum levels.TPO serum levels were measured in a bioassay using the murine IL-3–dependent 32D (clone 23) cells24 transfected with the human c-mpl.12 Serum samples were preabsorbed with the parental 32D (clone 23) cells to remove complement activity. For the assay, 32D/Mpl+ cells were washed free of IL-3 and plated (3,000 cells, 100 μL total volume per well) in flat-bottomed 96-well plates (Nunc, Wiesbaden, Germany) in RPMI 1640 medium supplemented with 5% heat-inactivated fetal calf serum in 100 μL serial dilutions of test sera in assay medium. After 72 hours, the microtiter cultures were pulsed with 3H-thymidine (0.5 μCi/well, specific activity 5 Ci/mmol) for another 4 hours. The radioactive uptake was determined in a liquid scintillation counter. Serial dilutions of rhTPO in preabsorbed human serum were used as standards, the concentrations of the samples were calculated from the standard curve by probit analysis. The sensitivity of the assay was 100 pg/mL.
Serum levels of IL-6, IL-11, and leukemia inhibitory factor (LIF ).Serum levels of IL-6 and IL-11 were measured in commercially available enzyme-linked immunosorbent assay (ELISA) systems (Quantikine, R&D systems, Abingdon, UK). Detection limits were 8 pg/mL for IL-6 and 30 pg/mL for IL-11. Serum levels of LIF were measured in a sandwich ELISA.25 Briefly, a monoclonal antibody (MoAb) against LIF served for capturing a polyclonal rabbit antiserum was used for detection. The detection limit of this assay was 30 pg/mL.
Assay for CFUs.Bone marrow mononuclear cells (BM-MNCs, 105) obtained by density gradient centrifugation with Ficoll were cultured in duplicates in 1 mL aliquots in a semi-solid medium containing 0.7% methyl cellulose (Methocel A4C, WAK-Chemie, Bad Homburg, Germany), 30% human fresh frozen plasma (Blood Bank, Medical School Hannover, Germany), 0.5 × 10−9 mol/L 2-mercaptoethanol in Iscove's modified Dulbecco's medium in 35-mm culture dishes. Hematopoietic growth factors were added as specified in the Results section. The cultures were incubated at 37°C in an atmosphere of 5% CO2 and 100% humidity for 14 days. After this time, colonies were analyzed and counted in an inversion microscope. Megakaryocytic colonies were picked, spinned on slides, and May-Grünwald/Giemsa stained for verifying the megakaryocytic morphology of the cells.
Costimulation of platelets with TPO.Blood of healthy volunteers or TAR patients was obtained from an antecubital vein through a 19-gauge needle with only a light tourniquet into a plastic syringe containing trisodium citrate (10 mmol/L final concentration). Stimulation experiments were started within 15 minutes after blood collection. Experiments were usually performed with whole blood, and in some cases (platelet counts < 50,000/μL) we used platelet-rich plasma (PRP). For preparing PRP, whole blood was centrifuged at 200g for 20 minutes and the supernatant PRP was removed. All incubations were done at 37°C. Five to 10 μL aliquots of blood or PRP containing 2 × 105 to 2 × 106 platelets were added to polystyrene tubes containing 60 μL of phosphate-buffered saline (PBS; 130 mmol/L NaCl, 10 mmol/L sodium phosphate, pH 7.5) with various concentrations of rhTPO (20 ng/mL, unless indicated otherwise). After a preincubation of 5 minutes, the platelet activators ADP (final concentration 50 μmol/L) or TRAP26 (final concentration 5 μmol/L) were added. The stimulation was stopped by addition of 1 mL of a solution of 1% formaldehyde in PBS. Unstimulated platelets serving as a negative control were fixed immediately after blood collection or preparation of PRP, respectively. The samples were stored for 30 minutes on ice before they were stained for flow cytometric analysis.
Flow cytometry.Fluorescein isothiocyanate (FITC)-conjugated MoAb anti P-selectin (CD62; clone CLB-Thromb/6) as an activation-dependent antibody and phycoerythrine (PE)-conjugated anti-gpIIb/IIIa (CD41; clone P2) as a pan-platelet marker were used for determination of platelet activation. FITC- and PE-labeled isotype control antibodies (Immunotech, Hamburg, Germany) were used as control. Fixed platelets were pelleted (5 minutes, 2,000g ), washed two times in FACS-buffer (PBS supplemented with 0.1% BSA and 0,1% sodium azide and then resuspended in 40 μL of a diluted human Ig solution (10 mg/mL GammaGard; Baxter, Unterschleiβheim, Germany) for blocking the Fc-receptors. After a short preincubation 10 μL of each antibody solution was added followed by a 20-minute incubation on ice. To determine the c-Mpl expression on patients' platelets we used an anti–c-Mpl MoAb (M1,17 Genzyme): Platelets were fixed and preincubated with human Ig as described before. Cells were incubated with the M1 antibody (10 μL in the Ig-solution) or a nonspecific isotype control antibody for 30 minutes on ice. Cells were washed in FACS-buffer and then incubated with FITC-conjugated rabbit antimouse Ig (Dako, Glostrup, Denmark) according to manufacturer's instructions.
After staining, platelets were washed two times in FACS-buffer and analyzed on a FACScan flow cytometer (Becton-Dickinson). FITC fluorescence was detected with a 530/30 nm and PE fluorescence was detected with a 585/42 nm band pass filter. Light scatter and fluorescence data were obtained with gain settings in the logarithmic mode. Platelets were distinguished from other cells, debris, and “machine noise” on the basis of their scatter profile. In some cases a fluorescence threshold was set to analyze only those blood cells that had bound the PE-conjugated anti-CD41 antibody.
Platelet preparation for gel electrophoresis and immunoprecipitation.PRP from whole blood was prepared as described above. PRP was incubated with acetyl salicylic acid (2 mmol/L) for 30 minutes at room temperature. Then PGE1 (1 μmol/L) was added from a 1-mmol/L stock solution in absolute ethanol. A soft pellet was obtained by centrifugation of the PRP at 800g for 10 minutes. The pellet was resuspended in a modified HEPES-Tyrode buffer as recently described21 also containing apyrase (2 U/mL) to avoid stimulation of platelets by ADP and washed only once to minimize additional physical stress. The platelets were resuspended in the same buffer, which was recalcified containing 1 mmol/L CaCl2 at 37°C.
Gel electrophoresis and Western blotting.The stimulation of platelets was terminated by adding an equal volume of 2×-concentrated sample buffer (10% glycerol, 1% sodium dodecyl sulfate [SDS], 5% 2-mercapto-ethanol, 50 mmol/L Tris-HCl pH 6.8, 10 mmol/L EGTA and 1 mmol/L sodium orthovanadate and 0.002% bromophenol blue). SDS-gel electrophoresis of platelet proteins was run on a 7.5% polyacrylamide gel after boiling the samples for 5 minutes.
The proteins were transferred onto a nitrocellulose membrane by semidry Western blotting with a buffer containing 50 mmol/L Tris, 40 mmol/L glycine, 0.04% SDS, and 17.5% methanol for 1.5 hours at room temperature.
For blocking unoccupied binding sites the membrane was incubated in PBS-T (0.1% Tween 20 in PBS) with 1% BSA (blocking buffer). For the detection of phosphotyrosine or c-Mpl the membrane was incubated in blocking buffer with MoAb against c-Mpl (clone M1, 1:1,000) or with HRP-conjugated recombinant MoAb against phosphotyrosine (RC20, 1:2,500) overnight at 4°C with gentle motion.
In the case of c-Mpl the MoAb was removed and the membrane was washed four times with PBS-T and incubated with HRP-conjugated second antibody (diluted 1:1,000 in PBS-T) for 1 hour at room temperature with gentle motion.
After washing the blots in PBS-T four times the antibody reactions were detected by chemiluminescence with the ECL reagents (Amersham) according to the manufacturer's instructions.
Immunoprecipitation.The platelet stimulation was terminated by adding an equal amount of lysis buffer [15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF ), 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate 0.8 mg/mL leupeptin, 2% (vol/vol) Triton X-100, pH 7.4). The samples were incubated for 20 to 30 minutes on ice and afterwards the debris was pelleted at 10,000g for 20 minutes at 4°C. The supernatant was removed and precleared by adding 50 μL of 50% slurry of protein A-agarose-beads (Upstate Biotechnology, New York, NY) for 1 hour in an orbital shaker at 4°C. The beads were centrifuged at 10,000g for 10 minutes, and the supernatant was transferred into a new tube. The MoAb against human c-Mpl (Genzyme) was added and incubated for 4 hours or overnight in an orbital shaker at 4°C. Then the complex was captured adding protein A-agarose-beads (50 μL of 50% slurry) for another 2 to 4 hours.
The immune complexes were washed three times with ice-cooled immunoprecipitation-buffer (50 mmol/L Tris-Cl; pH 7.4; 1% Nonidet P-40; 150 mmol/L NaCl; 1 mmol/L EGTA; 1 mmol/L PMSF; 1 μg/mL of the protease inhibitors aprotinin, leupeptin, and pepstatin; 1 mmol/L sodium vanadate; and 1 mmol/L sodium fluoride), resuspended in an equal volume of 2×-sample buffer, and detected by Western blotting after SDS-PAGE.
RESULTS
Serum levels of TPO, IL-6, IL-11, and LIF.In healthy control persons TPO serum levels normally were not detectable in the bioassay (detection limit: 100 pg/mL). In contrast, TPO serum levels were above the detection limit in all serum samples from TAR patients with the exception of the first serum sample of patient 2 (Table 2).
Levels of other cytokines known to influence thrombocytopoiesis were evaluated in the sera of the TAR patients. Serum levels of IL-6 and LIF were undetectable in all cases (detection limits 8 respectively 30 pg /mL). In contrast IL-11 serum levels were elevated above 300 pg/mL in three out of five TAR patients (Table 2).
CFU-mega assay.Colony forming assays were performed with bone marrow cells of one patient only (no. 4). We had no consent of the other patients' parents for bone marrow evaluation and there was no clinical need for bone marrow evaluation. In the patient tested, no megakaryocytic colonies were observed after stimulation of BM-MNC with rhTPO (10 ng/mL; Table 3). For comparison, in healthy controls the median number of megakaryocytic colonies was 11 (n = 12). In contrast, the numbers of CFU-GM and BFU-E colonies grown from the TAR patients' bone marrow were elevated as compared with healthy controls (Table 3).
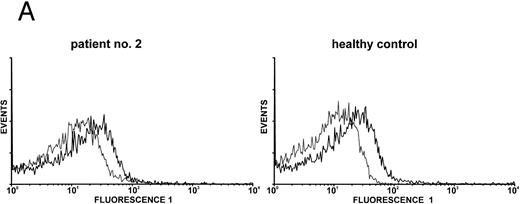
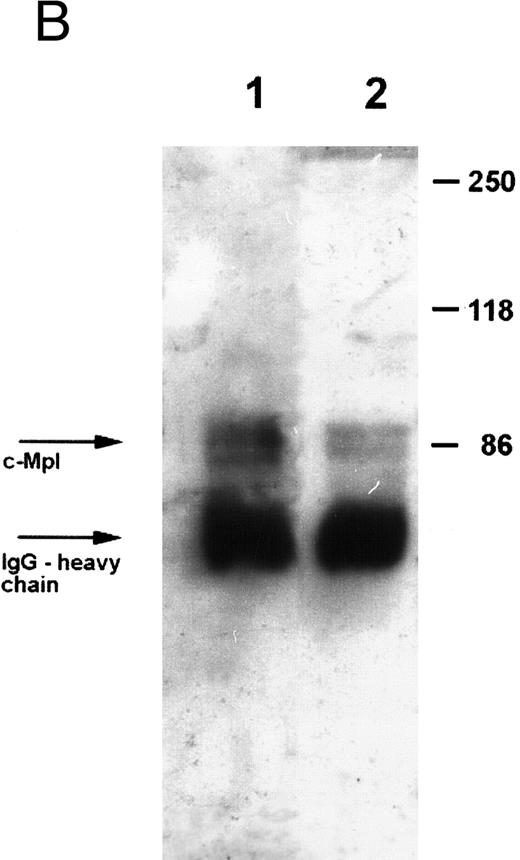
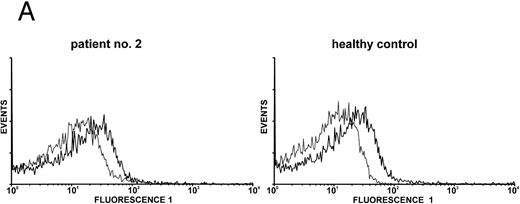
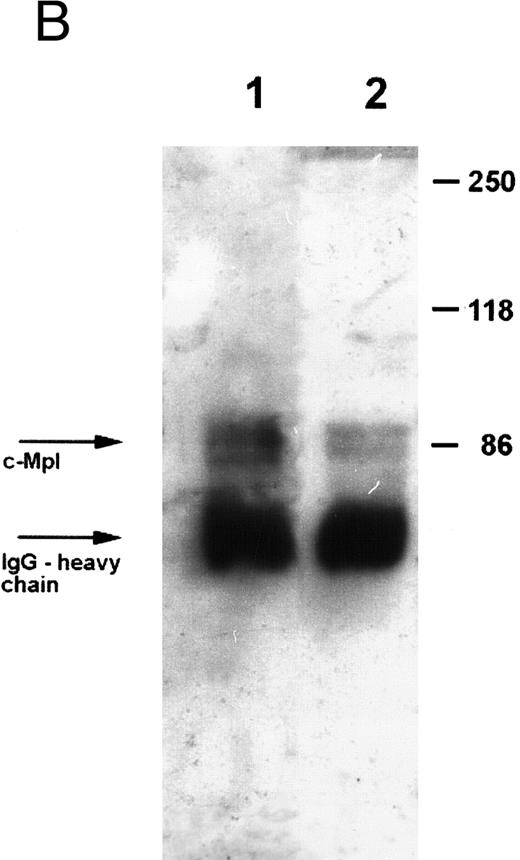
Expression of c-Mpl on platelets.Expression of the TPO receptor c-Mpl on platelets was determined by flow cytometry in all patients and by Western blotting in four out of five TAR patients (patients no. 1, 2, 3, and 5) (Fig 1). Because the receptor is presumably expressed on all cells of the megakaryocytic lineage, we used platelets as an example for c-Mpl expression in TAR patients. We could show a specific binding of a MoAb against the c-Mpl on platelets of all patients tested. Fluorescence intensity after staining with the anti–c-Mpl in platelets from the TAR patients was in the range of normal controls (Fig 1A). The molecular weight of the c-Mpl on the platelets from the TAR patients was found to be 86 kD as determined by SDS-PAGE and was not different from that of healthy controls (Fig 1B).
Expression of c-Mpl on platelets from TAR patients. (A) c-Mpl expression on the surface of platelets as measured by flow cytometry (see Materials and Methods). Gray, isotype control; black, anti c-Mpl. (B) Detection of c-Mpl in platelet lysates after immunoprecipitation and Western-blot analysis with anti-c-Mpl antibody M1 (chemoluminescence detection, see Materials and Methods). Lane 1, healthy donor; lane 2, TAR patient no. 1.
Expression of c-Mpl on platelets from TAR patients. (A) c-Mpl expression on the surface of platelets as measured by flow cytometry (see Materials and Methods). Gray, isotype control; black, anti c-Mpl. (B) Detection of c-Mpl in platelet lysates after immunoprecipitation and Western-blot analysis with anti-c-Mpl antibody M1 (chemoluminescence detection, see Materials and Methods). Lane 1, healthy donor; lane 2, TAR patient no. 1.
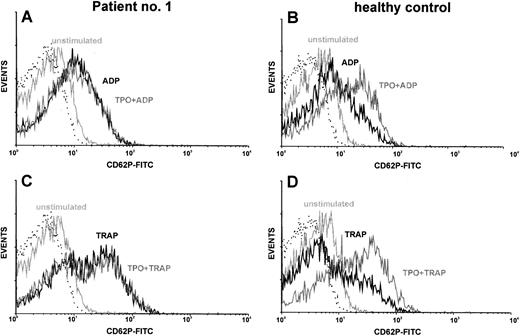
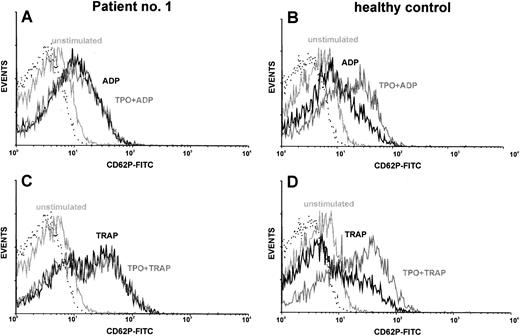
Costimulation of platelets with TPO.TPO synergizes with ADP or thrombin receptor agonists to activate platelets, as measured by increased expression of P-selectin (CD62P).27 We used this fact for in vitro testing of TPO reactivity of platelets from TAR patients. CD62P served as an activation-dependent marker. Using this method, platelets from normal individuals showed a synergism of rhTPO (5 - 10 ng/mL) with the platelet activators ADP and TRAP. Although there was a great variety in the reactivity of platelets to the activators ADP and TRAP, platelets from all TAR patients showed reactivity in the range of normal controls (Fig 2 and Table 4). However, we could not detect the expected synergism of these platelet activators with rhTPO up to concentrations of 1 μg/mL in these patients. The results of one representative experiment are shown in Fig 2. To quantitate the synergistic effect of TPO on platelet activation we calculated the ratio between the amount of CD62P-positive cells after TPO costimulation (with ADP or TRAP) and the amount of CD62P-positive cells after control stimulation without TPO (Table 4). TPO preincubation (20 ng/mL) lead to a 1.6-fold (±0.3) enhancement of ADP stimulation (50 μmol/L) or 1.4-fold (±0.3) enhancement of TRAP stimulation (5 μmol/L) in platelets of normal individuals (n = 6). In contrast, TPO stimulation indices in TAR patients were 1.0 ± 0.1 for ADP and 1.0 ± 0.2 for TRAP, respectively (Table 4).
Costimulation of platelets with rhTPO: TAR patient versus healthy control. Platelets of TAR patient no. 1 (A and C) and of a healthy control (B and D) were stimulated with either ADP (50 μmol/L; A and B) or TRAP (5 μmol/l; C and D) with or without preincubation with rhTPO (20 ng/mL). Flow cytometric analysis of platelet activation was performed using a MoAb against CD62P. CD62P expression of unstimulated platelets as well as the isotype control (dotted line) are shown in each histogram.
Costimulation of platelets with rhTPO: TAR patient versus healthy control. Platelets of TAR patient no. 1 (A and C) and of a healthy control (B and D) were stimulated with either ADP (50 μmol/L; A and B) or TRAP (5 μmol/l; C and D) with or without preincubation with rhTPO (20 ng/mL). Flow cytometric analysis of platelet activation was performed using a MoAb against CD62P. CD62P expression of unstimulated platelets as well as the isotype control (dotted line) are shown in each histogram.
Signal transduction of c-Mpl after TPO binding.Because platelets express c-Mpl they are used as a model for investigating signal transduction pathways of TPO. c-Mpl is a member of the cytokine receptor superfamily, which leads to tyrosine phosphorylation of cellular proteins.
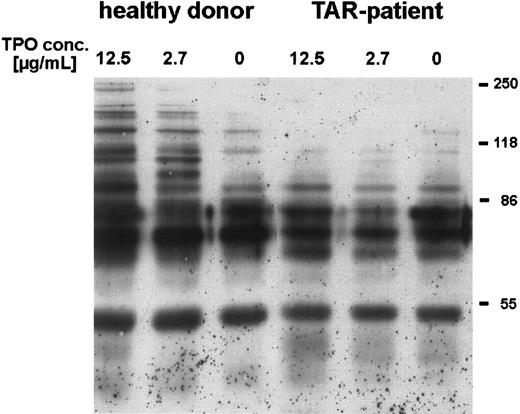
We could show that stimulation of platelets with high concentrations of rhTPO for 5 minutes induced phosphorylation of several proteins, especially at 86 kD and higher (Fig 3). Stimulation with only 50 ng/mL rhTPO lead to phosphorylation of a 110-kD protein (data not shown). In contrast, platelets from three out of four TAR patients (no. 1, 2, and 5) incubated with rhTPO concentrations up to 12 μg/mL showed no difference in tyrosine phosphorylation as compared with unstimulated cells (Fig 3), one other patient (no. 3) showed a weak phosphorylation of the 110-kD protein only after stimulation with rhTPO concentrations higher than 5 μg/mL.
Induction of tyrosine phosphorylation of platelet proteins after stimulation with rhTPO. Platelets (patient no. 5) were preincubated with different concentrations of rhTPO before lysis and Western-blot analysis with HRP-conjugated antiphosphotyrosine antibody (chemoluminescence detection) (see Materials and Methods)
Induction of tyrosine phosphorylation of platelet proteins after stimulation with rhTPO. Platelets (patient no. 5) were preincubated with different concentrations of rhTPO before lysis and Western-blot analysis with HRP-conjugated antiphosphotyrosine antibody (chemoluminescence detection) (see Materials and Methods)
DISCUSSION
The TAR syndrome is a rare congenital disorder characterized by the absence of radii on both arms and severe thrombocytopenia during the first years of life. Recent studies showed that the thrombocytopenia seen in TAR patients is caused by a defect in the megakaryocytopoiesis/thrombocytopoiesis.4 7 Different possible mechanisms for this bone marrow failure can be postulated: (1) absence of humoral or cellular stimulators of megakaryocytopoiesis, (2) absence of megakaryocytic progenitor cells, (3) cellular defects in megakaryocytic precursors (eg, receptor defects), or (4) presence of humoral or cellular inhibitors of megakaryocytopoiesis.
There are controversial reports concerning Mega-CSA or “TPO-like” activities in the sera of TAR patients. Our group6 and others4 found high Mega-CSA in the sera of TAR patients. However, de Alarcon et al7 reported Mega-CSA and “TPO-like” activities in the serum of one TAR patient, which were within the range of normal and below the high levels seen in amegakaryocytic subjects. Michalevicz et al5 even found an inhibitory effect of plasma of one TAR patient on the growth of multipotent and megakaryocytic progenitors. After the discovery of TPO and its receptor c-Mpl we were able to measure the activity of this major humoral regulator of megakaryocytopoiesis and thrombocytopoiesis in the sera of TAR patients. We found elevated levels of bioactive TPO in sera from all TAR patients tested, excluding a TPO production defect as the cause of thrombocytopenia in TAR syndrome. It is a common phenomenon that lineage specific hematopoietic growth factors are inversely regulated with the number of corresponding mature cells in peripheral blood. This has been shown for G-CSF,28 EPO,29,30 and TPO31,32 and was found to be the case in a number of congenital cytopenias as the severe congenital neutropenia, also.33 The first determination of TPO serum levels of patient 2 yielded no elevated level of this cytokine. We have no explanation of that discrepancy. However, the first determination has been made with a relatively old serum sample and we cannot exclude a loss of activity during inappropriate storage. On the other hand, variations of TPO levels in TAR syndrome might be the reason for differences in former reports.
The presence of colony forming units megakaryocyte (CFU-Mega) in the bone marrow of TAR patients is another point of controversy in the literature. Whereas most groups, like us, did not find any growth of megakaryocytic colonies from TAR patients,4,6 de Alarcon et al7 showed normal CFU-Mega counts with an abnormal colony morphology. In these colonies, cells were smaller and the number of cells per colony was much higher than in normal CFU-Mega derived colonies. In addition to the patient we tested earlier,6 we were able to test the presence of CFU-Mega in the bone marrow from only one TAR patient, a newborn baby-girl whose bone marrow was aspirated for diagnostic purposes. We could not detect any megakaryocyte colony growth after stimulation with TPO, indicating that megakaryocyte development is markedly impaired. However, more patients have to be tested to make a final statement concerning megakaryocytic colony growth in vitro. In contrast, the numbers of nonmegakaryocytic colonies (CFU-GM and BFU-E) stimulated by other growth factors (G-CSF, GM-CSF, IL-3, SCF, and EPO) were increased. This is in accordance with reports of episodes of leukocytosis and leukemoid reactions with white cell counts greater than 35,000/μL and a left shift of myeloid cells in infants with TAR syndrome.2,3 These reactions could be caused by elevated levels of other hematopoietic growth factors. In fact, we found elevated levels of IL-11 in at least three out of five patients affected with the TAR syndrome. IL-11 is a cytokine that is reported to promote the growth of myeloid and erythroid precursors.34 However, elevation of this cytokine also could have occurred independent of thrombocytopenia seen in these patients.
Platelet functions were reported to be normal in patients with TAR syndrome,3 although few patients had abnormal platelet aggregation and storage pool defects.8 9 Because of the high blood volume required in testing platelet aggregation from thrombocytopenic patients, we only measured the expression of the activation-dependent antigen CD62P on platelets after stimulation with ADP or TRAP. Under these experimental conditions, we found normal platelet activation in all TAR patients tested.
We examined the in vitro reactivity of platelets to TPO. Although TPO by itself does not cause an increase in α-granule secretion as assayed by CD62P expression, it upregulates the response to ADP or thrombin receptor agonists.27,35,36 However, TPO by itself causes significant tyrosine phosphorylations in a number of different proteins in platelets.21,35,37 In contrast, platelets from TAR patients showed no or very little reactivity to TPO: We found no synergism of TPO to the platelet activators ADP and TRAP. Furthermore there was no (3 of 4) or very weak (1 of 4) additional tyrosine phosphorylation of platelet proteins after stimulation with TPO. From this defective reactivity of platelets in response to TPO we assume a defect of all cells of the megakaryocytopoiesis including megakaryocyte progenitor cells. This defective response might be the cause of thrombocytopenia in the TAR syndrome. Signal transduction pathways downstream of c-Mpl should be essentially similar in platelets and their progenitors in the bone marrow, regardless of whether the signal transduction in platelets is a simple remnant from the megakaryocytes or whether there is a physiological role of TPO in platelet activation. Miyakawa et al21,37 showed phosphorylation of the signal transduction molecules Jak2, Shc, Stat3, and Stat5 in platelets in response to TPO. These data correspond to those obtained with cell lines normally expressing or engineered to express c-Mpl.22,23 38
We could show that c-Mpl is expressed on the surface of platelets from TAR patients in similar amounts as in healthy controls. In addition the molecular weight of the receptor corresponds to that of normal donors. However, point mutations in c-Mpl of TAR patients cannot be excluded as a cause for the defect in signal transduction. This scenario resembles very much the situation in congenital neutropenia patients in which we could show elevated G-CSF serum levels and normal G-CSF receptor expression but defective response to G-CSF in vitro and in vivo.39 Nevertheless, congenital neutropenia patients responded well to pharmacological dosages of G-CSF with an increase of neutrophils associated with a reduction of bacterial infections, suggesting that treatment of TAR patients with rhTPO might help to increase platelet numbers and improve clinical problems associated with thrombocytopenia.
ACKNOWLEDGMENT
We thank the following physicians for providing us with samples from their TAR patients: Dr H. Habenicht (Hamburg, Germany), Dr H. Jakobi, Dr H.-P. Haastert (Celle, Germany), Dr R. Burghard (Memmingen, Germany), Dr H. Schmidt, Dr U. Richter (Detmold, Germany), and Dr E. Koscielnak (Stuttgart, Germany). In addition we are grateful to Dr Michael Martin, Department of Molecular Pharmacology, MHH, for performing the IL-6 and IL-11 ELISAs and Dr Muhan Huang for performing the LIF ELISA.
Supported by Grant No. We 942/5-1 from Deutsche Forschungsgemeinschaft (DFG).
Address reprint requests to Karl Welte, MD, PhD, Abt Pädiatrische Hämatologie und Onkologie, Medizinische Hochschule Hannover, D-30623 Hannover, Germany.