Abstract
We and others have shown that both high and low molecular mass kininogens are able to inhibit the thrombin-induced aggregation of gel-filtered platelets, indicating that the locus for inhibition resides in the heavy chain. The inhibitory site is present in domain 3, confined to the C-terminal portion of the region encoded by exon 7 (K270-G292), and the minimal effective sequence is a heptapeptide (L271-A277; Kunapuli et al, J Biol Chem 271:11228, 1996). Kininogens inhibit thrombin binding to platelets and thus inhibit thrombin-induced aggregation. The molecular mechanism by which kininogens inhibit thrombin-induced aggregation of platelets is unknown. Thrombin has previously been shown to bind to two receptors on the platelet surface, glycoprotein (GP) Ib-IX-V complex and the hepta-spanning transmembrane receptor coupled to G protein(s). We now show that, unlike its effect on normal platelets, kininogen (2 μmol/L) did not inhibit the thrombin-induced aggregation of Bernard-Soulier platelets, which lack the GP Ib-IX-V complex, suggesting that kininogen interacts either directly or indirectly with that complex and restricts access by thrombin to this receptor. We further show that both recombinant K270-G292 polypeptide and the synthetic peptide L271-A277 derived from high molecular mass kininogen lower thrombin binding to platelets in a manner similar to monoclonal antibodies to or ligands (von Willebrand factor and echicetin) of GP Ib-IX. The anti–GP Ib-IX-V complex antibodies, TM-60 and SZ 2, can inhibit 125I-high molecular mass kininogen binding to platelets. Conversely, kininogen could block the binding of biotinylated TM-60 or of 125I-SZ 2. Kininogen inhibited the binding of biotinylated thrombin bound to a mouse fibroblast cell line transfected with the GP Ib-IX-V complex. These results indicated that kininogen binds to the GP Ib-IX-V complex modulating thrombin binding to platelets and the consequent platelet aggregation. Kininogen can thus serve as an important regulator of the early stages of platelet stimulation by thrombin.
TWO FORMS OF purified human plasma kininogen (kinin precursors) were described in plasma by Jacobsen and Kriz1: high molecular mass kininogen (HK; molecular weight [Mr] = 120 kD) and low molecular mass kininogen (LK; Mr = 68 kD). HK is an α-globulin with a plasma concentration of 80 μg/mL.2 LK is a β-globulin with a plasma concentration of approximately 220 μg/mL.3 Both HK and LK are encoded by the same gene4 through alternate splicing mechanisms. Both HK and LK are identical for the sequences encoded by the first 9 exons, the heavy chain (62 kD), and part of exon 10 that codes for bradykinin (BK) and 12 additional amino acids. However, the mRNA for HK continues through the complete exon 10, whereas the mRNA for LK is spliced to exon 11. Both HK and LK bind to unstimulated platelets in a zinc-dependent fashion with 3,300 and 650 sites per cell and kds of 9.9 nmol/L5 and 27 nmol/L,6 respectively. The number of binding sites for HK on platelets is increased by exposure to thrombin, but the kd is unchanged. Puri et al7 first demonstrated that HK inhibits thrombin-induced platelet shape change and increase in intracellular Ca2+ by inhibiting the agonist's binding to platelets. The presence of HK and LK in plasma resulted in a 10-fold increase in the concentration of thrombin needed to stimulate platelets. LK also inhibits thrombin binding to platelets and blocks thrombin-induced secretion.6 The action of both HK and LK to inhibit thrombin binding to platelets implies that the common heavy chain is involved.
The heavy chain is composed of three domains with similar disulfide bonding patterns, each highly homologous to the crystalline cysteine protein inhibitor, cystatin.8 Jiang et al9 have isolated domains 1, 2, and 3 of kininogens (D1, D2, and D3) by limited proteolysis of purified LK and shown that D3, but not D1 and D2, inhibited 125I-HK binding to platelets, indicating that this domain contains a cell-binding region. Herwald et al10 have defined a 13-residue segment in the region encoded by exon 9 that inhibits the binding of kininogens to endothelial cells. These investigators, using monoclonal antibodies (MoAbs), showed that there was an independent region of D3 responsible for inhibiting thrombin binding to platelets.
Because D3 is encoded by exons 7, 8, and 9 of the kininogen gene, we expressed D3 and each of these exons and all of their combinations as glutathione S-transferase (GST) fusion proteins in Escherichia coli and evaluated each for its ability to inhibit thrombin-induced platelet aggregation.11 D3 folds, as does the native protein, and is equally potent as HK. Only products containing exon 7 inhibited platelet aggregation induced by thrombin, with an IC50 of less than 30 μmol/L. A deletion mutant of exon 7 product (K236-K270) did not block thrombin-induced platelet aggregation, whereas L271-Q292 inhibited with an IC50 of 19 μmol/L. A heptapeptide L271-A277 inhibited thrombin-induced aggregation of platelets with an IC50 of 65 μmol/L. The effect is specific for the activation of platelets by thrombin, but not by ADP or collagen.
We considered three possibilities to account for the inhibition of thrombin binding to platelets by kininogens. (1) Kininogens might form a complex with thrombin. However, HK did not inhibit the activity of thrombin on chromogenic substrates or on fibrinogen-to-fibrin conversion. Moreover, enzyme-linked immunosorbent assay (ELISA) techniques failed to demonstrate binding to immobilized HK, and a heterobifunctional noncleavable cross-linking agent failed to produce a complex.11 Furthermore, Hasan et al12 also failed to show an HK-thrombin complex. (2) HK could bind to the hepta-spanning G-protein–linked thrombin receptor. However, HK does not inhibit the activation produced by SFLLRN, the newly exposed peptide after cleavage of this receptor by thrombin. Although this finding does not rule out the G protein-linked receptor, we propose a third hypothesis. Harmon and Jamieson13 have shown that glycoprotein (GP) Ib is the high-affinity binding site for thrombin with Bmax = 50 sites/platelet and a kd of 0.3 nmol/L. Previous studies have shown that considerably more thrombin is needed to stimulate Bernard-Soulier platelets14 lacking GP Ib-IX-V, similar to the finding with kininogens.6 7 Using MoAbs to and ligands of GP Ib-IX-V and cells transfected with this platelet receptor complex, as well as recombinant and synthetic peptides derived from kininogen D3, we tested the hypothesis that kininogen inhibits thrombin binding to GP Ib, thereby modulating the ability of thrombin to aggregate platelets.
MATERIALS AND METHODS
125I was purchased from ICN Pharmaceuticals, Inc (Irvine, CA). Avidin-fluorescein isothiocyanate (FITC) was purchased from Sigma (St Louis, MO). All other reagents were of the best purity available. Human α-thrombin was a generous gift of Dr John W. Fenton II (Division of Laboratories and Research, New York State Department of Health, Albany, NY) and had an activity of 2400 NIH U/mg and a purity of 99.24%. Human HK (1.6 mg/mL), migrating as a single band of 120,000 daltons (reduced) on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), was purchased from Enzyme Research Laboratories (South Bend, IN). Echicetin was a gift of Dr Manling Peng (Department of Biochemistry, Temple University School of Medicine, Philadelphia, PA). All statistical probabilities were assessed using the Student's t-test.
Platelet isolation and aggregation.Platelet isolation and aggregation were performed as described previously.11 Briefly, an aliquot of platelets (>100,000/μL) in a 0.5-mL cuvette at 37°C was incubated with stirring and added inhibitors for 2 minutes; then, aggregation was monitored for 5 minutes after the addition of 2 nmol/L thrombin. Platelets deficient in GP Ib-IX-V were obtained from a patient with Bernard-Soulier disease15 and prepared similarly, except that the final platelet concentration was 36,000/μL. Higher concentrations of platelets were not possible due to the very low concentration of platelets in the patient's plasma.
Iodination of thrombin and HK.Iodination of thrombin and HK was performed by the iodogen method.16 The resulting preparation of 125I-α-thrombin had a specific radioactivity of 1.9 × 106 cpm/μg. HK was prepared similarly, yielding a specific radioactivity of 1.3 × 108 cpm/μg. 125I-HK retained greater than 90% of its coagulant activity compared with the unlabeled protein.
MoAbs.The MoAbs AK-1, which binds to GP IX close to its site of interaction with GP Ib; AK-2, which recognizes the 45-kD ligand-binding peptide tail of GP Ib; and AK-3, which binds to the macroglycopeptide of GP Ib,17 were provided by Dr William J Booth (University of Sydney, Westmead, Australia). AK-2 blocks thrombin binding to GP Ib, but does not inhibit thrombin-induced platelet aggregation, whereas AK-1 and AK-3 have no effect on thrombin binding. The MoAb to GP IX, FMC-25 (which does not block thrombin binding), was supplied by Dr Manling Peng and developed by Berndt et al.18 SZ 2, which only blocks thrombin binding at low concentrations but can inhibit thrombin-induced platelet aggregation, was purchased from Biodesign International (Kennebunk, ME) and recognized GP Ib (extracellular domain).19 The biotinylated antibody TM-60 (260 μg/mL) that binds to the N-terminal 45-kD peptide of GP Ib and also inhibits thrombin-induced platelet aggregation20 was a generous gift of Dr Naomasa Yamamoto (The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). The antihuman α-thrombin MoAb EST-7 was purchased from American Diagnostica Inc (Greenwich, CT).
Recombinant proteins and synthetic peptides.Exon products from the D3 portion of HK were expressed as GST-fusion proteins in E coli, cleaved by thrombin, and purified by high-pressure liquid chromatography (HPLC). Peptides were synthesized, purified, and characterized as described.11
Platelet binding assays.Platelets were adjusted to yield 1 × 105 cells in 1 μL in a total volume of 50 μL, including all test reagents. HK, thrombin, or antibodies were incubated for 30 minutes. To measure specific binding, the total 125I-thrombin binding was determined and the binding in the presence of a 100-fold molar excess unlabeled ligand (nonspecific binding) was subtracted. All platelet binding assays were performed in 96-well MultiScreen DV 0.65 μmol/L hydrophilic plates and washed with 10 mg/mL bovine serum albumin (BSA) in HEPES/Tyrodes buffer, pH 7.3, on a MultiScreen Vacuum Filtration Manifold system (Millipore Corp, Bedford, MA). After washing and removal of the excess radioactive reagent by vacuum extraction, the filters were separated from the plate with a Millipore Multi-punch apparatus into individual tubes and counted for 1 minute in an LKB-Wallac 1274 RiaGamma counter (LKB-Wallac, Turku, Finland). In the fluorescence assays, biotinylated antibody binding was detected with FITC-labeled avidin (10 μL of a 1-20 dilution of the stock reagent) and incubated for 30 minutes at 23°C. After washing and removal of the supernatant by vacuum, plates were analyzed for fluorescent antibody bound to the platelets at an excitation wavelength of 485 nm and detected at an emission wavelength of 530 nm in a Millipore fluorescence microtiter plate reader.
Cell lines.L2H/V cells are transfected mouse L cells that stably express the full GP Ib-IX-V complex (GP Ibα, GP Ibβ, GP IX, and GP V).21 To make this cell line, we transfected the GP V gene22 into a previously isolated clone (L2H)23 that only expressed three components of the complex (GP Iba, GP Ibβ, and GP IX). The cells were cultured in a mixture of Dulbecco's modified Eagle's medium and F12 medium (DMEM/F12, 1:1; Life Technologies Inc, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS). The medium contained 1 mmol/L sodium hypoxanthine, 4 μmol/L aminopterin, 160 μmol/L thymidine (HAT; Life Technologies, Inc) and 500 μg/mL hygromycin. The cells were maintained in an atmosphere of 5% CO2 and 99% humidity.
Flow cytometry ligand binding assay.Thrombin binding to L2H/V cells was evaluated with flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Confluent L2H/V and parental L cells (negative control) were washed with 0.1 mol/L Na phosphate, pH 7.4, 0.15 mol/L NaCl (phosphate-buffered saline [PBS]) and incubated overnight with DMEM/F12 medium containing 1% BSA to eliminate any potential effects from von Willebrand factor (vWF ) or protease inhibitors that may have been present in the FBS. The cells were detached with 0.25 mmol/L EDTA, washed with PBS, and then suspended in PBS containing 1% BSA to a concentration of 2 to 2.5 × 106 cells/mL. HK, peptide, or buffer was then added and incubated for 30 minutes at 37°C. Thrombin was added to the cell suspension to a final concentration of 0.5 nmol/L and the cells were incubated at 37°C for 20 minutes. Unbound thrombin was removed by washing the cells twice with PBS. The thrombin that remained bound to the cells was recognized by staining with 0.15 μg/mL of the monoclonal antithrombin antibody EST-7 (American Diagnostica) for 60 minutes at room temperature followed by 1 mg/mL of FITC-conjugated rabbit antimouse IgG (Zymed, South San Francisco, CA) for 30 minutes at room temperature. At the end of the incubation, unbound antibodies were removed by washing the cells twice in PBS and binding was analyzed by flow cytometry. An argon ion laser was used to excite the fluorophor at 488 nm; a 520-nm bandpass filter was used to detect the emitted light.
Two methods were used to control for nonspecific binding. First, the binding of thrombin to L2H/V cells was compared with thrombin binding to parental L cells, which served as negative controls. A second control was to omit thrombin and incubate the cells only with the primary and secondary antibodies.
RESULTS
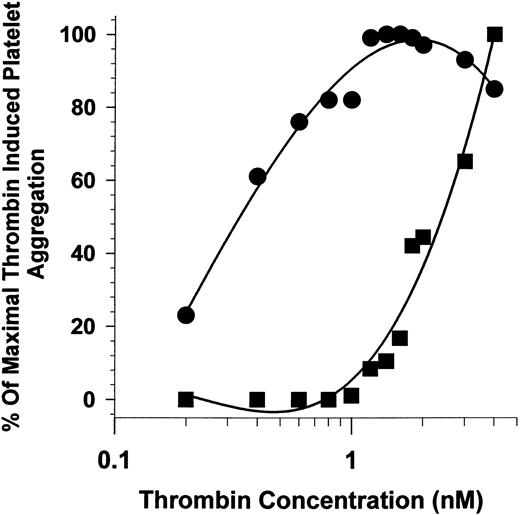
LNAENNA-induced shift of thrombin-induced platelet aggregation.The heptapeptide L271-A277 (LNAENNA) coded for by part of exon 7 of HK has been shown to inhibit thrombin-induced platelet aggregation in a concentration-dependent manner (IC50 = 65 μmol/L)11 at fixed thrombin concentrations. In this experiment, a fixed concentration of LNAENNA shifted the dose response of thrombin to induce platelet aggregation more than sevenfold, from an EC50 of 2.5 nmol/L in the presence of LNAENNA (400 μmol/L) compared with 0.35 nmol/L for platelets in its absence of LNAENNA (Fig 1). This finding is similar to the increased requirement for thrombin to activate platelets previously shown in the presence of plasma kininogens.7,11 These data recall the findings of reduced thrombin aggregation and a shift of concentration dependence seen in Bernard-Soulier platelets lacking the GP Ib-IX-V receptor complex.24 These results raised the possibility that the kininogen inhibition of thrombin-induced platelet aggregation may be due to interaction of kininogens with GP Ib-IX-V on the platelet membrane, modulating the high-affinity binding of thrombin to this receptor.
Effect of LNAENNA on platelet aggregation as a function of thrombin concentration. The extent of platelet aggregation, stimulated by α-thrombin at concentrations from 0.2 to 4 nmol/L, was measured in the presence (▪) and absence (•) of 400 μmol/L LNAENNA. Maximum platelet aggregation in the absence of the peptide was observed between 1 and 2 nmol/L thrombin and the results normalized with 2 nmol/L thrombin equal to 100%.
Effect of LNAENNA on platelet aggregation as a function of thrombin concentration. The extent of platelet aggregation, stimulated by α-thrombin at concentrations from 0.2 to 4 nmol/L, was measured in the presence (▪) and absence (•) of 400 μmol/L LNAENNA. Maximum platelet aggregation in the absence of the peptide was observed between 1 and 2 nmol/L thrombin and the results normalized with 2 nmol/L thrombin equal to 100%.
Thrombin-induced aggregation of Bernard-Soulier platelets.Blood was collected in ACD from a patient with well-characterized Bernard-Soulier disease whose platelets contained less than 3% of normal GP Ib-IX-V.15 The platelets were separated from plasma by gel filtration. These platelets are present at the normal concentration in plasma; therefore, the final platelet count was significantly reduced in the aggregation cuvette (36,000 platelets/μL). Preincubation with 2 μmol/L HK followed by stimulation with thrombin (4 nmol/L) resulted in an inhibition of the rate of platelet aggregation of only 14% compared with these platelets in the absence of HK. In contrast, as previously reported,7 11 thrombin aggregation of normal gel-filtered platelets was inhibited by the presence of HK. Under similar conditions (4 nmol/L thrombin and 2 μmol/L HK), the inhibition was 52%. Full inhibition was achieved when thrombin was reduced to 2 nmol/L in the normal platelets.
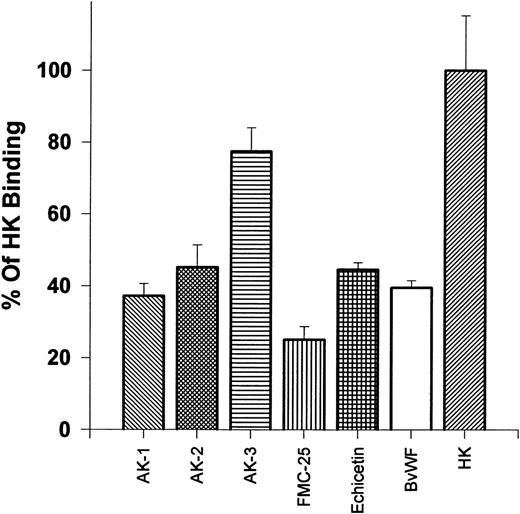
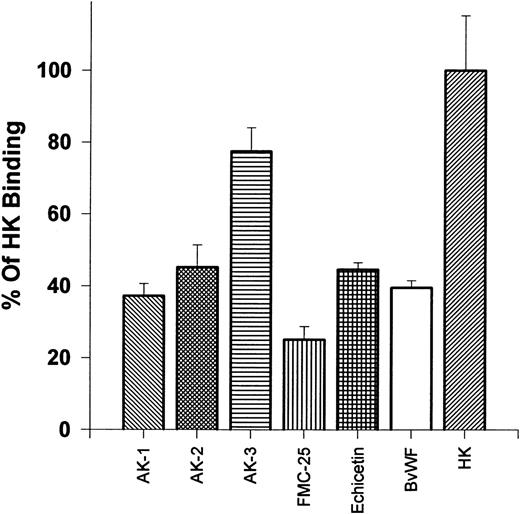
Effects of MoAbs and ligands of the GP Ib-IX-V complex on 125I-HK binding to platelets.We postulated that HK might bind to the GP Ib-IX-V complex on unstimulated platelets. To test this hypothesis, we evaluated the binding of 125I-HK to normal gel-filtered platelets in the presence of several MoAbs that recognize various regions of the GP Ib-IX-V complex (Fig 2). HK binding was reduced by more than 50% by 1.68 μmol/L of AK-2, which recognizes the 45-kD peptide tail region of the extracellular domain containing the thrombin and vWF binding sites of GP Ib, and 1.68 μmol/L of AK-1, which binds to GP IX near the ligand binding sites on GP Ib. In contrast, HK binding was minimally inhibited (<20%) by AK-3 (1.68 μmol/L), which recognizes the macro-glycopeptide core of the α-chain of GP Ib, which does not contain the 45-kD peptide tail.17 An MoAb directed to GP IX, FMC-25 (1.68 μmol/L), strongly (>75%) blocked HK binding. Echicetin (1.68 μmol/L), a snake venom protein that binds tightly to both the thrombin and vWF binding regions on the N-terminal portion of GP Ib,25 inhibited binding of HK to platelets greater than 50%. Bovine vWF (1.68 μmol/L), which binds to the N-terminal region of GP Ib in a region close to the thrombin binding site,26 blocks HK binding (40%). These results show that 125I-HK binding to platelets could be inhibited by antibodies directed to the GP Ib-IX-V complex or protein ligands that have been previously shown to bind to this high-affinity platelet receptor for thrombin. All antibodies and ligand means were significantly lower compared with 125I-HK alone (P < .0005), with the exception of AK-3.
Effects of antibodies: reaction on gel filtered platelets ability to bind 125I-HK. 125I-HK (9 nmol/L) binding to gel-filtered platelets was monitored after incubating for 30 minutes with the MoAbs AK-1, AK-2, AK-3, and FMC-25 or the protein ligands echicetin and bovine vWF (all additions at 1.68 μmol/L). The total binding defined as 100% was that observed in the absence of antibody or ligand. The results are the mean ± SEM of two experiments in quadruplicate. The differences are statistically significant (P < .0005), except for AK-3, compared with 125I-HK binding alone.
Effects of antibodies: reaction on gel filtered platelets ability to bind 125I-HK. 125I-HK (9 nmol/L) binding to gel-filtered platelets was monitored after incubating for 30 minutes with the MoAbs AK-1, AK-2, AK-3, and FMC-25 or the protein ligands echicetin and bovine vWF (all additions at 1.68 μmol/L). The total binding defined as 100% was that observed in the absence of antibody or ligand. The results are the mean ± SEM of two experiments in quadruplicate. The differences are statistically significant (P < .0005), except for AK-3, compared with 125I-HK binding alone.
Effect of HK on the ability of 125I-SZ 2 MoAb binding to platelets.The MoAb SZ 2, directed to the α-subunit of GP Ib and able to precipitate the GP Ib-IX-V complex, has been shown to partially inhibit thrombin binding to platelets.19 To determine if HK could directly block access to the GP Ib-IX-V complex by the MoAb, 125I-SZ 2 (53 nmol/L) was incubated in the absence or presence of increasing amounts of HK (0.55 to 1.01 μmol/L) and compared with the effect of excess unlabeled SZ 2 (0.88 μmol/L; Fig 3). A 10-fold molar excess of HK significantly (P < .002) inhibited the binding of SZ 2 to platelets (44% ± 11.7% standard error of the mean [SEM]) and could further reduce SZ 2 binding at increasing concentrations of HK with 84% ± 3.2% SEM inhibition found at 1.01 μmol/L. A 20-fold excess of unlabeled SZ 2 was capable of reducing labeled binding to 67% ± 7.9% SEM compared with incubation with 125I-SZ 2 alone. This experiment suggests that HK may bind directly to GP Ib and compete with the antibody for the high-affinity binding site for thrombin on the platelet surface.
HK blocks 125I-MoAb SZ 2 (anti-GP Ib) binding to platelets. The binding of the anti-GP Ib-IX-V MoAb 125I-SZ 2 (53 nmol/L) incubated with gel-filtered platelets was measured in the presence of increasing concentrations of HK. Unlabeled SZ 2 added at 0.88 μmol/L was assessed for its ability to reduced 125I-SZ 2 binding compared with 125I-SZ 2 alone, which was set equal to 100%. The results are the mean ± SEM of quadruplicate replicates of a single experiment. All points, compared with SZ 2 alone, were significant (P < .0005).
HK blocks 125I-MoAb SZ 2 (anti-GP Ib) binding to platelets. The binding of the anti-GP Ib-IX-V MoAb 125I-SZ 2 (53 nmol/L) incubated with gel-filtered platelets was measured in the presence of increasing concentrations of HK. Unlabeled SZ 2 added at 0.88 μmol/L was assessed for its ability to reduced 125I-SZ 2 binding compared with 125I-SZ 2 alone, which was set equal to 100%. The results are the mean ± SEM of quadruplicate replicates of a single experiment. All points, compared with SZ 2 alone, were significant (P < .0005).
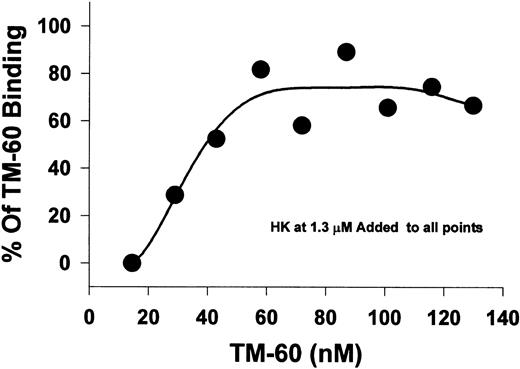
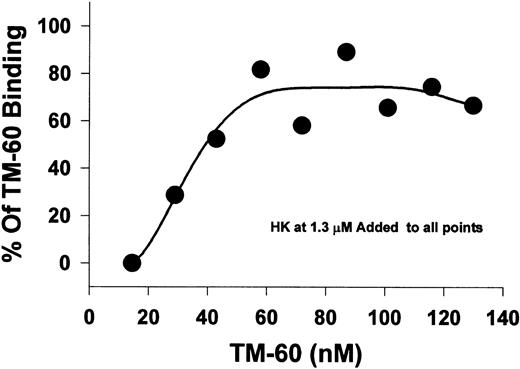
Effect of HK on biotinylated TM-60 binding to platelets.In this experiment, we used a fixed HK concentration (1.3 μmol/L) to measure the concentration dependence of the binding of the biotinylated MoAb, TM-60, which inhibits thrombin- and ristocetin-induced platelet aggregation directed to GP Ib-IX-V. A fluorescence assay was used to measure the amount of MoAb, TM-60, bound to platelets (Fig 4). At low concentrations of TM-60 (10 nmol/L), binding was completely inhibited by HK. As the ratio of TM-60 to HK was increased, additional binding of TM-60 was observed. Thus, HK competed for binding with TM-60 directed to GP Ib-IX-V, presumably by binding to GP Ib, and the inhibition was reversed by high concentrations of TM-60 (60 nmol/L or higher).
The displacement of platelet TM-60 binding to GFP by HK. Gel-filtered platelets were incubated with increasing concentrations of biotinylated TM-60 in the presence of 1.3 μmol/L HK at 23°C. Measurement of the bound fluorescence shows that HK could directly compete for TM-60 binding to platelets at low antibody concentrations. The effect could be almost abolished at higher antibody concentrations greater than 60 nmol/L.
The displacement of platelet TM-60 binding to GFP by HK. Gel-filtered platelets were incubated with increasing concentrations of biotinylated TM-60 in the presence of 1.3 μmol/L HK at 23°C. Measurement of the bound fluorescence shows that HK could directly compete for TM-60 binding to platelets at low antibody concentrations. The effect could be almost abolished at higher antibody concentrations greater than 60 nmol/L.
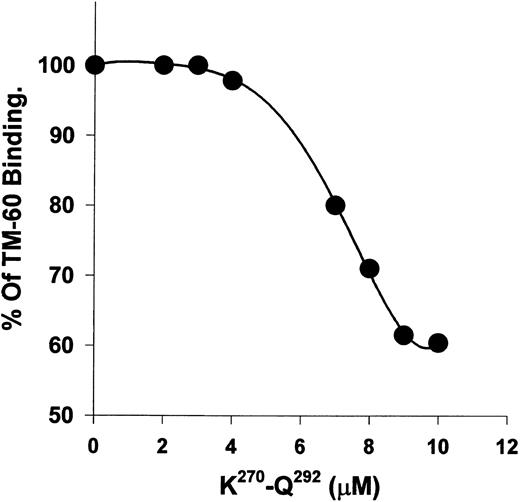
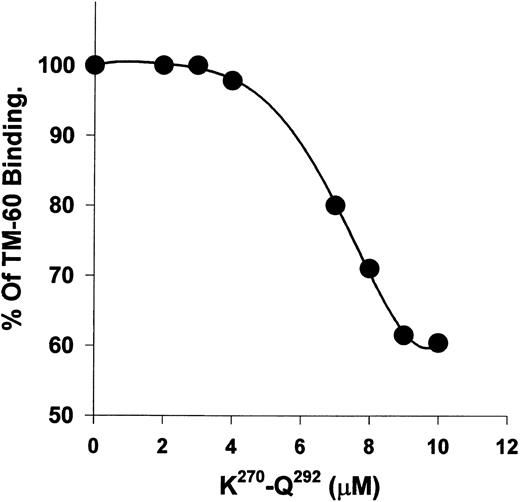
Recombinant polypeptide K270-Q292 from kininogen D3 alters the binding of TM-60 to platelets.The recombinant polypeptide, K270-Q292, was previously shown to inhibit thrombin-induced platelet aggregation with an IC50 of 19 μmol/L. When K270-Q292 was preincubated with platelets, TM-60 binding was blocked with increasing concentrations (2 to 100 μmol/L), reaching a plateau at a maximum binding of 60% of control (Fig 5). Similarly, when 100-fold molar excess (to TM-60) of D-Phe-Pro-Arg-chloromethyl ketone (PPACK)-inactivated α-thrombin was used, the antibody binding to platelets was also 60% of control. The polypeptide fragment of kininogens could only achieve partial blocking of TM-60 binding, unlike intact HK, which was a more potent blocker of this antibody directed to GP Ib. GST alone did not alter the binding of TM-60.
K270-Q292 from D3 of HK blocks TM-60 binding to platelets. Biotinylated TM-60 (58 nmol/L) binding to gel-filtered platelets was measured fluorometrically with binding in the absence of peptide set equal to 100%. The peptide concentration was from 2 to 10 μmol/L. Concentrations from 10 to 100 μmol/L of peptide did not result in further blocking greater than 50% and therefore are not shown. Note that the ordinate begins at 50% binding, reflecting the maximal inhibition encountered for the peptide effect on thrombin.
K270-Q292 from D3 of HK blocks TM-60 binding to platelets. Biotinylated TM-60 (58 nmol/L) binding to gel-filtered platelets was measured fluorometrically with binding in the absence of peptide set equal to 100%. The peptide concentration was from 2 to 10 μmol/L. Concentrations from 10 to 100 μmol/L of peptide did not result in further blocking greater than 50% and therefore are not shown. Note that the ordinate begins at 50% binding, reflecting the maximal inhibition encountered for the peptide effect on thrombin.
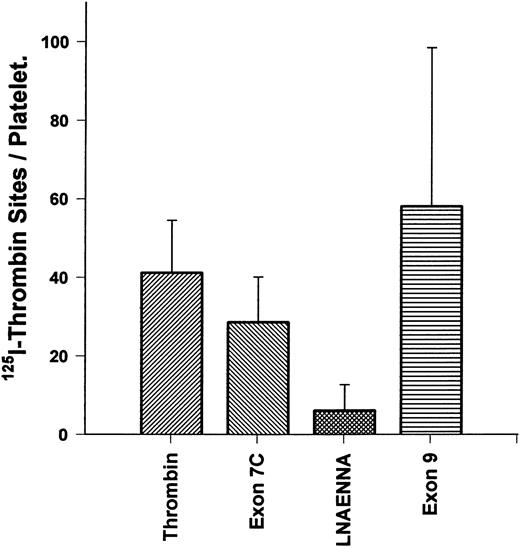
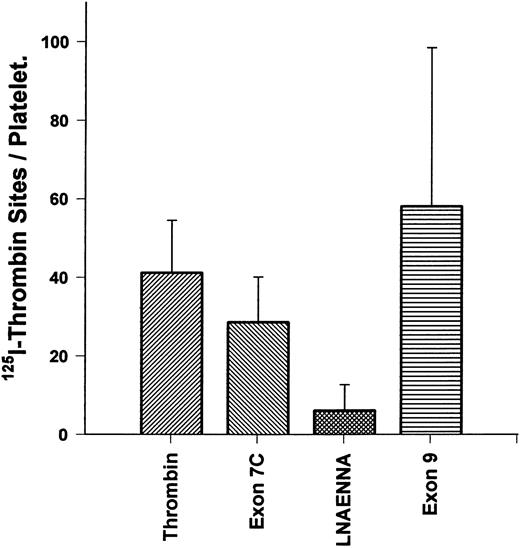
Kininogen derivatives alter 125I-thrombin binding to the platelet high-affinity site.Direct binding of 125I-thrombin to platelets was performed to determine if the number of high-affinity binding sites could be reduced by various fragments derived from kininogen D3 (Fig 6). Because the kd of the high-affinity site is 0.3 nmol/L, we first studied thrombin binding from 0.1 to 0.6 nmol/L as reported previously27; 0.3 nmol/L yielded approximately 50% of maximal binding in that range. Thrombin (0.3 nmol/L) binding to platelets alone yielded an average of 41 ± 13.3 SEM sites per cell, in good agreement with previous studies27 that found that GP Ib-IX-V complex only accounted for the highest affinity, lowest capacity binding site. We found that a peptide (C333NAEVYVVPGC343; 100 μmol/L), derived from exon 9 (with the penultimate W changed to G and terminal amino acid changed from E to C to allow disulfide formation and folding), similar to the binding site for HK to endothelial cells,10 did not significantly alter the number of high-affinity sites for thrombin (58 ± 40 SEM). In contrast, the peptide derived from the exon 7 product of HK, K270-Q292 (100 μmol/L), and the heptapeptide L271-A277 (100 μmol/L) could reduce high-affinity thrombin binding to 29 ± 11.5 SEM and 6 ± 6.6 SEM sites per platelet, respectively (P < .05). These results show that a primary site on kininogens for inhibition of the thrombin binding to the high-affinity receptor (GP Ib-IX-V complex) resides within the exon 7 product of kininogen D3.
HK exon 7 products alters 125I-thrombin binding sites/platelet. Gel-filtered platelets were incubated with 0.3 nmol/L 125I-α-thrombin to measure the high-affinity binding sites per cell to assess the effect of peptides from D3 of HK on thrombin binding. The first bar shows specific thrombin binding, in the absence of peptides. The second bar shows specific binding in the presence of K270-Q292 (100 μmol/L). The third bar shows specific binding in the presence of LNAENNA (100 μmol/L). The fourth bar shows specific binding in the presence of exon 9-coded peptide (100 μmol/L). Only products of exon 7 were able to block thrombin binding to the platelet high-affinity receptor for thrombin. The results are the mean ± SEM of six determinations in quadruplicate and, with the exception of the exon 9-coded peptide, are significantly different compared with the binding of thrombin alone (P < .05).
HK exon 7 products alters 125I-thrombin binding sites/platelet. Gel-filtered platelets were incubated with 0.3 nmol/L 125I-α-thrombin to measure the high-affinity binding sites per cell to assess the effect of peptides from D3 of HK on thrombin binding. The first bar shows specific thrombin binding, in the absence of peptides. The second bar shows specific binding in the presence of K270-Q292 (100 μmol/L). The third bar shows specific binding in the presence of LNAENNA (100 μmol/L). The fourth bar shows specific binding in the presence of exon 9-coded peptide (100 μmol/L). Only products of exon 7 were able to block thrombin binding to the platelet high-affinity receptor for thrombin. The results are the mean ± SEM of six determinations in quadruplicate and, with the exception of the exon 9-coded peptide, are significantly different compared with the binding of thrombin alone (P < .05).
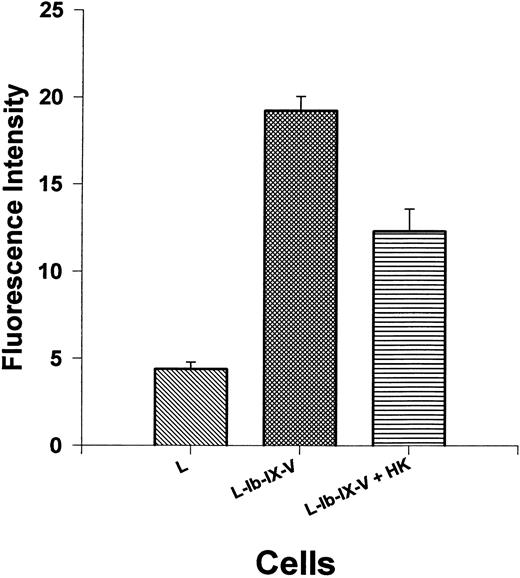
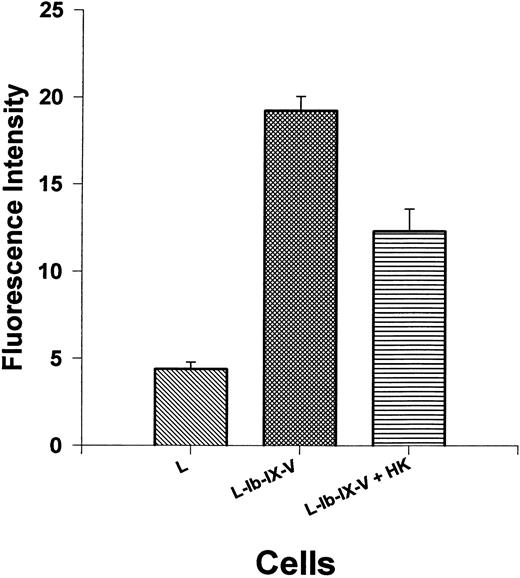
Thrombin binding to cellular GP Ib-IX-V complex is regulated by HK.The preceding experiments indicate that thrombin stimulation of isolated platelets is partially regulated by HK on the platelet surface, and the GP Ib-IX-V complex may be the primary site for this regulatory effect. However, the hepta-spanning G-protein–coupled receptor represents another potential site. Therefore, L cells, a cell line derived from mouse fibroblasts transfected with GP Ib-IX-V complex, were used to measure the interaction of thrombin and HK with this receptor complex. FITC-labeled thrombin (590 nmol/L) was incubated with L cells or L cells expressing the GP Ib-IX-V complex in the presence or absence of HK (1.3 μmol/L; Fig 7). These cells have been shown to bind thrombin with high affinity.27a Thrombin could be detected on L cells, by flow cytometry, expressing the GP Ib-IX-V complex with a fluorescent intensity of more than four times (19.2 ± 2.4 SEM) the levels of control L cells without GP Ib-IX-V (4.4 ± 1.2 SEM). HK significantly (P < .0001) reduced fluorescent thrombin binding to 12.3 ± 3.8 SEM or by 53% after subtraction of the contribution of the untransfected control cells. This experiment shows that HK competes with thrombin for high-affinity sites to the cellular GP Ib-IX-V complex.
Thrombin binding to GP Ib-IX-V expressed on leukemic mouse fibroblasts cells is inhibited by HK. Mouse fibroblast cell line transfected with GP Ib-IX-V complex was tested for the ability to bind thrombin with or without added HK. The first bar shows biotinylated thrombin (590 nmol/L) binding to untransfected cells. Thrombin binding to cells transfected with and expressing GP Ib-IX-V complex in the absence (bar 2) or presence (bar 3) of 1.3 μmol/L HK is shown. The results are the mean ± SEM of 10 determinations. The binding to L cells and the effect of HK addition to L-Ib-IX-V cells are statistically different from the binding to L-Ib-IX-V cells alone (P < .0001).
Thrombin binding to GP Ib-IX-V expressed on leukemic mouse fibroblasts cells is inhibited by HK. Mouse fibroblast cell line transfected with GP Ib-IX-V complex was tested for the ability to bind thrombin with or without added HK. The first bar shows biotinylated thrombin (590 nmol/L) binding to untransfected cells. Thrombin binding to cells transfected with and expressing GP Ib-IX-V complex in the absence (bar 2) or presence (bar 3) of 1.3 μmol/L HK is shown. The results are the mean ± SEM of 10 determinations. The binding to L cells and the effect of HK addition to L-Ib-IX-V cells are statistically different from the binding to L-Ib-IX-V cells alone (P < .0001).
DISCUSSION
One receptor involved in the ability of HK to modulate thrombin interaction with platelets appears to be the GP Ib-IX-V complex. Several lines of evidence support this conclusion. Platelets from patients with Bernard-Soulier disease require 10-fold the thrombin concentration to cause complete aggregation, and, unlike normal platelets, this thrombin-induced aggregation is not inhibited by HK. The 10-fold increase in thrombin concentration for platelet activation is similar to that observed when kininogen is added to either purified systems or to kininogen-deficient plasma.7 Certain antibodies to GP Ib, such as TM-60, also induced this shift of the thrombin dose-response curve.28 It is notable that the inhibitory effect of HK is only observable at thrombin concentrations less than 2 nmol/L, in the range of the known high-affinity binding site for thrombin to GP Ib. MoAbs to the extracellular portion of GP Ib, AK-2, which recognize the 45-kD peptide tail containing the ligand binding domain,17 and AK-1 to GP IX both block HK binding to platelets. Alternatively, AK-3, directed to the macroglycopeptide lacking the ligand-binding domain, causes minimal inhibition. Conversely, HK inhibits the binding of MoAb SZ 2 (directed to GP Ib) to platelets. The binding of TM-60 (directed to the GP Ib-IX-V complex and known to inhibit thrombin activation of platelets) to platelets is also inhibited by HK. The MoAbs, FMC-25 and AK-1, both to GP IX, inhibit the binding of HK to platelets, suggesting that GP IX and, thus, the heterodimeric complex are also involved in HK binding. Alternatively, antibodies to GP IX might interfere with higher order complexes with more than one copy of each component. Because antibodies can produce effects by steric hindrance, we also used a low molecular mass (28-kD dimer) ligand, echicetin, that is known to bind to GP Ib,25 inhibiting both thrombin and vWF29 interaction with that receptor. This naturally ocurring inhibitor blocks binding of HK to platelets. The vWF and thrombin binding sites, although not identical on GP Ib, are spatially proximate. We have also observed that bovine vWF inhibits HK binding. Bovine vWF was used because it does not require ristocetin for its binding,30 as does human vWF, thus obviating that complication. These studies all indicate that HK binds to the GP Ib-IX-V receptor on platelets.
To show that HK inhibits the high-affinity binding of thrombin to platelets, we have performed direct binding of 125I-thrombin to platelets at very low concentrations. We have reproduced the results of Harmon and Jamieson13 and De Marco et al27 and found 41 sites compared with 40 to 50 sites in their studies. We have shown that HK inhibited this binding. Finally, we have shown that HK partially inhibits the binding of thrombin to cells transfected with GP Ib-IX-V. The findings that minimal binding was observed in the untransfected cells and that the binding to the transfected cell was inhibited by HK, support the ability of HK to compete with thrombin for binding to GP Ib-IX-V. However, the inhibition of high-affinity thrombin binding was only 52%. We are uncertain of the explanation for why we were only able to inhibit thrombin binding to the L cells by 50%. The existence of other potential binding sites in mouse cells is not likely to be the reason because the control is an untransfected L cell that is basically equivalent to cells expressing the GP Ib-IX-V complex. The only differences between the two cells other than the presence of the complex is that those expressing the complex are also expressing the genes that mediate resistance to HAT medium (the herpes simplex thymidine gene) and hygromycin are grown in these selective media. It is possible, although unlikely, that expression of these resistance genes or growth in the media somehow increases the nonspecific thrombin binding of the complex-expressing cells. Another possibility is that because the ratio of HK to thrombin was about two to one, transfected L cells may have a higher number of copies of GP Ib-IX-V, resulting in an insufficient ratio for full inhibition.
Recently, Connolly et al31 showed that gene knockout of the G-protein–coupled thrombin receptor failed to abolish thrombin-induced aggregation in murine platelets but did abolish thrombin responses in the murine fibroblasts of that mouse. The discovery that a second protease-activated receptor (PAR2) is part of a thrombin receptor gene family32 suggests that existence of a putative second G-protein–linked thrombin receptor on platelets. However, it appears that murine fibroblasts (L cells) contain the original G-protein–coupled receptor.
In a recent study,11 we mapped the region on kininogen domain 3 to the N-terminal segment of exon 7, the product K270-Q292 (IC50 = 19 μmol/L), and defined the minimum necessary sequence as LNAENNA (IC50 = 65 μmol/L) for inhibition of thrombin-induced platelet aggregation. In contrast, a peptide encoded by exon 9 fails to modulate thrombin activation of platelets. That polypeptide is similar to a region reported by Hasan et al12 as a binding site on kininogens to endothelial cells. The same investigators found two other discontinuous sites on kininogen that make up a composite binding site. It is conceivable that the exon 9-encoded peptide contains a binding site on platelets distinct from GP Ib-IX-V that does not interfere with thrombin binding or activation of platelets. Our findings suggest that both K270-Q292 and LNAENNA could interact with GP Ib-IX-V. In favor of this theory is the demonstration that these polypeptides can shift the potency of thrombin about 10-fold, similar to HK in platelet-rich plasma.7 The polypeptides also directly inhibit 125I-thrombin binding to platelets. LNAENNA is a discrete region on the surface of D3 that probably represents a minimum sequence for kininogens to inhibit thrombin binding.11
The binding of thrombin to the high-affinity site of GP Ib may allow a local increase in thrombin to a level that can then cleave the intermediate-affinity seven-transmembrane receptor to expose SFFLRN, which then binds to the exosite on the hepta-spanning polypeptide. The precise mechanism by which the GP Ib-IX-V complex is involved in thrombin's action is unclear. One possibility is that the complex sequesters thrombin and increases its local concentration to a point at which it can cleave the intermediate-affinity seven-transmembered-domain receptor. Alternatively, the complex may itself be involved in thrombin-induced signaling, which may act synergistically with the contribution of the seven-transmembered-domain receptor. Kininogen seems to act as a competitor for the receptor, with 10-fold more thrombin being required to stimulate the platelet, a situation similar to that seen in Bernard-Soulier disease platelets.
The HK peptide, LNAENNA, contains an identical tetrapeptide, NAEN, found in GP Ib at residues 223-226 and located within a 24 amino acid sequence that has been shown to inhibit thrombin-induced platelet aggregation.33 This observation strongly suggests that this region of HK may compete with GP Ib, thus blocking thrombin binding. The presence of an identical sequence in GP Ib is seemingly contradictory to the observation that HK blocks thrombin by binding directly to GP Ib and preventing access to thrombin. This apparent contradiction might be reconciled if the HK-homologous sequence in GP Ib is involved in an intramolecular association with another region of the polypeptide. Competition for that region by HK would be expected to change the conformation of GP Ib and, thus, prevent thrombin binding. Thrombin binds to at least one other site distinct from NAEN,34 and the bound HK could interfere sterically with thrombin binding by competing with GP Ib for binding of thrombin. This hypothesis would also explain why HK blocks the binding of the antibody AK-2, which does not inhibit thrombin-induced platelet aggregation.
Similar to the findings of Greco and Jamieson35 with vWF, we have also observed a maximal inhibition of the GP Ib antibody, TM-60, by HK of only 50%. One explanation of this finding would be provided by a two-receptor model for HK binding to platelets. Stimulated platelets bind fivefold as much HK as do unstimulated platelets, and this increased binding is due to thrombospondin expressed on the activated platelet surface.36 The finding that HK blocking of thrombin antibodies or GP Ib antibodies did not demonstrate a requirement for zinc, in contrast to the absolute requirement for HK binding as reported by Greengard et al37 for stimulated platelets, again suggests the existence of two receptors for HK for platelets. The zinc requirement for HK may be unnecessary for the high-affinity receptor (GP Ib-IX-V complex), but essential for binding to stimulated cells.
The ability of HK to inhibit thrombin-induced aggregation of platelets has two important ramifications. First, in conditions in which HK is depleted, such as the adult respiratory distress syndrome (due to septic shock and multiple trauma),38 thrombin may stimulate platelets at lower concentrations, enhancing disseminated intravascular coagulation. Second, polypeptides from HK or GP Ib or peptomimetic derivatives of these sequences may serve to inhibit thrombin-induced platelet aggregation in situations such as reocclusion after angioplasty or thrombolysis39 without interfering with the other hemostatic functions of thrombin and/or its action on fibrinogen to form a fibrin clot.
NOTE ADDED IN PROOF
In a recent abstract, Joseph et al,40 using an HK affinity column, isolated GPIb from platelet membrane lysate, thus confirming our results by a totally different approach.
ACKNOWLEDGMENT
The authors thank Dr Yingzhang Lin and Leonard Rick for their assistance in producing the recombinant proteins used in this study.
Supported by National Institutes of Health (NIH) Grant No. HL54894 (R.W.C.), a Clinical Investigator Award HL02681 (R.A.D.) from the NIH, a grant from the W.W. Smith Charitable Trust (S.P.K.), an Established Investigator Award from the American Heart Association (national) (S.P.K.), and NIH Grants No. HL02463 and HL46416 (J.A.L.).
Address reprint requests to Robert W. Colman, MD, Sol Sherry Thrombosis Research Center, Temple University School of Medicine, 3400 N Broad St, Philadelphia, PA 19140.