Abstract
Bone marrow transplants for severe aplastic anemia were first performed in the 1970s. Transplant regimens, supportive care, and patient selection have changed substantially since then. Our objective was to determine the impact of these changes on transplant outcome. We studied 1,305 recipients of HLA-identical sibling transplants for aplastic anemia between 1976 and 1992, reported to the IBMTR by 179 centers. We compared survival of transplants performed in three intervals (1976 through 1980 [n = 186], 1981 through 1987 [n = 648], and 1988 through 1992 [n = 471]) using Cox proportional hazards regression. Five-year survival (±95% confidence interval) increased from 48% ± 7% in the 1976-1980 cohort to 66% ± 6% in the 1988-1992 cohort (P < .0001). Risks of graft-versus-host disease (GVHD) and interstitial pneumonia decreased over time, but the risk of graft failure did not. Higher long-term survival resulted primarily from decreased mortality in the first 3 months posttransplantation. Late mortality risks were low and changed little over the intervals studied. In multivariate analysis, changes in transplantation strategies accounted for most but not all of the improved outcome. Use of cyclosporine to prevent GVHD was the most important factor. Changes in patient selection did not seem to explain improved survival. Survival after HLA-identical sibling bone marrow transplantations for aplastic anemia has improved since 1976. Changes in GVHD prophylaxis account for much of this improvement. Other changes may also operate.
BONE MARROW transplantation from an HLA-identical sibling was first used to treat aplastic anemia in the 1970s.1,2 Early studies reported about 50% long-term survival.3-5 More recent studies report survival rates of 60% to 90%.6-16 It is not certain whether these higher survival rates reflect patient selection, earlier transplantation, changes in transplantation regimens, and/or supportive care or some combination of these factors.17 18
Several patient- and disease-related factors may influence outcome of transplantations for aplastic anemia, including recipient age, disease severity, numbers of pretransplantation transfusions, and donor-recipient sex-match.2,18 Major problems after transplantations are graft failure, graft-versus-host disease (GVHD), and infection. Graft failure occurs in 5% to 30% of patients.19,20 Substantial acute GVHD (grades II-IV) occurs in 30% to 50% and chronic GVHD in 25% to 60% of patients.21,22 The high incidence of graft failure is attributed to sensitization to minor histocompatibility antigens by prior blood transfusions. Strategies designed to reduce graft failure include earlier treatment, adding radiation or antithymocyte globulin (ATG) to cyclophosphamide for pretransplantation conditioning, and adding donor buffy coat cells to the graft.7,9,13,16,23,24 The main approach for preventing GVHD is posttransplantation immune suppression. Methotrexate (MTX) or cyclosporine (CSA), used alone, were common in the early 1980s. Combined MTX and CSA is now the most widely used regimen.6,11,25 26
Changes in supportive care measures since the 1970s include protective environments (laminar airflow [LAF] and high efficiency particulate air [HEPA] filtration) to decrease airborne infections, acyclovir to prevent reactivation of herpes viruses, cotrimoxazole to prevent pneumocystis pneumonia, bacterial prophylaxis with systemic antibiotics, intravenous immune globulins to decrease viral and other infections, and growth factors to accelerate bone marrow recovery.27-34
The purpose of this study was to determine whether outcome of transplantations for aplastic anemia improved from 1976 to 1992 and, if so, to determine whether improvement resulted from changes in patient selection or changes in transplantation technique or both.
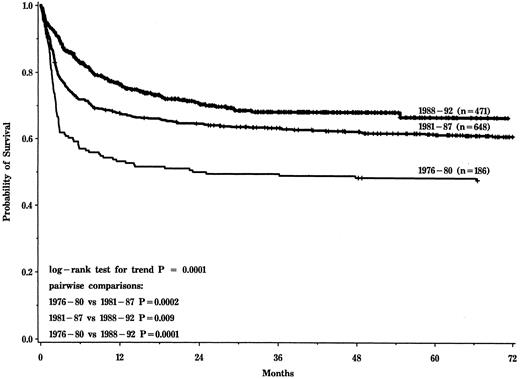
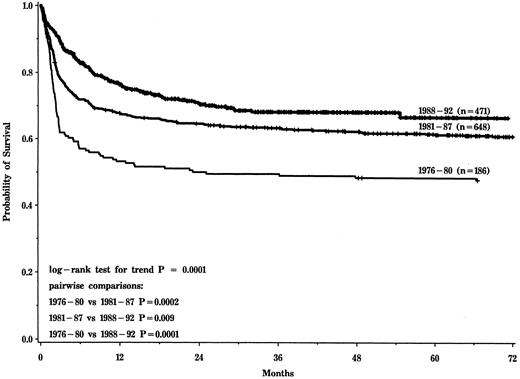
Probability of survival after HLA-identical sibling transplantation for aplastic anemia for cohorts transplanted in 1976-1980, 1981-1987, and 1988-1992.
Probability of survival after HLA-identical sibling transplantation for aplastic anemia for cohorts transplanted in 1976-1980, 1981-1987, and 1988-1992.
MATERIALS AND METHODS
Patients.A total of 1,577 patients receiving an HLA-identical sibling transplant for severe aplastic anemia between 1976 and 1992 were reported to the International Bone Marrow Transplant Registry (IBMTR) by 179 teams worldwide. Congenital forms of aplastic anemia were not considered. Of these patients, 1,305 had ≥2 of the following criteria for severe bone marrow failure: (1) a granulocyte count of less than 0.5 × 109/L; (2) a platelet count of less than 20 × 109/L; and (3) a hemoglobin level of less than 80 g/L or a hematocrit level of less than 20% or a reticulocyte count of less than 50 × 109/L. Patients were considered eligible if they met ≥2 criteria at time of diagnosis and/or pretransplantation. Two hundred and seventy-two patients were excluded, 131 because of insufficient data regarding pretransplantation hematologic parameters and 141 because they met only one or none of these criteria.
Patient-, disease-, and transplant-related characteristics of the 1,305 patients studied are shown in Table 1.
Outcomes.The primary study endpoint was survival. Graft failure was analyzed in patients surviving ≥21 days posttransplantation using published criteria.20 Acute GVHD (grade II-IV) was defined using published criteria35; patients surviving ≥21 days with engraftment were considered at risk. Chronic GVHD was defined by published criteria21; patients surviving ≥90 days posttransplantation with engraftment were considered at risk. Interstitial pneumonia was defined using published criteria36,37; etiology was determined by bronchoalveolar lavage, biopsy, or autopsy in 75% of cases.
Statistical methods.Patients were divided into three cohorts based on year of transplantation, using Cox proportional hazards regression to determine the time periods that best modeled changes in survival over time. Multiple cutpoints were examined and those giving the largest partial likelihood were selected: 1976 through 1980, 1981 through 1987, and 1988 through 1992. Within each of these intervals, survival by year of transplantation was relatively constant.
Patient-, disease-, and treatment-related variables in Table 1 were compared among the cohorts using the χ2 test for discrete variables and the Kruskall-Wallis test for continuous variables. Probabilities of graft failure, acute and chronic GVHD, interstitial pneumonia, and survival were calculated using the Kaplan-Meier estimator. A log-rank test for trend was used to determine if the three groups were ordered for increasing survival and decreasing graft failure, acute and chronic GVHD, and interstitial pneumonia.
A Cox model was fit, using a forward stepwise selection procedure to evaluate the impact of patient-, disease-, and transplant-related variables on survival.38 Data for some variables were collected only after 1985 and therefore were not available for all cohorts. The year of transplantation was included in all steps of model selection, which used a P ≤ .05 significance level for inclusion of other variables. Four models were constructed: (1) including only year of transplantation; (2) including year of transplantation and patient- and disease-related variables; (3) including year of transplantation and patient-, disease-, and treatment-related variables available for most patients; and (4) including year of transplantation and all patient-, disease-, and treatment-related variables. The last model included only patients undergoing transplantation in 1986 or later for whom all variables were available and evaluated only two time periods (1986-1987 and 1988-1992).
For all covariates in the final models, the assumption of proportional hazards was tested using time-dependent covariates. We found that year of transplantation did not meet the assumption of proportional hazards, because it affected early (≤3 months) but not late (>3 months) posttransplantation survival, ie, the relative effect was not constant at all time points. Consequently, year of transplantation was modeled as a time-dependent covariate, considering its effect on early and late survival separately. Within the two posttransplantation time periods (≤3 and >3 months posttransplantation), the proportionality assumption held. Because of multiple comparisons, we considered only P values less than .01 to be statistically significant.39
RESULTS
The three time periods that best modeled changes in posttransplantation survival were 1976-1980 (n = 186), 1981-1987 (n = 648), and 1988-1992 (n = 471). Five-year probabilities (±95% confidence interval) of survival for the three cohorts were 48% ± 7%, 61% ± 4%, and 66% ± 6%, respectively (P < .0001, Fig 1). Probabilities of graft failure, grade II-IV acute GVHD, chronic GVHD, and interstitial pneumonia are shown in Table 2. There was no significant change in the risk of graft failure over time. Risks of acute and chronic GVHD and interstitial pneumonia were significantly lower in more recent transplant recipients.
Table 1 shows patient-, disease-, and transplant-related variables for the three cohorts. Statistically significant differences over time were noted for etiology of aplastic anemia, severity of granulocytopenia, and extent and type of treatment of aplastic anemia before transplantation. Transplant characteristics significantly different among the three cohorts were pretransplantation conditioning, GVHD prophylaxis, use of donor buffy coat cells posttransplantation, and type of isolation.
A univariate Cox proportional hazards regression model showed significant differences in survival by year of transplantation with decreased risks of death in the first 3 months posttransplantation for cohorts transplanted in 1981-1987 (relative risk in first 3 months [95% confidence interval], 0.53 [0.40 to 0.71]) and in 1988-1992 (relative risk in first 3 months, 0.25 [0.18 to 0.36]) compared with the cohort undergoing transplanted in 1976-1980 (Table 3). The difference in risk of early death between the 1981-1987 and the 1988-1992 cohort was also statistically significant (P < .0001). The risk of death greater than 3 months posttransplantation did not differ by year of transplantation. A second model adjusting for patient- and disease-related characteristics showed the same association between year of transplantation and early survival (Table 3). In a third model, adjustment for treatment-related variables reduced the association between year of transplantation and survival to borderline statistical significance for the 1981-1987 cohort but not for the 1988-1992 cohort (Table 3). Consideration of treatment-related variables also led to a borderline association between year of transplantation and late risks of death for the 1988-1992 cohort. Adjusting for supportive care variables available for the period 1986-92 did not significantly alter results (Table 4). Patients transplanted in 1988-1992 had an early survival advantage with a relative risk of death of 0.49 (0.28 to 0.85; P = .01) compared with patients transplanted in 1986-1987 even after adjusting for transplantation regimen and supportive care; late risks of death were similar. Although none of the variables examined fully explained the improvement in outcome over time, several were significantly associated with posttransplantation survival, including age (P < .0001), number of pretransplantation transfusions (P < .0001), interval between diagnosis and transplantation (P = .03), donor-recipient sex-match (P = .006), donor buffy coat cells posttransplantation (P = .03), GVHD prophylaxis regimen (P = .0001), use of intravenous immune globulins (P = .0006), and cotrimoxazole (P = .004). Neither pretransplantation conditioning nor center size (number of transplantations performed annually) were statistically significant variables.
DISCUSSION
There were two major findings in this study. First, survival after HLA-identical sibling transplants increased from 48% in 1976-1980 to 66% in 1988-1992. This is consistent with reports from both single centers and the European Group for Blood and Marrow Transplantation.15,40 41 This increase reflects reduced early mortality associated with decreased risks of GVHD and interstitial pneumonia. Risk of graft failure was unchanged.
Second, although some patient- and disease-related variables changed over time, they did not explain changes in survival. Some, but not all, changes in treatment strategies contributed to improved survival, particularly GVHD prophylaxis using CSA. However, none of the variables or combination of the variables examined fully explained the improvement in survival observed during the study period.
In addition to reduced early mortality, the fully adjusted models also show somewhat higher late mortality risks in the 1988-1992 cohort, albeit of borderline significance. A possible explanation is that, for some patients, strategies that improved early survival may have simply delayed death beyond 3 months posttransplantation. For example, some patients with severe acute GVHD may now survive the early posttransplantation period only to later succumb from complications of chronic GVHD.
Changes in conditioning regimens, generally intensification to reduce graft failure, were not associated with improved survival. Decreased graft failure with more intensive conditioning has generally been offset by increased mortality from interstitial pneumonia and GVHD and a higher incidence of second cancers.20,42,43 Adding ATG to cyclophosphamide also did not improve survival, although the numbers of patients receiving ATG in this study were small. This contrasts with a recent report that conditioning with ATG and cyclophosphamide increased survival compared with historical controls treated with cyclophosphamide alone, although the benefit in that study was in decreased chronic GVHD and interstitial pneumonia rather than graft failure.16 Our study suggests that using historical controls may create a bias even after adjusting for patient-, disease-, and treatment-related factors. A randomized trial by the IBMTR comparing cyclophosphamide with or without ATG is in progress.
Cotrimoxazole prophylaxis and intravenous immune globulins were strongly associated with improved survival. In previous studies, intravenous immune globulins decreased GVHD, interstitial pneumonia, hepatic veno-occlusive disease, and transplantation-related mortality after transplantations for leukemia.44 45 This is the first time a benefit of immune globulins is reported in transplantations for aplastic anemia.
A previous IBMTR study showed that T-cell–depleted transplantations for aplastic anemia were associated with increased risks of graft failure.20 In the present study, survival of patients receiving T-cell–depleted transplants was comparable to patients receiving non — T-cell–depleted transplants with posttransplantation MTX and CSA. The number of patients receiving T-cell–depleted transplants was small (n = 43), and all but five received radiation as part of the conditioning regimen, making it difficult to draw any conclusions about the effects of T-cell depletion on outcome. Eleven of 39 evaluable patients receiving T-cell–depleted transplants had graft failure but some were rescued successfully with second transplants; 33 patients were alive at last follow-up.
None of our models fully explains changes in survival over time. Even the model adjusting for all available supportive care variables shows an independent effect of year of transplantation, with more recently treated patients having better survival. This finding suggests that there are other unmeasured factors contributing to improved survival. Possibilities include better transfusion support both pretransplantation and posttransplantation, including more frequent use of filtered, leukocyte-depleted blood products,46 new antibacterial and antifungal antibiotics, and other measures.
In summary, survival after transplantations for aplastic anemia has improved over the past 15 years. The data we review suggest that use of CSA for GVHD prophylaxis was an important factor in this progress. However, some of the improvement results from unknown factors. This underscores the need for concurrent controls to evaluate new strategies of bone marrow transplantation for aplastic anemia.
Submitted June 10, 1996; accepted March 7, 1997.
Supported by Public Health Service Grant No. PO1-CA-40053 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute of the US Department of Health and Human Services; and by grants from Alpha Therapeutic Corp; Amgen, Inc; anonymous; Astra Pharmaceutical; Basel Cancer League, Switzerland; Baxter Healthcare Corp; Bayer Corp; Biogen; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol-Myers Squibb Co; Frank G. Brotz Family Foundation; Cancer Center, Medical College of Wisconsin; CellPro, Inc; Centeon; Center for Advanced Studies in Leukemia; Chimeric Therapies, Inc; Ciba-Geigy Jubilaeums Foundation, Switzerland; Charles E. Culpeper Foundation; Eleanor Naylor Dana Charitable Trust; Eppley Foundation for Research; Free Academic Society, Basel, Switzerland; Genentech, Inc; Glaxo Wellcome Co; Hoechst Marion Roussel, Inc; Immunex Corp; Janssen Pharmaceutica; Kettering Family Foundation; Kirin Brewery Co; Robert J. Kleberg, Jr and Helen C. Kleberg Foundation; Herbert H. Kohl Charities, Inc; Eli Lilly Co Foundation; Nada and Herbert P. Mahler Charities; Milstein Family Foundation; Milwaukee Foundation/Elsa Schoeneich Research Fund; Samuel Roberts Noble Foundation; Ortho Biotech Corp; John Oster Family Foundation; Elsa U. Pardee Foundation; Jane and Lloyd Pettit Foundation; Alirio Pfiffer Bone Marrow Transplant Support Association; Pfizer, Inc; Pharmacia and Upjohn; RGK Foundation; Sandoz Pharmaceuticals; Schering-Plough International; Walter Schroeder Foundation; Searle; Stackner Family Foundation; Starr Foundation; Joan and Jack Stein Charities; Swiss National Science Foundation; and Wyeth-Ayerst Laboratories.
Address reprint requests to Mary M. Horowitz, MD, MS, International Bone Marrow Transplant Registry, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226.
REFERENCES
Author notes
Deceased.