Abstract
Various functions of human phagocytes are modulated by nitric oxide (NO). We transfected the human U937 monoblastoid cell line with an expression vector containing human endothelial NO synthase (eNOS) or murine inducible NOS (iNOS) cDNA to study the regulatory role of NO without the nonspecific effects associated with exogenous NO sources. Western blot confirmed expression of eNOS or iNOS in respectively transfected cells, but not in naive or empty-vector transfected cells. Transfectants expressing iNOS, a calcium-independent enzyme, but not eNOS, a calcium-dependent enzyme, spontaneously produced NO (P < .001). The NO release from iNOS-transfected cells, as measured by nitrite and nitrate accumulation and by cyclic guanosine monophosphate (cGMP) increases in rat reporter cells, was inhibitable (P < .01 for both) with Nω-methyl-L-arginine (L-NMA), a NOS inhibitor. The eNOS transfectants were shown to contain functional enzyme by the conversion of L-arginine to L-citrulline in fractionated cells (P = .0001) and by exposing intact cells to calcium ionophore using the cGMP reporter cell assay (P = .0001). After differentiation with phorbol-12-myristate-13-acetate (PMA), iNOS transfectants produced more tumor necrosis factor-α (TNF-α) (124.9 ± 25.4 pg/5 × 105 cells per 24 hours) than did empty-vector transfected cells (21.9 ± 1.9 pg/5 × 105 cells per 24 hours; P = .02). This effect was inhibited by 500 μmol/L L-NMA (54.4 ± 3.1 pg/5 × 105 cells per 24 hours; P = .05). However, in the presence of high concentrations of lipopolysaccharide (1 μg/mL), which further increased NO production in iNOS transfected cells (P = .044), TNF-α production was similar comparing PMA-differentiated iNOS and empty-vector transfectants (12.2 ± 0.8 and 13.1 ± 1.7 ng/5 × 105 cells per 24 hours, respectively; P = .5). The results show that under certain conditions endogenously produced NO can upregulate TNF-α production in human phagocytes.
NITRIC OXIDE (NO), produced by NO synthases (NOS) from L-arginine, is a free radical messenger1 that serves as a neurotransmitter,2 and vasodilator.3,4 NO also has an important, yet incompletely defined, role in host defense.5 Rodent phagocytes exposed to inflammatory mediators or pathogens produce large amounts of NO.6-11 In contrast, human phagocytes exposed to similar inflammatory mediators produce little or no NO.11-15 In the rodent, but probably not the human immune system, NO is an effector of antimicrobial and antitumor activity.6-15 For example, interferon-γ (IFN-γ) induces resistance to intracellular pathogens in both murine and human phagocytes. However, this effect of IFN-γ in mice is NO-dependent, whereas in humans, it appears unrelated to NO.15,16
Although human neutrophils, monocytes, and macrophages do not appear to use NO as a toxic metabolite in host defense, these cells do contain NO-sensitive guanylate cyclase and respond in a paracrine manner to NO signals.17,18 A role for NO as a regulator of human inflammatory responses is suggested by investigations showing that NO affects tumor necrosis factor-α (TNF-α) synthesis in neutrophils18 and peripheral blood mononuclear cells (PBMC).19,20 However, all of the studies on this question have, by necessity, used NO gas or NO donors,18-20 as the induction of biologically relevant endogenous NO production in human phagocytes in vitro has not been readily achieved.11-16 Further, the reported effects of NO on TNF-α production include increases, decreases, and no change, varying with the stimulus used to induce cytokine synthesis, the compounds chosen to donate NO, and the type of cell preparation examined.18-24
In this investigation, we transfected the human U937 monocytic cell line with eukaryotic expression vectors containing either human endothelial NOS (eNOS) or murine macrophage inducible NOS (iNOS) cDNA so that we might study the immune regulatory role of NO without the need for exogenous sources of NO. The effect of endogenous NO on TNF-α production was studied because exogenous NO has produced seemingly conflicting reports.18-24 Furthermore, the answer to this question has potential implications for new therapies undergoing clinical evaluation, such as the use of NOS inhibitors to raise blood pressure in septic shock5 and inhaled NO to treat hypoxemia in the adult respiratory distress syndrome (ARDS).25 Transfected U937 cells expressed functional eNOS or iNOS, respectively. Our findings suggest that these transfectants may be useful in exploring the regulatory role of NO in human phagocytes and in studying the potential of NOS gene therapy in malignancy and chronic infections.
MATERIALS AND METHODS
Culture Media and Reagents
All cell culture media and supplements were obtained from Biofluids Inc (Rockville, MD); all restriction endonucleases were obtained from United States Biochemical Corp (Cleveland, OH). HEPES was obtained from ICN Biochemicals (Cleveland, OH). Tetrahydrobiopterin was from Research Biochemicals, Inc (Natrick, MA). Flavin-adenine dinucleotide (FAD) and superoxide dismutase were from Calbiochem (La Jolla, CA). [14C]L-arginine was from Amersham (Arlington, Heights, IL). Other chemicals were from Sigma (St Louis, MO), unless otherwise stated.
Construction of NOS Expression Vectors
A 4-kb cDNA encoding the 1294 amino acids of human eNOS, at the EcoR I site of pUC19, was generously provided by Dr Kenneth D. Bloch.26 A Hind III/Not I fragment containing the entire open reading frame of eNOS was excised from the plasmid and ligated into the eukaryotic expression vector pCEP4 (Invitrogen, San Diego, CA). The mouse macrophage iNOS expression construct was subcloned into the same expression vector, pCEP4, in which both the 5′ and 3′ untranslated regions of the iNOS cDNA had been deleted.27 NOS gene transcription in the pCEP4 expression vector is driven by the human cytomegalovirus (CMV) immediate early enhancer/promoter of pCEP4. Although inherently activated in many cells types, the human CMV promoter also has a transcriptional activator protein binding site, AP-1, making it phorbol-12-myristate-13-acetate (PMA) and lipopolysaccharide (LPS) responsive in certain cells.28 29 The pCEP4 plasmid also contains a hygromycin-resistance gene that allows selection of stable transfectants. Both of the NOS expression vectors were partially sequenced to confirm the correct sequences and orientations.
DNA Transfection
U937 cells (American Type Culture Collection, Rockville, MD) were maintained in RPMI 1640 complete medium containing HEPES (25 mmol/L), 10% low endotoxin fetal calf serum (FCS), L-glutamine (2 mmol/L), penicillin (100 U/mL), and streptomycin (50 μg/mL). The expression vectors containing either human eNOS or murine iNOS were transfected into cells by electroporation as described.27 The parent pCEP4 vector not containing either NOS gene served as a control. Briefly, U937 cells were suspended at a density of 2 × 105 to 5 × 105 cells/mL in RPMI 1640 complete medium and cultured overnight. Cells (1 × 107) were then resuspended in 0.3 mL RPMI 1640 medium containing 25 mmol/L HEPES, and 10% FCS, but no L-glutamine or antibiotics. Plasmid DNA was added to the cell suspension in a cuvette (GIBCO-BRL, Gaithersburg, MD) and an electric pulse (240 V, 1180 μF ) was applied with a Cell-Porator (GIBCO-BRL). Cells were then cultured in RPMI 1640 complete medium at a density of 5 × 105 cells/mL for 3 days. This was followed by resuspension of the cells in RPMI 1640 complete medium, containing 275 U/mL of hygromycin B (Calbiochem, San Diego, CA), to select stable transfectants. Cells transfected with iNOS in this manner gradually lost their ability to produce NO over 12 to 16 weeks of continuous passage, even in the presence of hygromycin.
Western Blotting
To demonstrate the expression of NOS gene products, transfected U937 cells were collected by centrifugation and washed with phosphate-buffered saline (PBS) (GIBCO-BRL). The pellet was mixed with lysis solution [125 mmol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), and 5% glycerol] that contained protease inhibitors (one protease inhibitor set/100 mL of lysis solution; Boehringer, Mannheim, Germany); the mixture was boiled for 10 minutes. The samples were then centrifuged for 5 minutes to remove insoluble debris. Protein concentration was determined using the bicinchoninic acid method (Pierce, Rockford, IL). Samples (20 μg of protein/lane) were applied to an 8% SDS-polyacrylamide gel (NOVEX, San Diego, CA) and subjected to electrophoretic separation. Human endothelial cell lysate, and lysate from mouse macrophage RAW 264.7 cells induced with LPS and IFN-γ (obtained from Transduction Laboratories, Lexington, KY), served as positive controls for eNOS and iNOS, respectively. Separated protein was electrically transferred to a polyvinyldifluoridine membrane (NOVEX, San Diego, CA). The blot was blocked with 5% nonfat dry milk in PBS overnight at 4°C. The primary antibodies (mouse monoclonal antibody to eNOS or iNOS, both diluted 1:250 with 5% nonfat dry milk in PBS) and second antibody (horseradish peroxidase-coupled goat antimouse IgG diluted 1:2,000 with 5% nonfat dry milk in PBS) were obtained from Transduction Laboratories. The protein bands were detected using a chemiluminescence reagent (DuPont NEN, Boston, MA).
Determination of NOS Activity
A nitrite (NO−2 ) and nitrate (NO−3 ) assay, based on the Griess reaction, was performed as described previously to detect NO production by intact cells.12 Transfected U937 cells were washed with Hanks' balanced salt solution without calcium or magnesium [HBSS(−)] three times and resuspended (1 × 106 cells/mL) in NO−3 salt-free, α-modified minimum essential medium (AMEM), containing 10% FCS. These undifferentiated U937 cells were then incubated in six-well plates with or without the NOS inhibitor, Nω-methyl-L-arginine (L-NMA; Calbiochem). Cell-free medium was collected at 12, 24, 48, and 62 hours for NO−2 and NO−3 determinations. Other U937 transfectants (5 × 106 cells/mL) were first differentiated with 100 nmol/L PMA for 2 days. Plate wells were washed with HBSS(−) three times by transferring supernatants and washings into labeled tubes, spinning down the cells, resuspending, and finally transferring them back into their original wells. Adherent cells remaining in the wells were kept moist with a half volume of medium until the process was completed. The cells were then incubated in NO−3 salt-free AMEM with or without L-NMA, for 24 hours before measuring NO−2 and NO−3 .
In a separate set of experiments, we evaluated the effects of PMA (100 nmol/L) and LPS (1 μg/mL) on NO−2 and NO−3 production. This experiment, unlike those above, required direct comparisons between undifferentiated and PMA-differentiated U937 cells. As PMA both increases cell adhesion and decreases cell proliferation, the following procedure was used to control for cell number. Transfected U937 cells (5 × 107 to 1 × 108 per 180 cm2 flask) were incubated for 2 days in AMEM containing 10% FCS without and with 100 nmol/L PMA. Adhered cells were removed by incubation with 1 mmol/L EDTA in PBS for 30 minutes at 37°C. Nonadhered cells were exposed to the same treatment. Cells were then washed three times with HBSS(−), recounted, and plated into six-well plates at 1 × 107 cells per well in NO−3 salt-free AMEM (2 mL) without or with LPS (1 μg/mL). Cell-free supernatant was collected at 24 hours for NO−2 and NO−3 determinations.
Calcium-dependent NOS activity in fractionated eNOS transfectants was measured by the conversion of [14C]L-arginine to [14C]L-citrulline, as previously described.12 Cell lysates prepared by homogenization (400 μL) were combined with HEPES buffer (200 μL) to produce a reaction mixture containing 1.25 mmol/L CaCl2 , 1.0 mmol/L MgCl2 , 1.0 mmol/L nicotinamide adenine dinucleotide-reduced (NADPH), 80 μmol/L flavin adenine dinucleotide (FAD), 20 μmol/L tetrahydrobiopterin, 13 μg/mL calmodulin, 100 U/mL superoxide dismutase, 12 μmol/L L-arginine, 1 mmol/L L-citrulline, 60 mmol/L L-valine and 1.23 μCi/mL of [14C]L-arginine as final concentrations. Conversion was conducted at 37°C for 60 minutes. The specificity of the conversion assay was demonstrated by adding 250 μmol/L L-NMA, or leaving CaCl2 out of the reaction mixture.
In an alternative method for measuring NO release from intact transfectants, RFL-6 rat fetal lung fibroblasts (American Type Culture Collection, Rockville, MD), cultured to confluency in six-well plates, were used as reporter cells by measuring NO-stimulated cyclic guanosine monophosphate (cGMP) production according to the method described by Ishii et al.30 Briefly, RFL-6 cells were washed twice with 2 mL of Locke's buffer, containing 0.3 mmol/L 3-isobutyl-1-methyl-xanthine and were then equilibrated with the same buffer (2 mL) at 37°C for 30 minutes. In one set of experiments, the Locke's buffer used in the incubation step for some wells contained the calcium ionophore A23187. On addition of U937 cells (see below), the final concentration of A23187 was 1 μmol/L. The composition of the Locke's buffer was as follows: NaCl 154.0 mmol/L; KCl 5.6 mmol/L; CaCl 2 2.0 mmol/L; MgCl2 1.0 mmol/L; NaHCO3 3.6 mmol/L; glucose 5.6 mmol/L; L-arginine 0.05 mmol/L; and HEPES 10.0 mmol/L; pH 7.4. Transfected U937 cells (5 × 106) in 200 μL Locke's buffer, with or without 500 μmol/L of L-NMA, were then added to the RFL-6 cells in the presence of 100 U/mL of superoxide dismutase. After a 10-minute incubation, the reaction was terminated by aspirating the buffer, adding 1.5 mL of ice-cold 65% ethanol, and placing the cells on ice for 20 minutes. Supernatant was collected from the cells by centrifugation at 2,000g for 15 minutes, and cGMP was then quantitated by use of a cGMP enzyme immunoassay kit in accordance with the manufacturer's instructions (Amersham, Arlington Heights, OH).
TNF-α Production in Transfected U937 Cells After PMA Differentiation
For measuring TNF-α production in transfected U937 cells, 5 × 105 cells suspended in 1.5 mL of RPMI 1640 complete medium, containing 100 nmol/L PMA, were incubated in 24-well plates for 2 days. The differentiated cells were washed with HBSS(−) twice, and placed in 1 mL of RPMI 1640 complete medium in the presence or absence of LPS (1 μg/mL). After the cells were cultured for an additional 24 hours, cell-free supernatant was assayed for the presence of TNF-α by use of an enzyme-linked immunosorbent assay (R & D System, Minneapolis, MN) in accordance with the manufacturer's instructions.
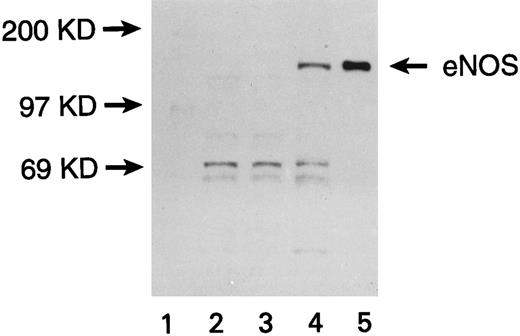
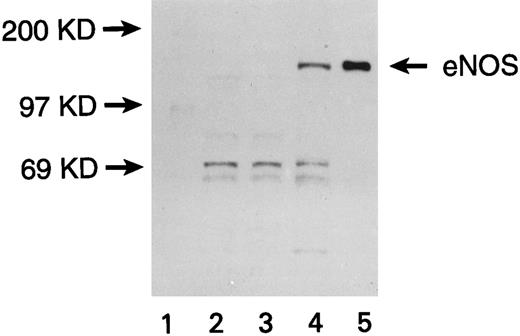
Expression of eNOS enzyme in transfected U937 cells. Western blot with a monoclonal antibody against human endothelial NOS (eNOS) is shown. Lanes are numbered as follows: (1) M.W. markers; (2) untransfected U937 cells; (3) U937 cells transfected with the pCEP4 vector without the eNOS gene; (4) U937 cells transfected with the pCEP4 vector containing the eNOS gene; (5) human endothelial cell lysate (positive control).
Expression of eNOS enzyme in transfected U937 cells. Western blot with a monoclonal antibody against human endothelial NOS (eNOS) is shown. Lanes are numbered as follows: (1) M.W. markers; (2) untransfected U937 cells; (3) U937 cells transfected with the pCEP4 vector without the eNOS gene; (4) U937 cells transfected with the pCEP4 vector containing the eNOS gene; (5) human endothelial cell lysate (positive control).
Statistics
All P values are two-sided. All summary data is the mean ± standard error (SE). The accumulation of NO−2 and NO−3 under different experimental conditions over time was compared by use of a repeated measures analysis of variance, with Bonferroni-corrected posthoc tests to compare various curves over the entire 62 hours. To simultaneously assess the influence of multiple factors, multiway analysis of variance was used, possibly with Bonferroni-corrected posthoc tests to compare selected pairs of conditions (eg, two factors of interest: different transfectant groups and presence/absence of L-NMA). In analyses involving multiple conditions in the same set of experiments, Bonferroni-adjusted paired t-tests were used to compare multiple treatments with the control group (eg, empty vector transfectants) and possibly with each other (thus correcting for the multiple-comparisons problem). To assess the influence of a single factor, such as L-NMA, in conjunction with various treatments, simple paired t-tests were used. In cases where the data did not come from the same set of experiments (eg, TNF production by PMA-differentiated U937 cells with and without addition of LPS), standard two-sample t-tests were used.
RESULTS
Expression of NOS Proteins in Transfected U937 Cells
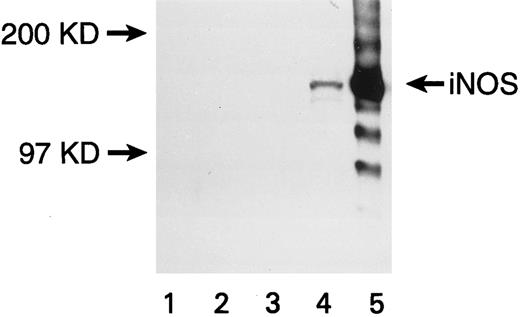
U937 cells transfected with the eNOS or iNOS genes expressed the respective proteins, as confirmed by Western blots. A band of 140 kD for eNOS (Fig 1) and a band of 130 kD for iNOS (Fig 2) were recognized by specific anti-NOS monoclonal antibodies. No NOS protein was detected in untransfected or empty-vector transfected U937 cells. The transfectants were morphologically identical to the parent cell line. The iNOS, eNOS, and empty-vector transfected cells had similar proliferation kinetics in the absence and presence of L-NMA (Table 1; P > .5). Although PMA decreased cell number (P = .001), this effect and cell proliferation in the absence of PMA was similar comparing iNOS and empty vector transfectants (Table 2; P = .80).
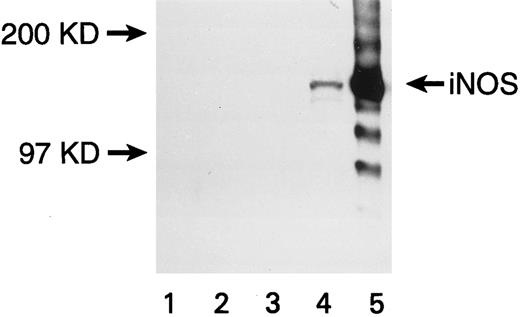
Expression of iNOS enzyme in transfected U937 cells. Western blot with a monoclonal antibody against mouse macrophage inducible NOS (iNOS) is shown. Lanes are numbered as follows: (1) M.W. markers; (2) untransfected U937 cells; (3) U937 cells transfected with the pCEP4 vector without the iNOS gene; (4) U937 cells transfected with the pCEP4 vector containing the iNOS gene; (5) lysate from murine macrophage RAW 264.7 cells induced with LPS and IFN-γ (positive control).
Expression of iNOS enzyme in transfected U937 cells. Western blot with a monoclonal antibody against mouse macrophage inducible NOS (iNOS) is shown. Lanes are numbered as follows: (1) M.W. markers; (2) untransfected U937 cells; (3) U937 cells transfected with the pCEP4 vector without the iNOS gene; (4) U937 cells transfected with the pCEP4 vector containing the iNOS gene; (5) lysate from murine macrophage RAW 264.7 cells induced with LPS and IFN-γ (positive control).
NO Production by U937 Cells
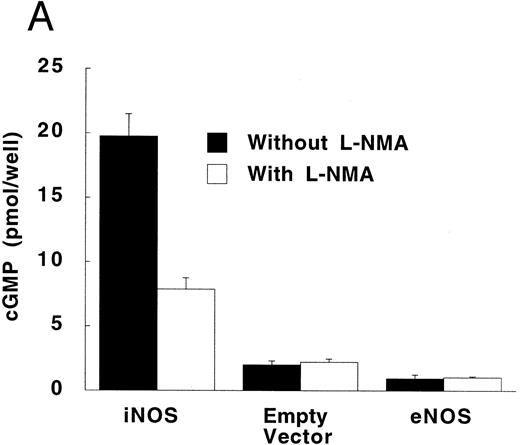
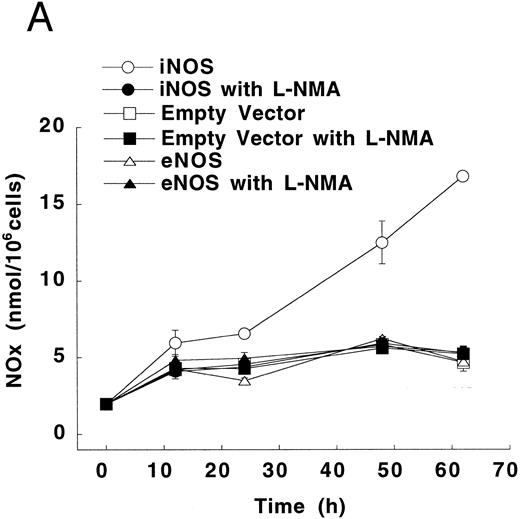
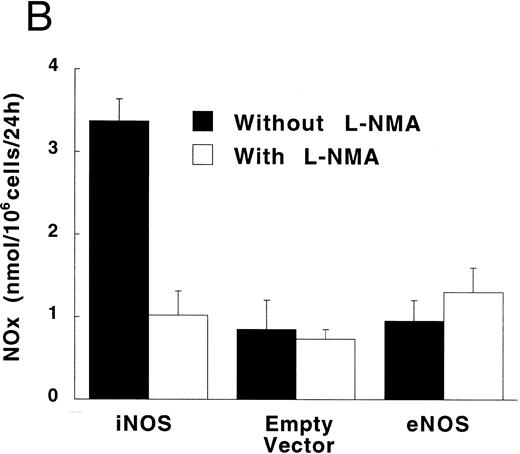
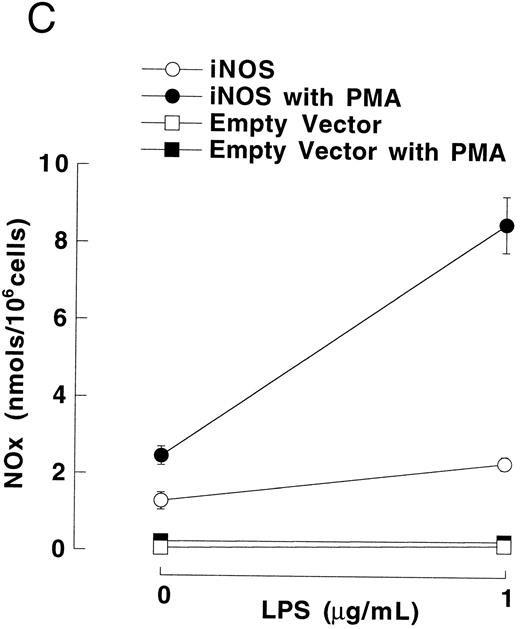
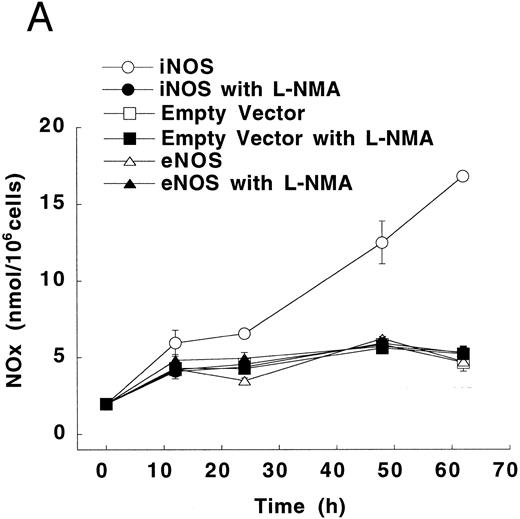
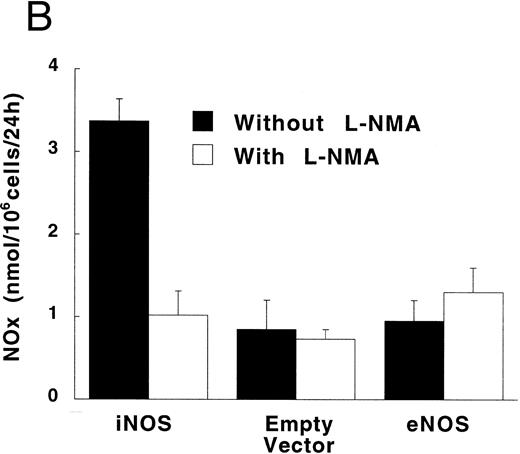
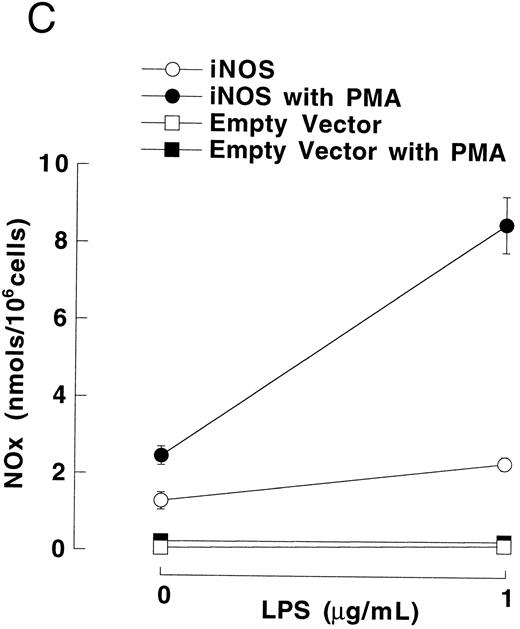
Figure 3A shows that cultured iNOS transfected U937 cells accumulated more NO−2 and NO−3 , metabolic products of NO, over 62 hours than cultures of empty vector transfected cells (P = .001). This NO production was inhibited by 1 mmol/L L-NMA (P = .001). Culture supernatants of eNOS and empty-vector transfected cells without or with L-NMA (1 mmol/L) had NO−2 and NO−3 concentrations that were similar and not different from iNOS transfected cells cultured in the presence of L-NMA (P > .9). In a separate experiment, it was found that PMA-differentiation, which was used to induce TNF-α production in U937 cells, did not alter these relationships between the different transfectants (Fig 3B). PMA-differentiated, iNOS transfected U937 cells accumulated more NO−2 and NO−3 over 24 hours of incubation than PMA-treated empty vector and eNOS transfected cells (Fig 3B; P = .01). This NO−2 and NO−3 accumulation in PMA-differentiated, iNOS transfectants was also inhibited (P = .01) by L-NMA (500 μmol/L). In an additional experiment (see Materials and Methods), we examined the effects of PMA (100 nmol/L) and LPS (1 μg/mL) on NO production by iNOS transfectants (Fig 3C). Again, we found that iNOS transfectants released more NO, as measured by NO−2 and NO−3 accumulation, than empty vector transfected cells (P = .001). However, in this direct comparison, both PMA (P = .008) and LPS (P = .044) were found to increase NO production by iNOS transfectants (Fig 3C). These results demonstrate that only the iNOS transfectants spontaneously produced L-NMA–inhibitable NO, and that both PMA and LPS upregulate this NO production.
NO−2 and NO−3 (NOx ) accumulation in transfected U937 cells. (A) Undifferentiated U937 cells without or with L-NMA (1 mmol/L; n = 3). (B) PMA-differentiated U937 cells without or with L-NMA (500 μmol/L). Cell-free supernatant was collected at 24 hours for measurement of NO−2 and NO−3 (n = 5). (C) Effects of PMA and LPS on NO−2 and NO−3 accumulation at 24 hours (see Materials and Methods; n = 3). All data are means ± SE.
NO−2 and NO−3 (NOx ) accumulation in transfected U937 cells. (A) Undifferentiated U937 cells without or with L-NMA (1 mmol/L; n = 3). (B) PMA-differentiated U937 cells without or with L-NMA (500 μmol/L). Cell-free supernatant was collected at 24 hours for measurement of NO−2 and NO−3 (n = 5). (C) Effects of PMA and LPS on NO−2 and NO−3 accumulation at 24 hours (see Materials and Methods; n = 3). All data are means ± SE.
The conversion of [14C] L-arginine to [14C] L-citrulline in transfected U937 cells. NOS cofactors were added to lysates of eNOS transfected, empty vector transfected, and untransfected U937 cells (see Materials and Methods). L-NMA (250 μmol/L) was added to or CaCl2 was omitted from some lysate preparations as indicated. Data are means ± SE (n = 3).
The conversion of [14C] L-arginine to [14C] L-citrulline in transfected U937 cells. NOS cofactors were added to lysates of eNOS transfected, empty vector transfected, and untransfected U937 cells (see Materials and Methods). L-NMA (250 μmol/L) was added to or CaCl2 was omitted from some lysate preparations as indicated. Data are means ± SE (n = 3).
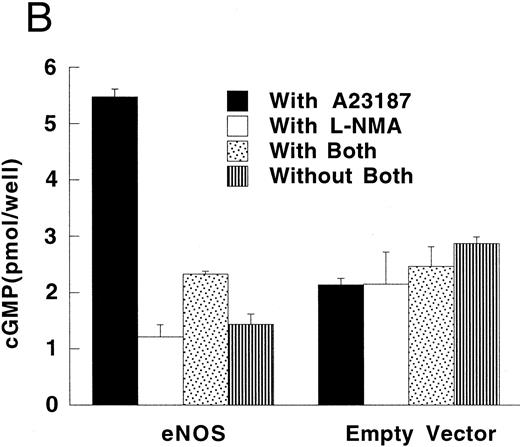
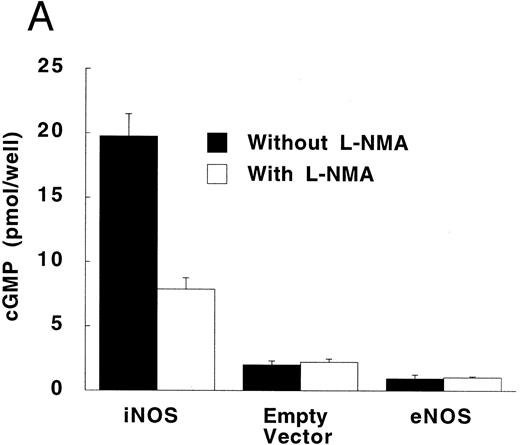
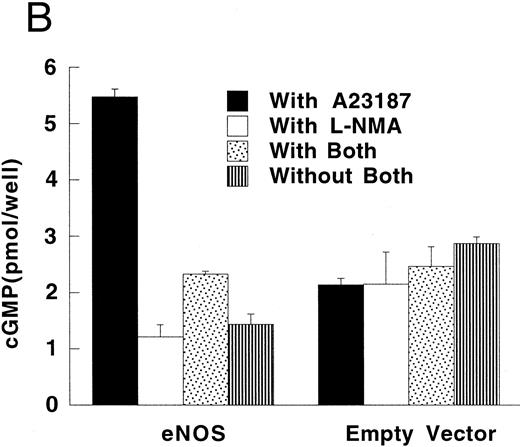
Production of cGMP in RFL-6 reporter cells coincubated with transfected U937 cells. (A) RFL-6 cells were incubated with U937 cells transfected with the iNOS gene, the empty vector, or the eNOS gene, without or with L-NMA (500 μmol/L) as shown (see Materials and Methods). (B) RFL-6 cells were incubated with eNOS or empty vector transfected cells in the presence or absence of A2318 (1 μmol/L), a calcium ionophore, and without or with L-NMA (500 μmol/L). Data are means ± SE (n = 3 for both experiments).
Production of cGMP in RFL-6 reporter cells coincubated with transfected U937 cells. (A) RFL-6 cells were incubated with U937 cells transfected with the iNOS gene, the empty vector, or the eNOS gene, without or with L-NMA (500 μmol/L) as shown (see Materials and Methods). (B) RFL-6 cells were incubated with eNOS or empty vector transfected cells in the presence or absence of A2318 (1 μmol/L), a calcium ionophore, and without or with L-NMA (500 μmol/L). Data are means ± SE (n = 3 for both experiments).
To investigate whether eNOS transfected U937 cells expressed a protein with functional NO synthetic activity, cell lysates were prepared. NOS activity was measured by the conversion of radiolabeled L-arginine to L-citrulline in the presence of NOS cofactors (calcium, calmodulin, NADPH, tetrahydrobiopterin, and FAD). NOS activity was elevated in fractionated eNOS transfected cells, compared with empty-vector transfected cells or untransfected cells (Fig 4; P = .001). L-arginine to L-citrulline conversion in eNOS transfected cells was blocked by the addition of L-NMA (250 μmol/L), a NOS inhibitor, or by leaving CaCl2 out of the reaction mixture (P < .001 for both), thus demonstrating that it represented calcium-dependent NOS activity. Conversely, neither L-NMA nor the absence of CaCl2 altered background levels of L-arginine conversion in empty vector transfected or untransfected cells.
RFL-6 rat fetal lung fibroblasts, which contain abundant amounts of soluble guanylate cyclase and produce cGMP in response to NO, were used as a sensitive reporter system to detect NO. Compared with empty vector transfectants, iNOS transfected U937 cells stimulated more cGMP production in RFL-6 cells (Fig 5A; P = .013). This increase of cGMP in RFL-6 cells was inhibited by 500 μmol/L L-NMA (P = .044). Similar to the results obtained using NO−2 and NO−3 accumulation in culture supernatants, eNOS transfected U937 cells did not release NO spontaneously as measured by cGMP production in RFL-6 cells (Fig 5A and B). However, coincubation of RFL-6 cells with eNOS, but not empty vector transfected cells in the presence of A23189 (1 μmol/L), a calcium ionophore, resulted in an increase in cGMP levels (Fig 5B; P = .0001) This increase in cGMP production was inhibited by 500 μmol/L L-NMA (P = .0002).
Effect of Endogenously Produced NO on TNF-α Secretion in Transfected U937 Cells
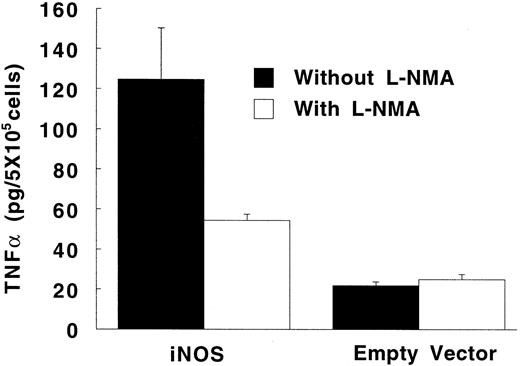
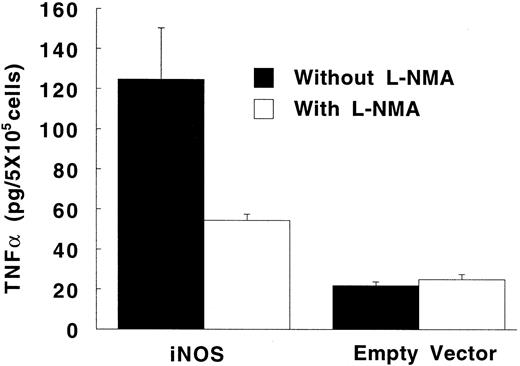
In the absence of LPS, TNF-α synthesis by PMA-differentiated U937 cells was significantly higher in iNOS transfectants than in empty vector transfected cells (Fig 6; P = .02). This upregulation of TNF-α production by endogenous NO was inhibited by 500 μmol/L of L-NMA (P = .05). Exposure to a high concentration of LPS (1 μg/mL) increased TNF-α production by PMA-differentiated U937 cells by more than 100-fold (P < .001 for both the iNOS and empty vector conditions). However, in the presence of LPS, TNF-α production was similar comparing iNOS and empty vector transfectants (12.2 ± 0.8 and 13.1 ± 1.7 ng/5 × 105 cells per 24 hours, respectively; P = .5; n = 5).
Effect of endogenously produced NO on TNF-α secretion in transfected U937 cells. PMA-differentiated cells transfected with either the iNOS gene or the empty vector were cultured in LPS-free medium without or with L-NMA (500 μmol/L) for 24 hours. Data are means ± SE (n = 4).
Effect of endogenously produced NO on TNF-α secretion in transfected U937 cells. PMA-differentiated cells transfected with either the iNOS gene or the empty vector were cultured in LPS-free medium without or with L-NMA (500 μmol/L) for 24 hours. Data are means ± SE (n = 4).
DISCUSSION
We have shown that it is possible to generate transfectants of U937 monoblastoid cells that express NOS activity. Of the two NOS transfectants, only the iNOS transfectants spontaneously accumulated NO−2 and NO−3 and stimulated cGMP formation in RFL-6 reporter cells. The eNOS transfectants expressed calcium-dependent NOS activity conditionally. Fractionated eNOS transfectants showed increased conversion of L-arginine to L-citrullin in the presence of added cofactors, including calcium, and intact eNOS transfectants released NO in the presence of a calcium ionophore, as measured by cGMP formation in RFL-6 cells. The reason why iNOS, but not eNOS transfectants, spontaneously release NO has not been fully determined. One explanation supported by our findings relates to the differences in calcium dependency of these two enzymes.1,5 Human eNOS is relatively inactive at resting levels of intracellular calcium. In contrast, murine iNOS binds calmodulin tightly and is functional at resting levels of intracellular calcium. Although eNOS is myristoylated in endothelial cells and thereby translocated to the cell membrane,31 we have not yet investigated whether eNOS is myristoylated in U937 transfectants. However, this posttranslation modification has not been found necessary for enzymatic activity.31
U937 cells differentiated with PMA develop monocytic cell characteristics and produce TNF-α.32 Our experiments showed that untransfected naive or PMA-differentiated U937 cells did not express NOS protein or produce NO. The source of small amounts of NO−2 and NO−3 in cultures of U937 cells not transfected with NOS is not entirely clear, but these background levels were not affected by NOS inhibitors. Notably, the maintenance medium that we and other investigators use for U937 cells contains calcium NO−3 . Although cells were washed and placed in medium free of NO−2 and NO−3, the cells release NO−3 as they equilibrate with the new medium. We observed this phenomenon previously with human neutrophils and noted that it may be misinterpreted as NO production.12 Further, the medium is supplemented with FCS, which contains variable amounts of NO−2 and NO−3 . Some investigators have reported NO production by U937 cells,33 but others have not.34,35 Likewise, whether or not freshly isolated human neutrophils, PBMC, monocytes, and macrohpages produce NO, and if so, how much, remains controversial.11-16,36-40 Recent work in this area has focused on differences in promoter characteristics39 and enzyme activity40 between species and may eventually help explain the variety of experimental results that have been obtained. Nonetheless, our results show that U937 cells, either naive or PMA-differentiated, did not express NOS enzymes until after they were transfected with plasmid vectors containing NOS genes.
We show that after PMA-differentiation, iNOS transfectants in the absence of LPS produce more TNF-α compared with empty vector transfectants. Previous studies in both human and murine phagocytic cells have concluded that NO regulates cytokine production.18-20,23,24 However, apparent conflicts within this data, which were obtained using a variety of exogenous NO sources, have been difficult to reconcile. Using human PBMC, Eigler et al20 found that 3-morpholino-sydnonimine (SIN-1), a NO donor that also releases superoxide, upregulated TNF-α synthesis induced by IL-1β, but downregulated that induced by LPS. Using LPS-stimulated murine RAW 264.7 cells that produce both NO and TNF-α, Eigler et al23 later showed that NOS inhibition increased TNF-α production; these results suggest that endogenous NO suppresses TNF-α production. However, in the human myeloid leukemia cell line HL-60, NO gas, sodium nitroprusside (SNP), and SIN-1 were shown to increase expression of mRNA for both TNF-α and IL-1β.21 Likewise, Lander et al19 reported that the NO donors, SNP and S-nitroso-N-acetylpencillamine, increased TNF-α production in unstimulated human PBMC through a cGMP-independent mechanism. It is not clear whether the differences among these studies are related to the source or dose of exogenous NO, the type of cell, its level of differentiation, its species of origin, or the cytokine-inducing agent used.
We previously found that SNP increased LPS-induced TNF-α production in human neutrophil preparations, but had no effect on cultured human macrophages.18 In the present experiments using transfected PMA-differentiated U937 cells, endogenous NO upregulated TNF-α production. These results support an upregulatory effect of NO on TNF-α production under certain conditions. However, it is important not to consider differentiated U937 cells to be identical to mature monocytes or macrophages, as they may differ in regards to the regulation and intracellular processing of cytokines.32 Further, the demonstration that a relatively high dose (1,000 μmol/L) of SIN-1, which releases both NO and superoxide, decreased LPS-stimulated TNF-α production by 61% in human PBMC20 suggests that, under some conditions, NO may limit TNF-α release. This potential downregulatory effect on cytokine synthesis was further shown using rat alveolar macrophages24 and murine macrophage RAW 264.7 cells, which were induced to produce NO.23 In addition, a recent in vivo experiment in mice with Staphylococcal enterotoxin B-induced shock suggested that NO protected the animals by decreasing TNF-α and IFN-γ release.41 Collectively, these investigations suggest that high concentrations of NO, perhaps in the presence of superoxide, may reduce TNF-α production.
The mechanism by which NO regulates TNF-α production has not been fully defined and remains an area of active investigation. In recent experiments, we found that NO donors increased TNF-α production in U937, at least in part, through a cyclic adenosine monophosphate (cAMP)-dependent signaling mechanism.42 In PBMC, the upregulation of TNF-α production by NO has been associated with changes in the binding activity of NF-κB, a transcriptional factor that increases TNF-α synthesis at the promoter level.19 Lander et al43 have also shown that NO can activate mitogen-activated protein kinases (MAP kinases), including p38, which can regulate the translation of TNF-α mRNA. However, in other cell types under other conditions, NO has been reported to decrease NF-κB activation.44-47 Importantly, the effects ascribed to NO in the above investigations involve fundamental signal transduction pathways. This suggests that NO will be found to regulate more than just TNF-α production in phagocytes. In the current investigation, endogenously produced NO increased TNF-α production in the absence, but not in the presence, of LPS. Notably, LPS was found to independently upregulate NO production in iNOS transfectants, probably via the AP-1 site contained in the CMV promoter of our constructs (see Materials and Methods).28 29 This suggests that NO at low concentrations may upregulate TNF-α production, but that this effect may be lost or even reversed at higher rates of NO release. This hypothesis requires further investigation. If correct, it may explain in part, the variety of results that have been obtained studying this question.
We have confirmed that NO regulates TNF-α production in human phagocytes using a NOS transfected cell line. Although this is an autocrine effect in these transfected cells, the regulatory role of NO in human phagocytes may function, at least at certain times, as a paracrine control mechanism. The importance of this NO regulatory mechanism to the clinical use of NOS inhibitors in septic shock and inhaled NO in ARDS warrants further investigation. NOS transfected cell lines should be useful in further defining NO regulatory pathways in human phagocytes and exploring the potential for NOS gene therapy.
Supported by National Institutes of Health, Bethesda, MD, intramural funds.
L.Y. and S.W. are co-first authors.
Address reprint requests to Robert L. Danner, MD, Critical Care Medicine Department, Bldg 10, Room 7D43, National Institutes of Health, Bethesda, MD 20892.

![Fig. 4. The conversion of [14C] L-arginine to [14C] L-citrulline in transfected U937 cells. NOS cofactors were added to lysates of eNOS transfected, empty vector transfected, and untransfected U937 cells (see Materials and Methods). L-NMA (250 μmol/L) was added to or CaCl2 was omitted from some lysate preparations as indicated. Data are means ± SE (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/3/10.1182_blood.v90.3.1160/4/m_bl_0046f4.jpeg?Expires=1768700100&Signature=Gc7lfQCC2jzABL04MjXy9BDOkqkC~wZH5BJQyOaErNgro5n1O96kHL3Ohek40u30-WMbrRgEMa7YB~breLYfVBKLualqt8LJcvIDI3uSSiumgaNDqa~CV~QMuC4yXnOvx2DxLkPEhmxu39EKMD~qdtOzE5gJAig~sY~tIsq0ePxZCYcD7BLM2hM3RLcdb9aFduaPYI7Z0Ps-h2CZ6-a82IWlAtzYWQXAwhUEGqlndVRzdgtoUABdIdlSwAA7FvYk0yA-tgs0SvNGZ~SUWLnYlaPeg-tnrr-K7RAI1I9HEchJea6v~c2CkALUACEE6-BjEAYS0avHdUMicSd6s8iMqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. The conversion of [14C] L-arginine to [14C] L-citrulline in transfected U937 cells. NOS cofactors were added to lysates of eNOS transfected, empty vector transfected, and untransfected U937 cells (see Materials and Methods). L-NMA (250 μmol/L) was added to or CaCl2 was omitted from some lysate preparations as indicated. Data are means ± SE (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/3/10.1182_blood.v90.3.1160/4/m_bl_0046f4.jpeg?Expires=1768700101&Signature=nU11BCYwkpUXUTn5A1Op2XmeSwB4tQDvm8inOTIbxZON3p9uHJBVWePavOAO5vFNH8cIUX-54~KC25clgmK1Q1IwxA6EbS9VHzgDfM~Qh0VOOVZNphXfmgfWlL-qR6qQ34KyYfIhg9KeSnAeCRZxmoe1QN2Ekakky~plsF3BELNaVLNmOtZQzU-moYcYND2~KznJyy7-Rm0VYm5Az5PKWpWi-HejtvX1fDft-4QsPfEUVbI8p3Q0y5WGWzbJ0IF3~zEDUMBRPdYNbMoOTppuvIPDM2h0mF80nMNpiQsqIHhm9u6~Ei4hL3hLzC7bWXwC7pqoikTbkW55jdstqwpJRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)