Abstract
Transforming growth factor-α (TGF-α) exerts several effects on target cells, such as neovascularization promotion and mitogenic signalling. Using immunoelectron microscopy, we show that monocytes and neutrophils, store TGF-α in cytoplasmic granules. In monocytes, TGF-α did not colocalize with components of peroxidase-positive granules or with albumin of secretory vesicles. Furthermore, no colocalization of TGF-α with components of azurophilic or specific granules or secretory vesicles was observed in neutrophils. Activated monocytes and tissue-macrophages contained much less TGF-α–positive granules, suggesting TGF-α release. Western blot analysis showed a protein of 10 kD in lysates of monocytes. TGF-α mRNA was detected in monocytoid cells from the bone marrow by in situ hybridization. This study shows for the first time that monocytes and neutrophils contain TGF-α in all stages of maturation and that TGF-α in monocytes is stored in a large population of peroxidase-negative granules suggesting a function for these granules. Monocytes and neutrophils are important effector cells in inflammatory reactions. The present finding that these cells contain TGF-α might explain complications such as fibrosis and neoplastic transformation, caused by chronic inflammation.
MONOCYTES and neutrophils originate from the myeloid line of differentiating cells in the bone marrow and enter the blood stream to play a role in host defense against invading microorganisms.1,2 However, these cells also have a pathophysiologic role in diseases such as asthma and rheumatoid arthritis.3,4 Monocytes change their phenotype after recruitment into tissues and develop into tissue-specific macrophages, for example Kupffer cells in the liver, Langerhans' cells in the skin, and alveolar and spleen macrophages.4 Tissue-macrophages can produce and release several inflammatory mediators such as coagulation factors, components of the complement system, plasma proteins, cytokines, enzymes, hormones and lipid mediators.4 In addition, they process and present antigen to T cells. Monocytes and macrophages, therefore, play a central role in the initiation and maintenance of inflammation and in subsequent tissue clean up and wound healing.4 Both monocytes/macrophages and neutrophils have cytotoxic capabilities and store digestive proteins in cytoplasmic granules. Monocytes contain two distinct populations of granules, peroxidase-positive and peroxidase-negative granules, in approximately equal numbers.5 The peroxidase-positive granules contain proteins such as myeloperoxidase, elastase, and cathepsin G.6 The content and function of the peroxidase-negative granules are as yet unknown. Neutrophils are terminally differentiated effector cells and also contain peroxidase-positive (azurophil) and peroxidase-negative (specific and gelatinase) granules. Azurophilic granules contain proteolytic and bactericidal proteins. The contents of the specific and gelatinase granules are also known.7
Transforming growth factor-α (TGF-α) is a secreted mitogen originally detected in the culture supernatants of transformed rodent fibroblast. TGF-α is synthesized by several cells, for instance epidermal keratinocytes,8 fibroblasts,9 and cells of human hematopoietic origin like eosinophils10 and stimulated macrophages.11 TGF-α is a polypeptide belonging to the family of epidermal growth factor (EGF )-related proteins. It binds to the EGF-receptor12 and elicits different effects in target cells such as mitogenic signaling13 and promotion of neovascularization.14 It has been suggested that TGF-α is involved in wound healing15 and in tumor development.12
We initiated the present study to determine whether TGF-α is present in human monocytes in all stages of maturation from promonocyte in bone marrow to peripheral blood monocyte and tissue macrophage, as well as in peripheral blood neutrophils and their bone marrow progenitors using immunoelectron microscopy. The presence of TGF-α in these cells was confirmed by enzyme-linked immunosorbent assay (ELISA) and in monocytes, the presence of TGF-α mRNA was studied by in situ hybridization.
MATERIALS AND METHODS
Reagents
The antibodies used were mouse monoclonal antibody (MoAb) Ab 1, directed against the region around residue 16 of the mature part of TGF-α (Oncogene Science Inc, Manhasset, NY). Rabbit antiproteinase 3 polyclonal antibody (poAb) was a gift from Dr Jörgen Wieslander, Lund, Sweden. Rabbit antihuman elastase poAb was a gift from Dr Kjell Ohlsson, Malmö, Sweden. Rabbit-antihuman cathepsin G poAb was supplied by Dr Inge Olsson (Lund, Sweden). Rabbit-antihuman lactoferrin poAb was purchased from Cappel Laboratories (Cochranville, PA). Rabbit-antihuman gelatinase poAb was a gift from Dr Niels Borregaard (Copenhagen, Denmark). Mouse CD63 MoAb and rabbit-antihuman albumin poAb were obtained from CLB (Amsterdam, The Netherlands). Goat-antirabbit IgG linked to 5-nm gold particle and goat-antimouse IgG linked to 5-nm or to 10-nm gold particles (Amersham Nederland's-Hertogenbosch, The Netherlands), heparin and varidase (Lederle, Madrid, Spain), Dextran (Dextran T 500) and Percoll (Pharmacia Fine Chemicals, Uppsala, Sweden), interleukin-10 (IL-10) and interferon-γ (IFN-γ) (Genzyme, Boston, MA), TGF-α ELISA kit (Oncogene Science Inc), and Yssel's medium (Gemini Bioproducts, Calabasas, CA) were obtained from the manufacturers. Incubation medium for the cells contained 132 mmol/L NaCl, 6.0 mmol/L KCl, 1.0 mmol/L CaCl2 , 1.0 mmol/L MgSO4 , 1.2 mmol/L potassium phosphate, 20 mmol/L HEPES, 5.5 mmol/L glucose, and 0.5% (wt/vol) human serum albumin, pH 7.4.
Localization of TGF-α in promonocytes. Ultrathin cryosection of a promonocyte of the bone marrow labeled to detect TGF-α. Many granules are present strongly labeled for TGF-α (large arrows). Mitochondria (m) and nucleus (n) are negative. Small arrows are pointing to a large bundle of filaments characteristic for promonocytes. Bar = 300 nm.
Localization of TGF-α in promonocytes. Ultrathin cryosection of a promonocyte of the bone marrow labeled to detect TGF-α. Many granules are present strongly labeled for TGF-α (large arrows). Mitochondria (m) and nucleus (n) are negative. Small arrows are pointing to a large bundle of filaments characteristic for promonocytes. Bar = 300 nm.
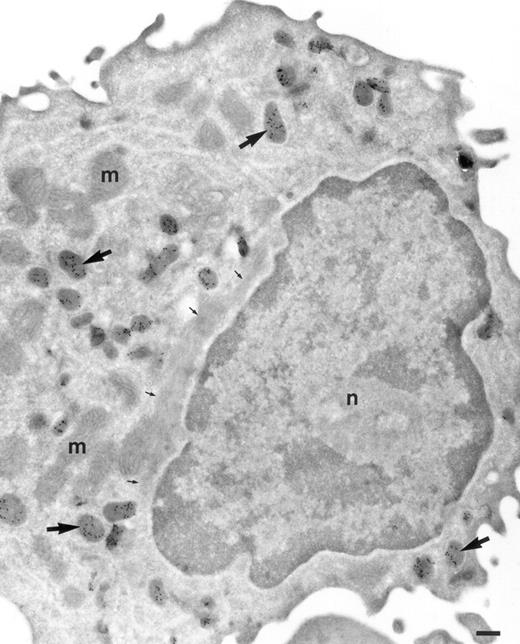
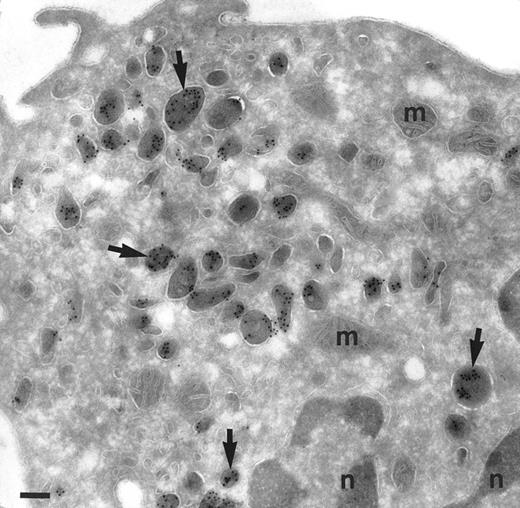
Localization of TGF-α in monocytes. Cryosection of a monocyte from the peripheral blood incubated as in Fig 1. The same pattern of distribution of TGF-α is found as in promonocytes. Abundant electron-dense granules are intensively labeled (arrows). No background is observed in cytosol, mitochondria (m) and nucleus (n). Bar = 200 nm.
Localization of TGF-α in monocytes. Cryosection of a monocyte from the peripheral blood incubated as in Fig 1. The same pattern of distribution of TGF-α is found as in promonocytes. Abundant electron-dense granules are intensively labeled (arrows). No background is observed in cytosol, mitochondria (m) and nucleus (n). Bar = 200 nm.
Isolation of Monocytes and Neutrophils
Blood was obtained after informed consent from healthy volunteers and approval of The Institutional Review Board. The blood was collected in 0.4% (wt/vol) trisodium citrate (pH 7.4) for immediate anticoagulation. After 1:1 dilution in phosphate-buffered saline (PBS) containing human serum albumin (0.5% wt/vol), the blood was fractionated by density gradient centrifugation over isotonic Percoll (specific gravity of 1.077 g/mL, at 800g, for 18 minutes, 20°C). The interphase, containing the mononuclear cells, was separated into pure lymphocyte and monocyte fractions by countercurrent centrifugal elutriation as described16; each fraction was more than 90% pure. After two washes in PBS, the monocytes were resuspended in incubation medium. The pellet fraction, containing erythrocytes and granulocytes, was treated for 10 to 15 minutes with ice-cold isotonic NH4Cl solution (155 mmol/L NH4Cl, 10 mmol/L KHCO3 , 0.1 mmol/L EDTA, pH 7.4) to lyse the erythrocytes. The cells were centrifuged at 4°C (270g for 4 minutes) and residual erythrocytes were lysed for another 3 minutes. The remaining granulocytes were further purified by negative selection with immunomagnetic beads and CD9 MoAbs against the contaminating eosinophils. Purity of the neutrophils was greater than 99%. The cells were resuspended in incubation medium at a final concentration of 107 cells/mL. Monocytes (3 × 106/mL) were incubated with IL-10 (400 U/mL) and IFN-γ (400 U/mL) in Yssel's medium. The cells were incubated for 24 hours at 37°C in a humid atmosphere containing 5% CO2 and processed for electron microscopy.
Isolation of Immature Monocytes and Neutrophils
Bone marrow was obtained from healthy volunteers. Bone marrow-aspiration was performed by iliac crest or sternal puncture after informed consent. The aspirates were mixed with 3 mL McCoy's medium (Sigma, St Louis, MO) containing 100 IU of heparin and 75 U of Varidase. The marrow cells were layered on 12 mL of isotonic Percoll with a specific gravity of 1.068g/mL. After centrifugation (20 minutes, 1,000g, 20°C), the cells on top of the Percoll layer were collected and processed for immunoelectron microscopy.
Cryoultramicrotomy and Immunolabeling
Bone marrow cells, monocytes, and neutrophils from peripheral blood were prepared as described above and were fixed with a mixture of 0.5% glutaraldehyde and 4% paraformaldehyde (PFA) in 0.1 mol/L PBS (pH 7.2) for 1 hour and pelleted in 10% gelatin in PBS. Nasal polyps obtained from patients suffering from allergic rhinitis undergoing polypectomi, were cut in small pieces and fixed as described above immediately after extraction. The gelatin slabs and the pieces of polyps were stored at 4°C in PBS containing 1% PFA. Small pieces of gelatin and polyps were infiltrated with 2.3 mol/L sucrose/polyvinylpyrrolidon-10 in PBS for 4 hours, mounted on pins, and frozen in liquid nitrogen. About 80 nm cryosections were made on a MT-7 cryoultramicrotome (Research and Manufacturing Co, Tucson, AZ) and incubated at room temperature with mouse MoAb to TGF-α (dilution 1:20) for 1 hour followed by 1 hour incubation with 10 nm gold-conjugated goat antimouse IgG (1:40). In double-labeling experiments when the sera used derived from different species, the cryosections were first incubated with a mixture of the sera, followed by incubation with a mixture of goat-antirabbit IgG linked to 5 nm gold and goat-antimouse IgG linked to 10 nm gold (both diluted 1:40) when both sera used were derived from mouse, the sections were first incubated with mouse anti–TGF-α and antimouse IgG linked to 5 nm gold and then treated with 1% glutaraldehyde for 10 minutes to prevent any interference between the different antibody gold complexes on the sections,17 followed by incubation with the other mouse antibody and antimouse IgG linked to 10 nm gold. For the controls, the primary antibody was replaced by a nonrelevant murine or rabbit antiserum. After immunolabeling, the cryosections were embedded in a mixture of methylcellulose and uranyl acetate. All sections were examined with a Philips CM10 electron microscope (Eindhoven, The Netherlands).
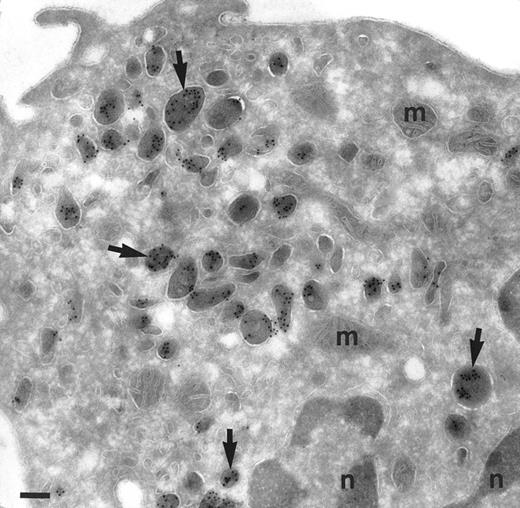
Localization of TGF-α in monocytes after incubation with IL-10 and IFN-γ for 24 hours and labeled for TGF-α. Monocytes are polarized and degranulated. Still some granules are observed mainly underneath the cell surface, where the cell membrane is ruffled (large arrows). The granules are labeled (small arrows and inset). Inset is a higher magnification of the labeled area with asterisk. Bars = 500 nm, inset = 100 nm.
Localization of TGF-α in monocytes after incubation with IL-10 and IFN-γ for 24 hours and labeled for TGF-α. Monocytes are polarized and degranulated. Still some granules are observed mainly underneath the cell surface, where the cell membrane is ruffled (large arrows). The granules are labeled (small arrows and inset). Inset is a higher magnification of the labeled area with asterisk. Bars = 500 nm, inset = 100 nm.
Localization of TGF-α in macrophages. Cryosection of a macrophage from a nasal polyp labeled for TGF-α. A few granules are labeled for TGF-α (arrows). Nucleus, (n); bundles of collagen (C). Inset is a higher magnification of the marked granules (asterisk) to show the gold labeling. Bars = 500 nm, inset = 100 nm.
Localization of TGF-α in macrophages. Cryosection of a macrophage from a nasal polyp labeled for TGF-α. A few granules are labeled for TGF-α (arrows). Nucleus, (n); bundles of collagen (C). Inset is a higher magnification of the marked granules (asterisk) to show the gold labeling. Bars = 500 nm, inset = 100 nm.
Morphometry
Size measurements and counts were performed on electron micrographs of monocytes at different stages of differentiation or after cell activation. The cells were selected at random and at least 10 cell sections were examined for each stage. The TGF-α–positive granules were counted and the surface of the cytoplasm was measured with an X-Y tablet (Kontron, München, Germany; MOP-Videoplan).
Determination of TGF-α Protein
Pellets of monocytes (>90% pure, mainly contaminated with lymphocytes and basophils) and neutrophils (>99% pure) were lysed in ice-cold PBS containing 0.1% Tween 20, 0.2% cetyltrimethylammonium bromide (CTAB), 0.2% bovine serum albumin, 20 mmol/L EDTA for 30 minutes. The TGF-α content was measured using a quantitative ELISA kit. This assay uses affinity-purified goat poAbs specific for human TGF-α.
Western Blot
Monocytes and neutrophils were lysed in 1% Triton (50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 0.02% sodium azide, 100 μg/mL phenylmethylsulfonylfluoride, and 1% Triton X-100) for 30 minutes on ice, mixed with an equal amount of a solution containing 1% sodium dodecyl sulfate (SDS) and 1% β-mercaptoethanol, and heated for two minutes at 95°C. Gel electrophoresis of the lysates were performed on 14% Tris-Glycine gels. Proteins were transferred to nitrocellulose membranes and probed with MoAb to TGF-α as described.18 Briefly, nonspecific binding was blocked with 5% nonfat dry milk in T-TBS (50 mmol/L Tris, pH 8.0, 150 mmol/L sodium chloride, 0.05% Tween 20) and the MoAb to TGF-α was used at a dilution of 10 μg/mL in T-TBS plus 1% gelatin. Blots were developed in TM (200 mmol/L Tris, pH 9.5, plus 10 mmol/L MgSO4 ) with 300 μg/mL nitro blue tetrazolium and 100 μg/mL 5-bromo-4-chloro-3-indolyl-phosphate. As a control, also recombinant TGF-α protein (Mw 5,500 kD, Oncogene) was run under similar conditions and showed a clear band around 5 kD. Probing with a control MoAb to human semenogelin (a kind gift of Dr Johan Malm, Malmö, Sweden) was negative.
In Situ Hybridization
Tissues.Formalin-fixed paraffin-embedded archival bone marrow sample from an 80-year-old man with myelodysplastic syndrome, was obtained from the Department of Pathology, Karolinska Hospital, Stockholm, Sweden. This man had 31% of monocytes in peripheral blood and the bone marrow was highly cellular showing a preponderance of promonocytic cells. Sections of 5 μm from bone marrow, were dried at 42°C on Superfrost Plus slides (Fischer Scientific, Nederland, Den Bosch, The Netherlands). Monocytes from healthy volunteers, isolated as described above, were centrifuged onto Superfrost Plus slides and then fixed by immersion in 4% paraformaldehyde at 4°C for 10 minutes and stored at 4°C in 70% ethanol until use.
Probe.A 4.29-kb fragment of human TGF-α from the American Type Culture Collection (ATCC, Rockville, MD) was subcloned in Bluescript SK+ vector. To produce antisense probe, the insert was linearized with Xho and transcribed with T3 RNA polymerase and to produce sense control probe, cleavage was performed with BamHI transcribing from the T7 promoter. In vitro transcribed RNA was labeled with α-(35S) uridine triphosphate (UTP) under conditions recommended by and with reagents from Promega (Madison, WI).
In situ hybridization was performed essentially as described previously.19 Briefly, sections were treated with proteinase K (Sigma Chemicals) and washed in 0.1 mol/L triethanolamine buffer containing 0.25% acetic anhydride. Sections were hybridized overnight with 2.5 × 106 cpm of labeled antisense or sense probe at 55°C. Washed slides were dipped in Kodak NTB-2 emulsion (Eastman-Kodak, Rochester, NY), prediluted 1:1 with distilled water, and processed for autoradiography as described.19 After development of the photographic emulsion, slides were stained with hematoxylin and eosin.
RESULTS
Using immunoelectron microscopy, the subcellular localization of TGF-α was studied in monocytes and neutrophils; their progenitors from bone marrow and mature cells from peripheral blood, and macrophages present in nasal polyps. The cellular specificity of the immunogold localization of TGF-α in monocytes and neutrophils was confirmed by the absence of staining in lymphocytes present in the same sections. No labeling was found in the controls of each experiment where the primary antibody was replaced by a nonrelevant murine or rabbit antiserum.
TGF-α in Monocytes/Macrophages
In promonocytes from the bone marrow, as well as monocytes from bone marrow and peripheral blood, TGF-α was localized in abundant electron-dense granules (Figs 1 and 2). The pattern of granules was similar in bone marrow promonocytes (Fig 1) and mature monocytes (Fig 2). These cells can be distinguished by the presence of bundles of filaments in the cytoplasm and a rounded or oval nucleus in promonocytes. Indications for the release of TGF-α–positive granules came from monocytes incubated for 24 hours with IL-10 and IFN-γ and thereafter labeled with anti–TGF-α. At the ultrastructural level, these monocytes have an activated appearance (Fig 3): ruffles on the cell membrane on one side, the nucleus localized at the opposite side and less TGF-α–positive granules than in untreated cells. Moreover, the remaining TGF-α containing granules are located underneath the cell membrane, mainly under the ruffles. Macrophages in nasal polyps (Fig 4) contained very few TGF-α–positive granules.
To assess the decrease of the TGF-α–positive granules in different stages of monocyte development or after activation of monocytes, the number of the positive granules in each stage of development or after activation was counted from at least 10 cell sections. As is clear from Table 1, the mean number of TGF-α–positive granules per cross-section is the highest in promonocytes (24.5), decreases slightly for the monocytes present in peripheral blood (18) and sharply decreases for the macrophages (3.3) or after incubation of the monocytes with IL-10 and IFN-γ (7.05). To rule out that this decrease of the positive granules after activation is only apparent and related to cell spreading, we have also measured the surface of the cytoplasm and calculated the density of TGF-α–positive granules: promonocytes, 10.3 granules per 10 μm2 (10 cross-sections analyzed); monocytes, 6.5 granules per 10 μm2 (21 cross-sections analyzed); macrophages, 1 granule per 10 μm2 (10 cross-sections analyzed), and monocytes incubated with IL-10 and IFN-γ, 2.5 granules per 10 μm2 (20 cross-sections analyzed). Again the density of positive granules is the highest in promonocytes and decreases at the same rate as shown for the amount of granules in Table 1.
Characterization of the TGF-α–Positive Granules in Monocytes
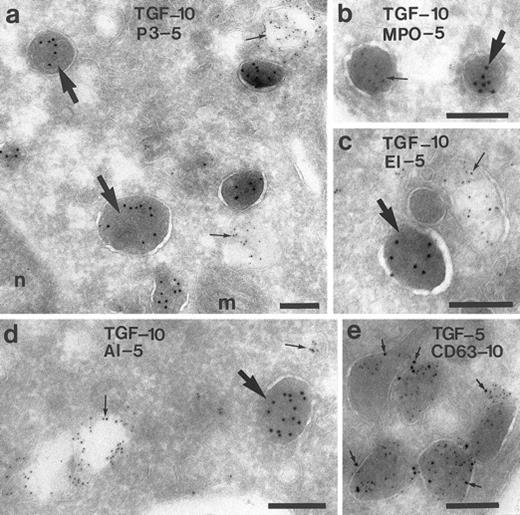
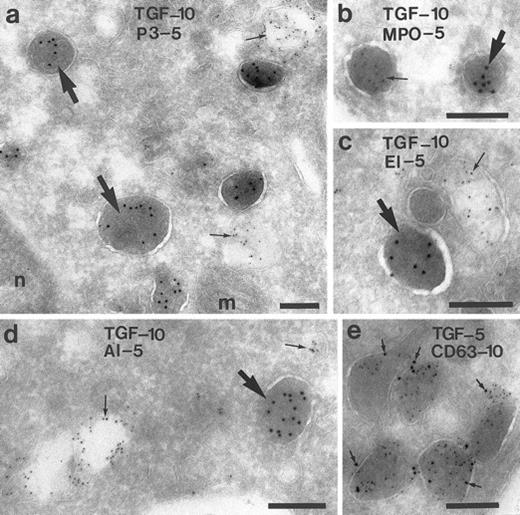
To characterize the TGF-α–positive granules in monocytes from peripheral blood, double-labeling experiments were performed using antibodies against TGF-α, in combination with antibodies to components of the peroxidase-positive granules: myeloperoxidase, proteinase-3, and elastase6,20; or antibodies against albumin, a marker for a rapidly mobilizable pool of secretory vesicles21; or antibodies against CD63, a membrane-anchored glycoprotein present in many membrane-bound granules of granulocytes.22 23 No colocalization of TGF-α was found with proteinase-3 (Fig 5a), myeloperoxidase (Fig 5b), elastase (Fig 5c), or albumin (Fig 5d). These results indicate that the TGF-α–positive granules differ from the peroxidase-positive granules and from the secretory vesicles. CD63 labeling was found on the membrane of the TGF-α–positive granules (Fig 5e). To study whether CD63 is also present in peroxidase-positive granules, monocytes were double-labeled with antiproteinase-3 and anti-CD63 and colabeling with both antibodies was also found (not shown).
Characterization of the TGF-α–positive granules in monocytes. Monocytes of the peripheral blood were double-labeled for TGF-α and for several components of the granules and vesicles, respectively. In (a) through (c), it is shown that TGF-α is localized in granules other than proteinase-3 (P-3), myeloperoxidase (MPO), and elastase (EL), markers of the peroxidase-positive granules of the monocyte. (d) No colocalization with albumin (AL), marker for a secretory compartment. (e) Colocalization with CD63, a membrane-bound glycoprotein. Bars = 200 nm
Characterization of the TGF-α–positive granules in monocytes. Monocytes of the peripheral blood were double-labeled for TGF-α and for several components of the granules and vesicles, respectively. In (a) through (c), it is shown that TGF-α is localized in granules other than proteinase-3 (P-3), myeloperoxidase (MPO), and elastase (EL), markers of the peroxidase-positive granules of the monocyte. (d) No colocalization with albumin (AL), marker for a secretory compartment. (e) Colocalization with CD63, a membrane-bound glycoprotein. Bars = 200 nm
TGF-α in Neutrophils
In the neutrophilic promyelocytes from the bone marrow, a few TGF-α–positive vesicles were found (not shown). These vesicles were more abundant in the neutrophilic myelocytes (Fig 6a). In Fig 6b, an area of a neutrophilic myelocyte is shown where the TGF-α–positive vesicles look like multivesicular bodies and multilaminar structures,24 in both, TGF-α is bound to the membrane. TGF-α was also found in cytoplasmic vesicles in peripheral neutrophils (Fig 7). To characterize these vesicles, sections were double-labeled with anti-TGF–α and antibodies against lactoferrin of neutrophil specific granules25 (Fig 7a), gelatinase of neutrophil gelatinase-containing granules26 (Fig 7b), cathepsin G of neutrophil azurophilic granules27 (Fig 7c), and albumin of secretory vesicles28 (Fig 7d). TGF-α did not colocalize with any of these markers, suggesting that the TGF-α–containing granules differ from the three main kinds of granules in neutrophils and from the rapidly mobilizable pool of secretory vesicles.
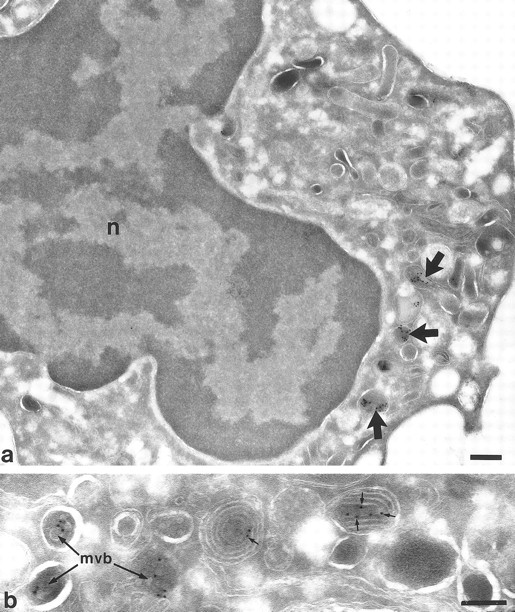
Localization of TGF-α in neutrophil myelocytes from bone marrow. In (a) at lower magnification, a survey of a myelocyte showing a few positive vesicles (arrows). No labeling is shown elsewhere. Nucleus (n). (b) Higher magnification of an area from another myelocyte showing TGF-α labeling in multivesicular bodies (mvb) and multilaminar structures (small arrows). Bars a = 300 nm, b = 200 nm.
Localization of TGF-α in neutrophil myelocytes from bone marrow. In (a) at lower magnification, a survey of a myelocyte showing a few positive vesicles (arrows). No labeling is shown elsewhere. Nucleus (n). (b) Higher magnification of an area from another myelocyte showing TGF-α labeling in multivesicular bodies (mvb) and multilaminar structures (small arrows). Bars a = 300 nm, b = 200 nm.
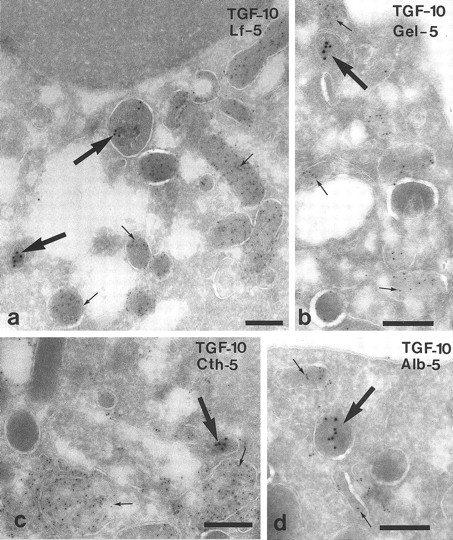
Characterization of the TGF-α–positive granules in neutrophils from peripheral blood by double-labeling. (a) TGF-α (large arrows) does not colocalize with lactoferrin (Lf ) (small arrows), a marker of neutrophil specific granules. (b) No colocalization with gelatinase (Gel), a marker of gelatinase-granules. (c) TGF-α (large arrow) is detected in a compartment other than cathepsin G (Cth) (small arrows), which is present in azurophil granules. (d) TGF-α (large arrow) is detected in a compartment other than albumin (Alb) (small arrows) of secretory vesicles. Bars = 200 nm.
Characterization of the TGF-α–positive granules in neutrophils from peripheral blood by double-labeling. (a) TGF-α (large arrows) does not colocalize with lactoferrin (Lf ) (small arrows), a marker of neutrophil specific granules. (b) No colocalization with gelatinase (Gel), a marker of gelatinase-granules. (c) TGF-α (large arrow) is detected in a compartment other than cathepsin G (Cth) (small arrows), which is present in azurophil granules. (d) TGF-α (large arrow) is detected in a compartment other than albumin (Alb) (small arrows) of secretory vesicles. Bars = 200 nm.
TGF-α in Monocytic and Neutrophil Lysates
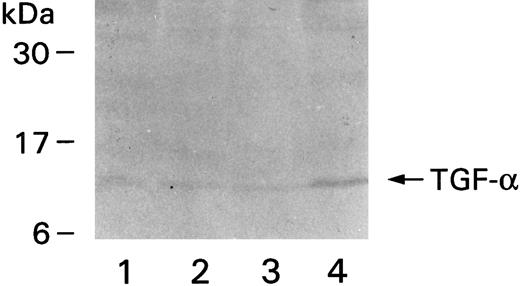
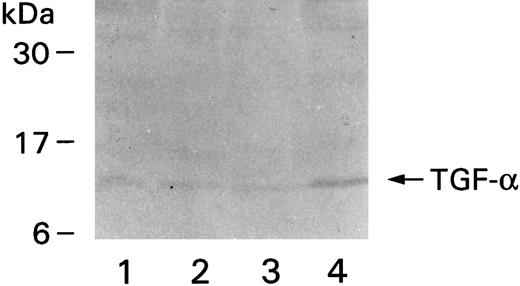
Monocytes and neutrophils were pelleted and lysed to quantify TGF-α using ELISA. Monocytes were found to contain 257 ± 40.5 pg of TGF-α/106 cells, but neutrophils much less: 2.5 pg of TGF-α/106 cells. Lysates from four different donors were also run on gels and transferred to nitrocellulose membranes for Western blotting. A band corresponding to a molecular weight of approximately 10 kD was observed in all lanes containing lysates from monocytes (Fig 8). Each lane represents one donor. No bands could be detected in the lanes containing neutrophil lysates (not shown), probably because of the relatively small amount of TGF-α in these cells.
Western blot showing immunoreactive TGF-α. Monocytes were purified from four different donors, the denatured proteins from the cell extracts were run on gels, transferred to nitrocellulose membrane, and detected by MoAb to TGF-α and secondary alkaline phosphatase-conjugated goat antimouse antibody. The positions of the molecular weight markers are on the left.
Western blot showing immunoreactive TGF-α. Monocytes were purified from four different donors, the denatured proteins from the cell extracts were run on gels, transferred to nitrocellulose membrane, and detected by MoAb to TGF-α and secondary alkaline phosphatase-conjugated goat antimouse antibody. The positions of the molecular weight markers are on the left.
Detection of TGF-α mRNA in Bone Marrow and in Monocytes by In Situ Hybridization
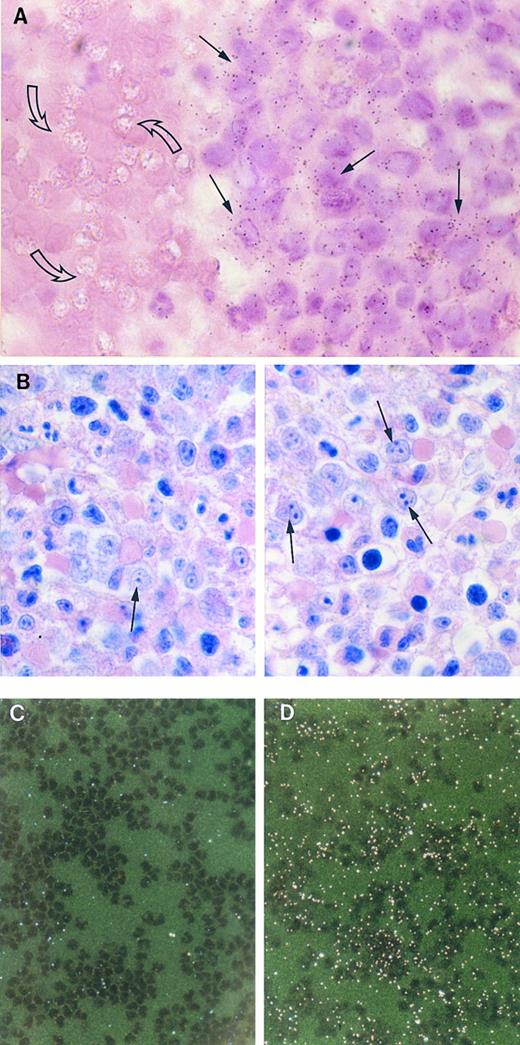
The expression of the TGF-α gene was studied in monocytes from peripheral blood and in their bone marrow progenitor. For this we used bone marrow from a patient with myelodisplastic syndrome, with a preponderance of promonocytes. Figure 9A shows that monocytoid cells from the bone marrow hybridized specifically with 35S-labeled antisense RNA for TGF-α, whereas no specific autoradiographic signal was found in red blood cells present in the same sections. Figure 9B shows a Wright-Giemsa stained section from a surrounding region of the same bone marrow, a predominance of monocytoid cells is clearly visible. In monocytes from peripheral blood of healthy donors, no TGF-α mRNA could be detected (Fig 9C) whereas, in the same monocytes, specific signal was evident for β-actin mRNA (Fig 9D).
Detection of TGF-α mRNA in bone marrow and peripheral blood monocytes by in situ hybridization. (A) Bright-field photomicrograph of a section of bone marrow from a patient with myelodysplastic syndrome hybridized with 32S-labeled antisense RNA for TGF-α and stained with hematoxylin and eosin. Positive signal for TGF-α is seen in numerous monocytoid cells (closed arrows), but not in the red blood cells (open arrows). Autoradiography was for 30 days. (×1,000). (B) Wright-Giemsa stained section of a surrounding region of the bone marrow. Monocytoid cells (arrows) are abundant in this area (×100). (C) and (D) Dark-field photomicrographs of circulating monocytes, prepared as described in Materials and Methods. There is no autoradiographic signal for TGF-α mRNA (C), whereas specific signal appearing as bright dots under dark-field microscopy, was evident for β-actin mRNA (D). Autoradiography was for 32 days. (×256).
Detection of TGF-α mRNA in bone marrow and peripheral blood monocytes by in situ hybridization. (A) Bright-field photomicrograph of a section of bone marrow from a patient with myelodysplastic syndrome hybridized with 32S-labeled antisense RNA for TGF-α and stained with hematoxylin and eosin. Positive signal for TGF-α is seen in numerous monocytoid cells (closed arrows), but not in the red blood cells (open arrows). Autoradiography was for 30 days. (×1,000). (B) Wright-Giemsa stained section of a surrounding region of the bone marrow. Monocytoid cells (arrows) are abundant in this area (×100). (C) and (D) Dark-field photomicrographs of circulating monocytes, prepared as described in Materials and Methods. There is no autoradiographic signal for TGF-α mRNA (C), whereas specific signal appearing as bright dots under dark-field microscopy, was evident for β-actin mRNA (D). Autoradiography was for 32 days. (×256).
DISCUSSION
In this study, we show that TGF-α is stored in a large number of peroxidase-negative granules of promonocytes and monocytes and in a few granules in tissue macrophages. Incubation of monocytes with IL-10 and IFN-γ decreased the number of TGF-α containing granules suggesting that these factors induce exocytosis of these granules. In addition, TGF-α was detected by ELISA in extracts from purified peripheral monocytes and neutrophilic granulocytes, and TGF-α mRNA was found to be present in monocytoid cells from the bone marrow. In neutrophils, TGF-α was seen in a cytoplasmic granule compartment that is not related to azurophilic, specific, or gelatinase granules or secretory vesicles.
This is the first time that TGF-α is shown to be present in granules of monocytes and neutrophils, as well as their bone marrow progenitors. These findings indicate that the gene is expressed early in maturation and the protein is stored in a granule. This was also confirmed, in the case of the development of the monocyte, by the identification of TGF-α mRNA in monocytoid cells from the bone marrow by in situ hybridization. Using this technique, we could not detect TGF-α mRNA in monocytes from the peripheral blood, also not by using the more sensitive reverse trancriptase polymerase chain reaction (PCR) method (data not shown). The expression of the TGF-α gene seems to reappear during monocytic conversion to stimulated tissue-macrophages because macrophages exposed to lipopolysacharide (LPS) induction show TGF-α gene expression,11 as do wound macrophages.15 Taken together, this would imply that the TGF-α gene is expressed and the polypeptide synthesized in bone marrow progenitors of monocytes and stored in granules, while the gene is downregulated in the resting monocyte transported in the bloodstream. TGF-α gene expression is turned on during monocytic conversion to stimulated tissue macrophage where it can play a role in processes such as inflammation and wound repair. However, the regulation of the secretion of TGF-α by monocytes/macrophages not only occurs at the transcriptional level, but also by induction of exocytosis of the TGF-α–containing granules. We show that TGF-α is stored in a large number of granules in monocytes and their number is reduced after incubation with IL-10 and IFN-γ. Also the number of TGF-α–containing granules is reduced in tissue macrophages. Moreover, we could detect by ELISA (data not shown) a release of TGF-α in the medium after the incubation of monocytes with the combination of phorbol ester and ionomycin, fMLP or with the combination of cytochalasin B and fMLP. In all cases, the release of TGF-α was at least four times higher than the release from monocytes incubated with medium alone. The exocytosis of TGF-α–containing granules can be the explanation why Madtes et all11 detected a basal level of TGF-α in the medium of macrophages cultured without LPS, whereas no transcription of the gene could be demonstrated.
TGF-α is synthesized as a transmembrane polypeptide of 160 amino acids that is subsequently cleaved. The resulting mature soluble TGF-α consists of 50 amino acids.12 Several species of TGF-α have been identified suggesting differential processing of the precursor.29,30 In macrophage-conditioned medium, two different species of 8.5 to 12 and 28.5 kD have been identified.11 However, the smaller form was more abundant, which could explain why we detected only this variant in immunoblots of monocyte extracts. In the electron microscopic studies, TGF-α was found to be located in the matrix of the granules of monocytes and neutrophils, whereas the membrane-anchored glycoprotein CD63 was located in the membrane of the same granules in monocytes. These results suggest that the polypeptide in these granules is the mature and not the membrane-bound form of TGF-α. However, in neutrophilic myelocytes, TGF-α was observed on the membrane of multilaminar structures and multivesicular bodies, suggesting that in these cells also the membrane-anchored protein is present. Membrane-bound TGF-α has also been found in erythroblasts from human bone marrow and its cognate receptor, the EGF receptor, was found on a blast-like cell of myelomonocytic origin.31 Based on the latter findings, a role for TGF-α in hematopoiesis was suggested. However, a role of TGF-α in the regulation of hematopoiesis has, to our knowledge, never been demonstrated.
Monocytes contain two distinct populations of granules,5 peroxidase-positive and peroxidase-negative granules, in approximately equal numbers. The content and function of the peroxidase-negative granules are as yet unknown. In the present study, we show that TGF-α is a component of the peroxidase-negative granules. We could not show, by immunoelectron microscopy, that other cytokines produced by monocytes, for instance IL-6 and IL-1 were stored in granules (data not shown). Another compartment in the monocyte cytoplasm is the secretory vesicle, which contains albumin and CR3.21 However, this compartment did not contain TGF-α because it did not colocalize with albumin. In neutrophils, TGF-α was localized in electron-dense vesicles. In double-labeling experiments, no colocalization of TGF-α was found with either lactoferrin in specific granules,25 gelatinase in gelatinase granules,26 cathepsin G in azurophilic granules,27 or albumin in secretory vesicles.28 This implies that in neutrophils, TGF-α is stored in a novel, not previously described compartment.
Local release of TGF-α from monocytes and neutrophils could contribute to characteristic features of chronic inflammatory diseases, eg, the development of fibrosis of the lung seen in asthmatic patients,32 proliferation of synovial and subsynovial connective tissue cells causing destruction of cartilage in rheumathoid arthritis,33 cirrhosis of the liver and the development of hyperplastic polyps, fibrosis, and increased risk of malignant disease in inflammatory bowel disease.34 Therefore, the present finding that monocytes and neutrophils are sources of TGF-α is important in explaining the development of complications, such as fibrosis and neoplastic transformation, which are caused by chronic inflammation, but also beneficial effects such as wound healing.35 The specific localization of TGF-α in monocytes provides a reason for the existence of peroxidase-negative granules.
ACKNOWLEDGMENT
We thank Nico Ong for the preparation of the micrographs and Dr van der Borden from Andreas Hospital, Amsterdam, The Netherlands, for supplying the nasal polyps.
Address reprint requests to Jero Calafat, PhD, Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands.